Activation of Liver X Receptor α Sensitizes Mice to T-Cell Mediated Hepatitis
Supported by the National Institute of Environmental Health Sciences (Grant/Award No. ES023438).
Potential conflict of interest: Nothing to report.
Abstract
Autoimmune hepatitis (AIH) is an inflammatory disease of the liver. Liver X receptors (LXRs), including the α and β isoforms, are previously known for their anti-inflammatory activities. The goal of this study is to determine whether and how LXR plays a role in AIH. LXRα gain-of-function and loss-of-function mouse models were used, in conjunction with the concanavalin A (ConA) model of T-cell mediated hepatitis. We first showed that the hepatic expression of LXRα was decreased in the ConA model of hepatitis and in human patients with AIH. In the ConA model, we were surprised to find that activation of LXRα in the constitutively activated VP-LXRα whole-body knock-in (LXRα-KI) mice exacerbated ConA-induced AIH, whereas the LXRα−/− mice showed attenuated ConA-induced AIH. Interestingly, hepatocyte-specific activation of LXRα in the fatty acid binding protein–VP-LXRα transgenic mice did not exacerbate ConA-induced hepatitis. Mechanistically, the sensitizing effect of the LXRα-KI allele was invariant natural killer T (iNKT)–cell dependent, because the sensitizing effect was abolished when the LXRα-KI allele was bred into the NKT-deficient CD1d−/− background. In addition, LXRα-enhanced ConA-induced hepatitis was dependent on interferon gamma. In contrast, adoptive transfer of hepatic iNKT cells isolated from LXRα-KI mice was sufficient to sensitize CD1d−/− mice to ConA-induced AIH. Conclusion: Activation of LXRα sensitizes mice to ConA-induced AIH in iNKT and interferon gamma–dependent manner. Our results suggest that LXRα plays an important role in the development of AIH.
Abbreviations
-
- αGalCer
-
- alpha-galactosylceramide
-
- Abcg5
-
- ATP-binding cassette gene
-
- AIH
-
- autoimmune hepatitis
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CD
-
- cluster of differentiation
-
- ConA
-
- concanavalin A
-
- FABP
-
- fatty acid binding protein
-
- FACS
-
- fluorescence-activated cell sorting
-
- Fasn
-
- fatty acid synthase
-
- Fsl
-
- Fas ligand
-
- Gzmb
-
- granzyme B
-
- H&E
-
- hematoxylin and eosin
-
- HFCD
-
- high-fat, high-cholesterol diet
-
- Icam1
-
- intercellular adhesion molecule 1
-
- IFN-γ
-
- interferon gamma
-
- IgG
-
- immunoglobulin G
-
- Il-1β
-
- interleukin 1 beta
-
- IL-2
-
- interleukin 2
-
- IL-4
-
- interleukin 4
-
- iNKT
-
- invariant natural killer T cells
-
- KC
-
- Kupffer cell
-
- KI
-
- knock-in
-
- LXR
-
- liver X receptor
-
- MNC
-
- mononuclear cell
-
- mRNA
-
- messenger RNA
-
- N.S.
