Improving Medication-Related Outcomes in Chronic Liver Disease
Supported by the Health Innovation, Investment and Research Office of Queensland Health (Clinical Research Fellowship to K.H.).
Potential conflict of interest: Nothing to report.
Abstract
Patients with chronic liver disease (CLD) are becoming increasingly complex due to the rising prevalence of multimorbidity and polypharmacy. Medications are often essential to manage the underlying liver disease, complications of cirrhosis and portal hypertension, and comorbidities. However, medication-related problems (MRPs) have been associated with adverse patient outcomes, including hospitalization and mortality. Factors that can contribute to MRPs in people with CLD are variable and often entwined. This narrative literature review discusses key barriers and opportunities to modify risk factors and improve medication-related outcomes for people with CLD.
Abbreviations
-
- ACEi
-
- angiotensin-converting enzyme inhibitor
-
- ADE
-
- adverse drug event
-
- ADR
-
- adverse drug reaction
-
- AKI
-
- acute kidney injury
-
- ARB
-
- angiotensin-receptor blocker
-
- CLD
-
- chronic liver disease
-
- GP
-
- general practitioner
-
- HE
-
- hepatic encephalopathy
-
- ICU
-
- intensive care unit
-
- MRP
-
- medication-related problem
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NSAID
-
- nonsteroidal anti-inflammatory drug
-
- PD
-
- pharmacodynamic
-
- PIM
-
- potentially inappropriate medicine
-
- PK
-
- pharmacokinetic
-
- PPI
-
- proton pump inhibitor
-
- QoL
-
- quality of life
-
- SBP
-
- spontaneous bacterial peritonitis
Chronic liver disease (CLD) is gaining recognition in the global community as a rising cause of health and economic burden. While the term CLD encompasses a broad range of liver pathologies, morbidity and mortality are highest among people with advanced-stage hepatic fibrosis (cirrhosis) and progressive portal hypertension. People with cirrhosis often experience debilitating complications of portal hypertension, impaired synthetic function, and reduced metabolism (of endogenous and exogenous substances), including development of ascites and hepatic encephalopathy (HE). Patients also frequently suffer non-liver-related comorbidities, including diabetes mellitus and cardiovascular and renal diseases.(1, 2)
To manage complications of CLD and comorbidities, patients consume on average nine medications each day from a variety of therapeutic classes.(3) Optimal use of medications is essential for positive health outcomes: treatment or cure of modifiable diseases, prevention and management of complications, reduced hospitalization, symptom control for quality of life (QoL), and lower risk of preventable death. However, taking more medications and inappropriate use of specific medications have been associated with negative outcomes, including hospitalization.(4-7) With the rising prevalence of multimorbidity and polypharmacy in CLD (particularly among patients with nonalcoholic fatty liver disease [NAFLD]),(8, 9) identification and resolution of medication-related problems (MRPs) that can contribute to negative health outcomes are essential.
MRPs are defined as events or circumstances involving drug therapy that actually or potentially interferes with desired health outcomes.(10) MRPs in cirrhosis can include nonadherence, adverse drug reactions (ADRs), drug–drug and drug–disease interactions, and indication, dosing, and monitoring issues (Table 1).(7, 11) Harm from an MRP may arise due to consumption of a medication (e.g., adverse drug event [ADE]) or failure to consume a medication. More than one type of MRP can contribute to an outcome (e.g., acute kidney injury [AKI] resulting from supratherapeutic diuretic use and suboptimal monitoring in a patient coprescribed an angiotensin-converting enzyme inhibitor [ACEi] for a cardiac comorbidity).
| Medication Group | Example MRPs | Potential Clinical Impact | Patient-Dependent Risk Classification* | ||
|---|---|---|---|---|---|
| Severity | Likelihood and Timeframe | Composite Risk | |||
| Diuretics | Dose too high | Mild electrolyte derangement | Minor-moderate |
Likelihood: possible-probable Harm may occur within days to months |
Medium-high |
| Monitoring issue | Mild dehydration | Minor-moderate | Medium- high | ||
| Drug–drug interaction (e.g., ACEi/ARB) | Severe dehydration; AKI; HRS | Severe-catastrophic | High | ||
| Nonadherence | Fluid overload | Moderate | Likelihood: unlikely-probable, dependent on history of ascites and prescribed diuretic dose | Medium-high | |
| Dose too low | Harm may occur within days to months | ||||
| Nonselective beta-blockers | Indication issue (e.g., primary prophylaxis of grade 1 varices) | Dizziness; postural hypotension | Minor-moderate | Likelihood: possible, with greater risk of severe harm in patients who are older and frail | Medium-high |
| Dose too high | Hypotension with falls | Severe | Harm may occur within days to months | High | |
| Drug–drug interaction (e.g., calcium channel blocker) | |||||
| Nonadherence | Variceal hemorrhage; ICU admission; death | Catastrophic | Likelihood: unlikely-probable, dependent on size of varices and history of bleeding; | Medium-high | |
| Harm may occur within weeks to years | |||||
| Spontaneous bacterial peritonitis (SBP) prophylaxis | Nonadherence | SBP recurrence | Severe | Likelihood: possible, dependent on history of ascites, SBP, and concomitant medications | High |
| Harm may occur within weeks to months | |||||
| Proton pump inhibitors | Dose too high | Abnormal absorption (e.g., some minerals, nutrients, medications) | Minor-moderate | Likelihood: possible, with greater risk of harm from nutritional deficiency in patients who are malnourished | Low-medium |
| Indication issue (e.g., no longer indicated) | Infection (e.g., SBP, Clostridium difficile) | Severe | Harm may occur within weeks to years | Medium-high | |
| Lactulose | Nonadherence Dose too low | Constipation | Minor-moderate | Likelihood: unlikely-probable | Medium-high |
| HE | Severe | Harm may occur within days to months | Medium-high | ||
| Dose too high | Diarrhea | Minor | Likelihood: possible-probable, but many patients self-cease due to diarrhea before severe harm can occur | Medium-high | |
| Electrolyte derangement; dehydration; AKI | Minor-severe | Harm may occur within days to months | Medium-high | ||
| Opioids | Dose too high | Constipation | Minor-moderate | Likelihood: possible-probable Harm may occur within days to months | Medium-high |
| Drug–drug interaction (e.g., benzodiazepines) | Sedation | Moderate | High | ||
| Confusion; HE | Severe | High | |||
| NSAIDs |
Drug–drug interaction (e.g., diuretics, ACEi/ARB) Drug–disease interaction (e.g., ascites, renal disease) |
AKI; HRS | Severe-catastrophic | Likelihood: possible-probable Harm may occur within days to months | High |
| Glucocorticoids | Adverse drug reaction | Irritability, mood disturbances | Minor-moderate | Likelihood: unlikely-possible | Medium-high |
| Harm may occur within days to months | |||||
- * Composite risk classification for the given examples was determined using a risk matrix tool(7) comprising individual measures of severity, likelihood, and duration of time until potential harm may occur. Patient-dependent risk of harm in the real world may be further influenced by age, concomitant medications, comorbidities, severity of liver disease, decompensation history, and other factors.
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; ICU, intensive care unit; NSAIDs, nonsteroidal anti-inflammatory drugs; SBP, spontaneous bacterial peritonitis.
The prevalence and impact of MRPs on outcomes in people with CLD have been variably studied in the literature. In a retrospective cross-sectional study of 400 admitted patients with cirrhosis, 21.5% experienced ≥1 potential drug–drug interaction, 46.0% were receiving ≥1 medication at an inappropriate dose, and 30.0% experienced ≥1 ADR.(12, 13) ADRs were the reason for hospital admission in 6.0% of patients; the authors deemed 78.1% of identified ADRs to be preventable.(13) In a prospective cohort study, 66.7% of 57 ambulatory patients with decompensated cirrhosis had ≥1 instance of nonadherence, 54.4% were taking ≥1 medication at an inappropriate dose, and 52.6% had ≥1 monitoring issue identified.(7) Within a 12-month follow-up period, 30.5% of unplanned admissions were medication related and 64.7% of these were considered preventable.(7) In 78 patients critically ill with decompensated cirrhosis admitted to a medical intensive care unit (ICU), 27% of MRPs identified by a clinical pharmacist were associated with preventable harm, including contribution to death in 3.8% of patients.(14) The incidence rate of “high-risk” MRPs has been linked with unplanned admissions and mortality in decompensated cirrhosis.(7)
Factors that can contribute to MRPs and medication-related outcomes in people with CLD are variable and often entwined. These factors may relate to patients, the health care system, and other social and economic barriers.(11) This narrative review discusses key barriers and opportunities to modify risk factors and improve medication-related outcomes for people with CLD.
Patient-Related Factors
People with CLD are heterogeneous in terms of disease etiology, severity, clinicodemographic characteristics, and multimorbidity. The risk of experiencing an adverse medication-related outcome may therefore be influenced by these variables.
Illness severity and polypharmacy
Unsurprisingly, the incidence rate of MRPs is higher in patients with more severe liver disease, greater comorbidity burden, and those taking more medications because this creates greater opportunity for problems to arise.(7) In some CLDs (e.g., viral hepatitis), disease-modifying agents can be used to treat the underlying cause of liver injury. However, most patients require therapeutic or prophylactic management of risk factors and liver-related complications throughout the course of disease. Patients with cirrhosis are also becoming increasingly complex with a greater comorbidity burden,(8, 15) requiring coprescription of multiple non-liver therapies. Polypharmacy (≥5 medications consumed daily) and hyperpolypharmacy (≥10 medications consumed daily) are prevalent in patients with various CLDs.(3, 5, 8, 9, 16, 17)
In people with decompensated cirrhosis, the number of medications prescribed at discharge has been shown to predict the rehospitalization rate and time to readmission, independently of disease severity.(5) Potential reasons for this include increased opportunity for (i) miscommunication between patients and clinicians about current therapies, (ii) confusion about dosing and monitoring requirements, and (iii) intentional and unintentional medication administration errors (by patients or caregivers). While a known risk factor for negative medication-related outcomes, the number of medications taken by patients with cirrhosis often increases over time.(18) Development of decompensation events (e.g., ascites, HE, variceal bleeding) often requires new pharmacotherapy. Side effects of prescribed medicines (e.g., nausea, headaches) are often managed by additional therapies. Other medicines such as proton pump inhibitors (PPIs) and antibiotics may be commenced prophylactically to prevent adverse effects of temporary medications (e.g., glucocorticoids) but inadvertently continued beyond the intended timeframe. Accumulation of medications and frequent changes to drug therapy can result in a complex regimen prone to medication discrepancies, mismanagement, and ADRs. Regular review of pharmacotherapy is therefore essential to reduce unnecessary drug burden and identify potentially inappropriate medicines (PIMs).
Knowledge, beliefs, and medication management behavior
Patient behavior in medication management is complex. This is reflected in the variety of objective and subjective measures used throughout the literature to assess and describe nonadherence. Regardless of the methodology employed (e.g., pill counts, medication possession ratio, self-reported questionnaires), nonadherence is relatively common among patients with various liver diseases.(16, 19-22) Nonadherent behavior, including with specific medications, has been associated with potentially preventable hospital admissions,(5-7) greater symptom burden, and lower QoL.(17, 19) Nonadherence is often thought of as an “unintentional” behavior; however, it can also be the result of an “intelligent” decision to not take medication as prescribed. In patients awaiting liver transplantation, taking more medications has been associated with nonadherence due to side effects, forgetfulness, and errors.(16) Lower self-perceived understanding of liver disease and negative beliefs about medications have also been linked with “low” medication adherence in decompensated cirrhosis.(17)
Factors that may influence health behaviors in CLD, especially adherence, are depicted in Fig. 1. For example, comorbidities (such as depression or drug and alcohol abuse) and competing pressures for limited financial resources can impact health decisions, including medication adherence. Poor patient knowledge (lack of appropriate disease and medicines education); low health literacy (poor understanding of sequelae); cultural and personal beliefs about illness, medications, and health care professionals (“doctors overuse medications,” “medications are not helpful or safe”); and the undesirable stigma surrounding liver disease may also impact patients’ ability or confidence to engage with health services to obtain treatment.(17, 23-26) This is particularly important in the management of viral hepatitis as nonadherence can lead to viral resistance, treatment failure, and increased risk of developing cirrhosis.
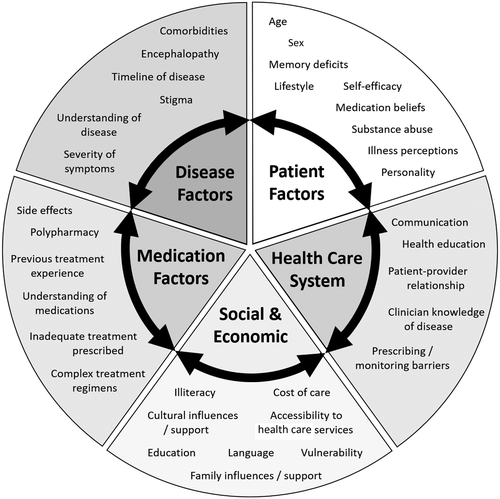
Many patients compare their perception of liver disease severity (including symptoms, timeline of progression, development of complications) with the perceived helpfulness and harms of treatment (side effects, complexity of therapy, long-term benefits of treatment).(17) Patients without fibrosis or those with early or compensated cirrhosis who are asymptomatic may display “intelligent” nonadherence due to adverse medication effects, which impact QoL. Even patients with decompensated cirrhosis who experience many symptoms related to disease may omit diuretics due to urinary urgency, withhold beta-blockers due to worsened lethargy and dizziness, or stop lactulose due to abdominal discomfort and flatulence.(19, 27, 28) Patient education about disease and medications, particularly consequences of disease progression, may help to address these issues for some patients. However, people with HE often have reduced cognitive activity and impaired learning, alertness, and memory,(29, 30) which can lead to confusion and negatively impact disease insight. Strategies to improve medication-taking behavior and reduce nonadherence in this group should consider reduced cognition and involve the caregiver or a family member if appropriate. It has been proposed that improved patient knowledge and adherence to therapy, especially diuretics and lactulose, may prevent unplanned hospitalization for management of ascites and encephalopathy and reduce disease impact on QoL.(5, 31)
Pharmacokinetic and pharmacodynamic changes
In people with CLD, pharmacokinetic (PK) and pharmacodynamic (PD; together, PKPD) alterations are driven by loss of hepatocyte density and function and development of vascular abnormalities. This most commonly occurs in patients with cirrhosis. PK changes may arise due to portosystemic shunting and diminished first-pass metabolism, resulting in higher bioavailability and peak plasma concentration of medicines. Decreased plasma proteins (e.g., albumin) and fluid retention (e.g., ascites) enlarge the volume of distribution and the fraction of unbound medicine. Loss of hepatocyte function decreases the activity of drug-metabolizing enzymes, resulting in reduced clearance and a longer elimination half-life. Overall, these PK alterations result in increased exposure (area under the plasma concentration time curve) to certain medications or metabolites.(32) Patients with cirrhosis are therefore at increased risk of ADRs, which may further contribute to intelligent nonadherence.
Increased susceptibility to ADRs has been demonstrated in a prospective study of 1,280 patients.(33) ADRs occurred with equal frequency in people with noncirrhotic liver disease and those without liver disease but significantly more frequently in patients with cirrhosis.(33) One third of ADRs have been associated with inappropriate drug dosing.(13) Patients may also have increased susceptibility to toxicologic effects of medications (PD change) due to existing disease complications, including thrombocytopenia (specific antibiotics, aspirin, nonsteroidal anti-inflammatory drugs [NSAIDs]), impaired immunity (prednisolone, azathioprine), and renal impairment (diuretics, angiotensin therapies, NSAIDs).(32) With regard to susceptibility to hepatotoxicity, an increased risk of drug-induced liver injury (DILI) in CLD has only been evidenced in literature for some specific medicines (e.g., methotrexate, antituberculosis drugs, and antiretroviral therapy).(34-37) Examples of clinically important PKPD changes in select medications (including PIMs) that occur in patients with cirrhosis are provided in Table 2.(38-53) To prevent ADRs due to these changes, health care professionals should carefully select an appropriate medication and dose adjust when prescribing.
| Medication | PKPD Change | Clinical Impact | Remarks |
|---|---|---|---|
| Alimentary tract medication | |||
| Metoclopramide(38) |
In Child-Pugh C cirrhosis:
|
Risk of somnolence, extrapyramidal symptoms | Use half the normal dose in Child-Pugh B/C cirrhosis |
| Pantoprazole(39) |
In Child-Pugh A/B cirrhosis:
|
Associated with development of HE and infection (especially SBP, C. difficile) | Esomeprazole appears to be the safest PPI choice in cirrhosis |
| Analgesics | |||
| Weak opioids: Codeine, tramadol(40, 41) |
Codeine:
|
Inadequate pain relief, risk of (worsening) HE |
Codeine: suboptimal analgesia due to reduced codeine activation to morphine. Tramadol is preferred (prolong dosing interval to 12 hours and in Child-Pugh C cirrhois also reduce starting dose) |
|
Tramadol:
|
Risk of (worsening) HE | ||
| NSAIDs(42, 43) |
|
Decreased glomerular filtration rate by ~30%; risk of AKI, hepatorenal syndrome |
Altered renal hemodynamics in people with ascites increases risk of harm. Avoid use in patients with cirrhosis |
| Antithrombotic agents | |||
| Clopidogrel(44) |
|
Therapeutic effect unpredictable |
Clopidogrel is a prodrug that requires hepatic activation and inactivation; both processes decrease with increasing severity of cirrhosis. Avoid in Child-Pugh C cirrhosis due to unpredictable effects |
| Rivaroxaban(45, 46) |
In Child-Pugh B cirrhosis:
|
Increased risk of bleeding | Apixaban, dabigatran, or edoxaban may be used with caution in Child-Pugh B cirrhosis; contraindicated in Child-Pugh C cirrhosis |
| Cardiovascular medication | |||
| Statins: Atorvastatin, simvastatin(47, 48) |
Atorvastatin:
|
Increased risk of muscle toxicity and rhabdomyolysis at higher doses | Low-dose simvastatin (20 mg/day) appears to be safe |
|
Simvastatin:
|
|||
| Nifedipine(48) |
|
Risk of hypotension, edema | Use half the normal initiation and maintenance dose; in Child-Pugh C cirrhosis, also double the dosing interval |
| Psychotropic medication | |||
| Sertraline(50, 51) |
|
Risk of nausea and vomiting, somnolence, and serotonin syndrome | Choose a selective serotonin reuptake inhibitor with lower hepatic extraction (e.g., citalopram or fluvoxamine) |
| Use half the normal citalopram/fluvoxamine dose in Child-Pugh B/C cirrhosis | |||
| Sleep aids: Zolpidem,(52) melatonin(53) |
Zolpidem:
|
Excessive drowsiness, risk of (worsening) HE |
Generally avoid all sleep aids, including “Z drugs” and melatonin. If a hypnotic is needed, use a benzodiazepine with a short half-life and a “simple” hepatic metabolism to minimize potential impact of PKPD changes (e.g., oxazepam or temazepam) |
|
Melatonin:
|
Unlikely beneficial in cirrhosis due to elevated endogenous melatonin levels | ||
- Abbreviation: PT, prothrombin time.
Health care professional factors
Safe prescribing
While the PKPD profile of most drugs is often unchanged in noncirrhotic CLD, many medications require dose adjustment, additional monitoring, or have relative contraindications in cirrhosis.(54) In a large retrospective cohort study of 5,618 patients with cirrhosis, 40% used a medication for which there are limited data to support safety and 60% used a potentially “unsafe” medication, such as pantoprazole or NSAIDs.(3) Nonspecialist clinicians prescribed (or repeated the prescription following initiation by a specialist) approximately two thirds of these potentially unsafe drugs.(3) Other studies demonstrated a lack of awareness among nonspecialist prescribers and community pharmacists about safe analgesia.(55, 56) In addition, general practitioners (GPs) felt uncertain about managing patients with liver disease due to the complexity and a lack of subspecialty knowledge.(57, 58) This is concerning as the rising prevalence of CLDs worldwide increases reliance on nonspecialist clinicians to manage liver- and non-liver-related therapies.
To support health care professionals in safe prescribing, product information summaries provided by drug companies are often used. However, safety advice and dosing recommendations in these product information summaries on medication use in patients with hepatic impairment have historically been unavailable or ambiguous.(59, 60) Dedicated studies to assess the impact of hepatic impairment on PK are heterogeneous, and study reports often poorly discriminate between type and severity of CLD.(59) Therefore, many nonspecialist health care professionals are uncertain about dose adjustments and relative contraindications for people with hepatic impairment.
There are several well-written and practical reviews that translate PK changes in cirrhosis to practical prescribing recommendations for clinicians.(34, 61-63) Some reviews focus on specific drug groups (e.g., diabetes mellitus)(61, 63) while others describe several drug groups.(34, 62) To support clinicians in diagnosing DILI and the culprit drug, the LiverTox website(64) developed by the National Institute of Diabetes and Digestive Diseases Liver Disease Research Branch is also a well-known resource.
In addition to these resources, evidence-based recommendations on safe drug use in cirrhosis have recently been developed using a standardized published method.(54, 65) The authors used a combination of PK data (including in people with cirrhosis vs. controls where available), PD literature (ADR frequency/severity in patients with cirrhosis, including comparison to controls where available), and expert opinion to develop safety recommendations (including dose adjustments) for 209 medications. For example, it was recommended to use half of the normal initiation and maintenance dose for both metoclopramide and nifedipine in patients with Child-Pugh B/C cirrhosis based on a doubled exposure compared to healthy controls.(38, 49) Medicines for which evidence of harm outweighed potential benefits (e.g., NSAIDs) were categorized as unsafe and recommended to avoid. An important component of the authors’ methodology, which is often lacking in published reviews, is the plan for regular revision and formal updates of recommendations based on new insights from practice and literature.(65)
Monitoring and review
In addition to the importance of prescribing an appropriate medicine at an appropriate dose in patients with CLD, regular review and monitoring are essential steps in the medication management cycle. In the previously mentioned medication safety guidance,(54) lactulose and diuretics were classified as safe to use in patients with cirrhosis based on the PK profile. However, failure to appropriately titrate and monitor these safe medications has been shown to result in patient harm, including hospitalization for ADEs (e.g., dehydration, electrolyte derangement, AKI).(5, 6) In addition, there are a number of key PIMs that are repeatedly recognized for their risk of ADRs and/or precipitating a decompensation event in patients with cirrhosis, despite having a valid indication. Where use of these PIMs cannot be avoided, it is vital to monitor and review therapy regularly.
Yet, safe prescribing and appropriate monitoring are difficult to achieve without knowledge of a patient’s entire medication regimen. In a cohort study of 50 ambulatory patients with cirrhosis (n = 30 compensated, n = 20 decompensated), 54% had at least one discrepancy between patient-reported and clinician-documented medicines, including PIMs (benzodiazepines, NSAIDs, opioids) consumed by patients that the treating clinician was unaware of.(66) Medication discrepancies can contribute to drug interactions and ADEs and lead to clinical decisions based on incorrect information (i.e., medication adjustment, prescription of potentially unnecessary therapy, or inadvertent duplication of therapy). Therefore, regular medication reconciliation should be conducted to improve communication about medicines and reduce discrepancies as part of the medication review process. Instructing patients to always bring their medications to medical appointments (including CLD and non-CLD medications, complementary and alternative therapies, vitamins and supplements) can aid medication review and improve communication about optimal medication use.
Key PIMs
All medications for which there is limited evidence to support efficacy and/or known risks for patient harm can be considered PIMs. The decision to initiate these therapies should be made on a case-by-case basis with a plan for regular review and monitoring.
For example, benzodiazepines are indicated for treatment of alcohol withdrawal symptoms. However, benzodiazepines are also known potential precipitants of HE, with one study reporting up to a 5-fold increased risk of first-time HE among benzodiazepine users compared to nonusers when taken for 3 to 10 days.(67) HE is a debilitating complication of advanced CLD and a potent risk factor for falls, accidental trauma, hospitalization, and mortality.(68-71) Gabapentin and pregabalin, opioids, and PPIs have also been linked with an increased risk of incident HE.(15) Benzodiazepines and antipsychotic medicines have further been associated with a 3-fold to 6-fold increased risk of falls, independently of HE(70) and with worse clinical outcomes.(69)
PPIs are extensively metabolized by the liver, and PK changes increase the risk of ADEs in people with compensated and decompensated cirrhosis.(39) There has been an observed trend toward increased use of PPIs in people with cirrhosis over the past 2 decades.(3) PPIs are often prescribed by GPs(3) but also by specialists for hospitalized patients following a variceal bleed or for “gastric protection.”(72) The risk of developing new HE or SBP is higher in patients who consume PPIs.(73) Greater PPI exposure has also been reported to increase the risk of liver-related hospitalization and mortality.(74, 75)
In critically ill patients with decompensated cirrhosis admitted to a medical ICU, NSAIDs were associated with 46.1% of the instances of preventable medication-related harm, including melena or hematemesis and worsening renal function (reduced creatinine clearance).(14) Other studies demonstrated a decreased glomerular filtration rate by ~30% in patients with compensated cirrhosis,(42, 43) explained by the inhibition of prostaglandin synthesis, which decreases vasodilation of the afferent renal arterioles. As AKI is a strong predictor of short-term mortality in cirrhosis,(76) NSAIDs are generally contraindicated. Similar concerns have been raised about the safety of ACEis and angiotensin-receptor blocker (ARB) therapies. In a nation-wide cohort study, the 10-year cumulative incidence rate of end-stage renal disease was 5 times higher in patients with cirrhosis and ascites prescribed ACEis/ARBs compared to patients taking calcium channel blockers.(77) This effect was not observed in patients without ascites.(77) Use of ACEi/ARB therapies in individuals with cirrhosis should therefore be supported by a strong clinical indication and careful monitoring.
Medication interventions in CLD
There have been a number of intervention studies aimed at improving adherence and medication management in patients with CLD, with inconsistent impact on health behavior and clinical outcomes.(18)
Patient knowledge
In a Japanese study by Kadokawa et al.,(78) patients with liver disease and their family members (n = 80 participants) self-reported greater knowledge of hepatocellular carcinoma prevention and treatment, branched-chain amino acid therapy, and dietary iron restriction (for patients with hepatitis C) following an outpatient education class, with a trend toward higher knowledge in people who had attended a greater number of classes. While the authors did not report an effect of greater knowledge on real-world self-management or patient outcomes, the findings suggest generalized education delivered over multiple sessions is effective for improving knowledge of key elements of liver disease management.
Volk and colleagues(26) investigated a less resource-intensive outpatient education modality in the form of a concise disease education booklet. Provision of the booklet (and emphasizing its importance) to outpatients with cirrhosis (without overt HE) was shown to effectively improve knowledge of cirrhosis self-management tasks on a cued-recall test completed by 115 participants 3 months later (correct response rate increased from 53% to 67%). A similar but smaller United Kingdom study (n = 39) also reported improved knowledge following provision of an educational leaflet (mean score 38% increased to 83%).(79) However, an Australian study (n = 40) suggested some patients with cirrhosis have difficulty retaining the more technical information from an educational booklet.(80)
Goldsworthy’s group(81) investigated the effectiveness of a 12-minute educational screencast tailored to patients with cirrhosis that patients viewed on a single occasion when attending their hepatology clinic. Among 35 patients who completed the follow-up questionnaire (between 1 and 6 months later), the median correct response rate improved from 25% to 67%. However, similar to the studies discussed above, this study did not report the impact of improved knowledge on health behaviors or patient outcomes.
Health behaviors
A systematic review of 14 studies that evaluated education interventions for patients at risk of acquiring or currently infected with viral hepatitis also found outpatient intervention (often multidisciplinary or nurse led) improved patients’ knowledge of disease.(82) The authors concluded that complex, multimodal, educational interventions had greater influence on patients’ health behaviors, such as increased rates of testing, vaccination, and willingness to commence and adhere to treatment.
In the era of direct-acting antiviral therapies for hepatitis C, multiple nurse-led, pharmacist-led, and collaborative care models have successfully modified barriers to access and improved patients’ health behaviors to optimize treatment in primary and secondary care.(83-86) However, all these studies focused on virus-specific health behaviors and outcomes (e.g., adherence with antivirals, reducing drug interactions with antivirals, sustained virologic response rates) as opposed to patient-oriented interventions. Rising complexity in CLD can confound the effectiveness of such targeted interventions, as was found in one study that reported lower sustained virologic response rates and no improvement in adherence following implementation of specific pharmacy support services for patients with hepatitis C.(87)
Patient-oriented intervention
To optimize care in complex patients with CLD, patient-oriented interventions should be considered. In multimorbid people with NAFLD, pharmaceutical intervention to optimize control of metabolic comorbidities that contribute to NAFLD progression (type 2 diabetes mellitus, hyperlipidemia, hypertension, and obesity) is feasible(88) and should be included in multifaceted lifestyle-focused models of care.
A small number of patient-oriented interventions that mention a dedicated medication component have been conducted in people with cirrhosis (Table 3).(7, 89-94) Most involved implementation of new multidisciplinary “chronic disease management” models or “patient care management” programs to improve postdischarge care and prevent readmission. Three studies delivered face-to-face interventions(89, 92, 93) and two studies used modern technologies(90, 91) to assist with remote management of medications and liver disease complications. While outcome measures varied, most studies reported reduced readmission rates (including for potentially medication-preventable complications, like ascites and HE) but not medication-specific outcome measures, like adherence or MRPs.
| Study | Study Design | Population and Setting | Intervention | Impact on Patient Outcomes |
|---|---|---|---|---|
| Wigg et al.(89) | Single-center prospective, randomized, controlled, parallel-group study design; | Admitted patients with decompensated cirrhosis (intervention n = 40; usual care n = 20); | Nurse-led chronic disease management model, including intensive postdischarge care, home visits, and telephone reviews to deliver self-management support, patient and caregiver education about diet, medications and investigations | No significant difference in median length of stay, liver-related occupied bed days, or total, planned, or unplanned liver-related admissions; |
| 12-month follow-up | South Australia, Australia | nonsignificant trend toward increased all-cause admission rate (IRR, 1.77; 95% CI, 1.0-3.0; P = 0.06); | ||
| no change in QoL; | ||||
| no difference in mortality | ||||
| Ganapathy et al.(90) | Pilot study to determine feasibility and acceptability of a smartphone application (Patient Buddy App) to support self-management; | Inpatients with cirrhosis (n = 40) and their caregivers (n = 40); no control group; | Smartphone app (and inpatient education on its use) to monitor medication and dietary adherence, signs/symptoms of decompensation, and enhance communication with the health care team | 30-day readmission rate 42.5% (no comparison group); |
| 30-day follow-up | Virginia, United States | HE-related admission potentially prevented in 8 patients | ||
| Khungar et al.(91) | Pilot study of a wireless mobile support system to enable telehealth monitoring; | Admitted patients with cirrhosis (intervention n = 19; control n = 143); | Patients provided with a 4G tablet, wireless blood pressure monitor, pulse oximeter, and weighing scale to monitor medication adherence, hepatic encephalopathy, fluid overload, bleeding, and infections | No significant difference in 30-day readmission rate; |
| 90-day follow-up | Philadelphia, United States | reduced 90-day readmission rate for potentially preventable causes (HE and volume overload) (0% vs. 33.8%; P = 0.02) | ||
| Morales et al.(92) | Quasi-experimental cohort study comparing patient outcomes before and after implementation of a dedicated Hepatology Day Hospital; | Discharged patients with decompensated cirrhosis (intervention n = 80; control n = 112); | A dedicated patient monitoring program, including follow-up within 7 days of discharge for clinical evaluation, procedures, and/or therapy and to ensure adherence with medications and diet | Reduced 30-day readmission rate (RR, 0.38; 95% CI, 0.19-0.75; P = 0.003) and shorter length of stay during readmissions (mean ± SD, 10.4 ± 3.7 vs. 18.36 ± 14.4 days; P = 0.007); |
| minimum 7-month follow-up or until death (average ± SD follow-up, 10.7 ± 6.8 months) | Badalona, Spain | reduced odds of 30-day readmission (adjusted* OR, 0.21; 95% CI, 0.08-0.53; P = 0.001); | ||
| fewer emergency department visits (mean ± SD, 1.10 ± 1.64 vs. 1.71 ± 2.36; P = 0.035); | ||||
| lower 60-day mortality rate (3.8% vs. 14.3%; P = 0.016) but not 30-day, 90-day, or end of follow-up mortality | ||||
| Ramachandran et al.(93) | Retrospective observational cohort study between two tertiary hospitals (unit 1 vs. unit 2); | Admitted patients with decompensated cirrhosis (unit 1 n = 69; unit 2 n = 54); | Coordinated chronic disease management model, including targeted postdischarge care with education and self-management support (as per Wigg et al.(89)) at unit 1 vs. standard care at unit 2 | No significant difference in 30-day or 90-day readmission rate; |
| 3-year follow-up | South Australia, Australia | reduced incidence rate of unplanned liver-related admissions (per person-year; adjusted† IRR, 0.52; 95% CI, 0.28-0.98; P = 0.042); | ||
| higher incidence rate of elective liver-related admissions (adjusted† IRR, 4.42; 95% CI, 1.69-11.6; P = 0.002); | ||||
| higher adjusted‡ transplant-free survival (67.7% vs. 37.2%; P = 0.009) at 3 years | ||||
| Hayward et al.(7, 94) | Single-center, randomized, controlled trial in a tertiary hospital hepatology outpatient center (comprising seven general hepatology clinics); | Patients who were ambulatory with decompensated cirrhosis (intervention n = 57; usual care n = 59); | Pharmacist-led, patient-oriented education, and medication management intervention targeted to outpatients with decompensated cirrhosis | Reduced incidence rate of unplanned all-cause admissions (adjusted§ IRR, 0.52; 95% CI, 0.30-0.92; P = 0.025); |
| 12-month follow-up | Queensland, Australia | improved QoL within the intervention group (P = 0.044) but not usual care; | ||
| no difference in mortality |
- * Adjusted for MELD-Na score, Charlson comorbidity index, serum albumin, and number of medications at discharge.
- † Adjusted for age, sex, MELD score, alcohol-related etiology, and comorbidity burden (Charlson comorbidity index).
- ‡ Adjusted for age, sex, comorbidity burden (Charlson comorbidity index), MELD score, occurrence of any liver-related emergency admission, liver-related emergency admissions within 30- and 90-days of discharge, and nonalcohol-related etiology.
- § Adjusted for Child-Pugh score, number of medications, history of variceal bleeding, and alcoholic liver disease.
- Abbreviations: CI, confidence interval; IRR, incidence rate ratio; MELD, Model for End-Stage Liver Disease; OR, odds ratio; RR, relative risk.
One randomized controlled trial evaluated the effectiveness of a pharmacist-led patient-oriented education and medication management intervention in ambulatory patients with decompensated cirrhosis.(7, 94) At follow-up, patients who received the intervention (n = 57) had greater knowledge of cirrhosis and key self-care tasks compared to usual care (n = 59).(94) Intervention patients also experienced improved QoL(94) and had a 48% reduced incidence rate of unplanned admissions during the 12-month follow-up period, which coincided with a 68.9% resolution rate of high-risk MRPs.(7)
Improving safe prescribing
As previously discussed, safe prescribing in CLD may be improved by providing additional support to nonspecialist clinicians who are increasingly relied on to manage these patients day-to-day. Telemedicine case-based training and mentoring models, like the Project Extension for Community Healthcare Outcomes (Project ECHO), have been shown to improve GP knowledge and treatment of hepatitis C in primary care.(95, 96) Unlike the situation for other high-risk patient groups,(97) there is paucity of literature describing primary care-based interventions that specifically aim to reduce MRPs in CLD and/or support patients and GPs in medication management.
In the Netherlands, guidance for choosing medication and dose adjustments in patients with cirrhosis has been integrated with clinical decision support systems for both primary and secondary health care providers.(54) The effect of this intervention on prescribing behavior and medication safety has not yet been evaluated. However, a survey of community pharmacists’ experiences highlighted initial difficulties applying the guidance due to limited available patient history in some primary health care settings(98); this warrants further consideration. Future research will assess the impact of the intervention on medication safety and patient-related outcomes following a suitable implementation period.
In people with life-limiting illness and limited life expectancy, deprescribing drugs with anticholinergic properties and those with lower evidence for benefit has been shown to reduce falls, hospitalization, and mortality and improve quality of life.(99, 100) Given the degree of frailty that is commonly present in people with advanced liver diseases, reduction in medication burden by deprescribing nonessential therapies and PIMs may also prevent medication-related harm. A prospective, randomized, controlled trial of a PPI deprescribing intervention for hospitalized patients with cirrhosis is planned to commence in late 2020; this will provide valuable safety data surrounding PPI discontinuation for nonevidence-based indications.(101) Future specific or general deprescribing interventions could be considered in select patient groups to generate evidence on feasibility and patient outcomes.
Conclusion
While medications are essential in the management of many CLDs, MRPs are an important source of preventable harm. Patients may be at risk of adverse medication-related outcomes due to several patient-related (polypharmacy, illness severity, knowledge, and beliefs) and health care professional-related (inadequate prescribing and monitoring of therapy) factors. Available evidence supports multifaceted, collaborative, and multidisciplinary models of care to improve outcomes for people with complex medication needs, such as those with cirrhosis. Further research to expand our knowledge of MRPs and PIMs in the real world will support safe medication choices in this increasingly multimorbid group of patients.