-
- statistically not significant
-
- RNA-seq
-
- RNA-sequencing
-
- RPMI-1640
-
- Roswell Park Memorial Institute 1640
-
- Scd-1
-
- stearoyl-CoA desaturase-1
-
- Srebp-1c
-
- sterol regulatory element-binding protein 1c
-
- Tnf-α
-
- tumor necrosis factor alpha
-
- Vcam1
-
- vascular adhesion molecule 1
-
- WT
-
- wild type
Autoimmune hepatitis (AIH) is an inflammatory disease of the liver that occurs in children and adults with a female predominance.(1) The clinical manifestations of AIH include destruction of hepatocytes, appearance of circulating autoantibodies, and elevated serum levels of liver transaminases and immunoglobulin G (IgG).(2) Although several risk factors have been proposed, the molecular mechanism for the pathogenesis of AIH remains to be clearly defined. Treatment with Concanavalin A (ConA), a lectin (carbohydrate-binding protein) originally extracted from the jack-bean, is a widely used mouse model to investigate the cellular and molecular mechanisms of immune-induced hepatitis.(3) The ConA model is featured by marked increases in serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), simultaneous infiltration of cluster of differentiation (CD)4+ T cells, Kupffer cells (KCs) and eosinophils, as well as necrotic area and massive apoptosis in the liver tissue.(4, 5) The hepatic invariant natural killer T (iNKT) cells also play a pathological role in ConA-induced liver injury by producing an array of pro-inflammatory cytokines, including interleukin (IL)-4, IL-2, and interferon gamma (IFN-γ).(4-8)
The liver X receptors, including LXRα (Nr1h3) and LXRβ (Nr1h2), are members of the nuclear receptor family of transcription factors that have diverse physiological and pathophysiological functions. Both endogenous and synthetic LXR agonists, such as GW3965, have been discovered or developed. Although the functions of LXRs in regulating the metabolism of cholesterol, fatty acids, and phospholipids have been well established, the role of LXRs in immune-related diseases has been intriguing and often controversial.(9-11) For example, mice lacking LXRs exhibited a breakdown in self-tolerance and developed autoantibodies and autoimmune glomerulonephritis. In contrast, treatment with an LXR agonist ameliorated disease progression in a mouse model of lupus-like autoimmunity.(12) A subsequent study by the same group suggested that cholesterol accumulation in CD11c+ immune cells as a result of decreased LXR-mediated cholesterol efflux is the reason for the sensitization of LXR knockout mice to autoimmune disease.(11) Activation of LXR has also been shown to alleviate ocular inflammation in an experimental model of autoimmune uveitis.(13) However, independent reports showed that treatment of mice with the LXR agonist GW3965 exacerbated collagen-induced rheumatoid arthritis, and the sensitizing effect of GW3965 was abolished in LXR knockout mice.(14, 15) These reports suggested controversial effects of LXRs on the development of immune-related diseases. AIH is an autoimmune disease of the liver, and LXRs are highly expressed in the liver, but the role of LXRs in the pathogenesis of AIH has not been sufficiently explored.
In this study, we have uncovered a function of LXRs in ConA-induced AIH. Our results demonstrate that activation of LXRα sensitized mice to ConA-induced AIH in an iNKT cell–dependent and IFN-γ-dependent manner.
Methods
Patient Samples
Liver biopsy samples and de-identified clinical data were collected from patients with AIH (n = 16, 11 females and 5 males, mean age 49.4 years). AIH was diagnosed according to criteria established by the International Autoimmune Hepatitis Group in 2008.(16) Subjects diagnosed with primary biliary cholangitis–AIH overlap syndrome according to the Paris criteria(17) were excluded. Tumor-free portions of livers removed from patients with hepatocellular carcinoma were used as controls. All subjects provided written informed consent, and the study was approved by the Ethics Committee of Peking University People’s Hospital.
Mice
Eight-week-old to 10-week-old female wild-type (WT), LXRα−/−, VP-LXRα knock-in (LXRα-KI), and fatty acid binding protein (FABP)-VP-LXRα transgenic mice in the C57BL/6 background were used. The LXRα−/− mice (Cat #013763) and CD1d−/− mice (Cat #008881) were purchased from the Jackson Laboratory (Bar Harbor, ME). The creation and characterization of the whole-body constitutively activated VP-LXRα knock-in (LXRα-KI) mice and hepatocyte-specific constitutively activated LXRα (FABP-VP-LXRα) transgenic mice have been previously described.(18, 19) In brief, VP-LXRα was generated by fusing the VP16 activation domain of the herpes simplex virus to the amino terminus of mouse LXRα sequence. The LXRα-KI mice were generated by knocking-in VP-LXRα complementary DNA into the mouse LXRα locus.(18) The homozygous LXRα-KI mice express VP-LXRα, whereas the expression of the endogenous LXRα is disrupted. The expression of the transgene in FABP-VP-LXRα mice was under the control of the hepatocyte-specific FABP gene promoter.(19) Mice were housed ad libitum on a 12-hour/12-hour light/dark cycle under pathogen-free conditions. All experimental procedures were performed in accordance with relevant federal guidelines and with the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
ConA Model of AIH
Concanavalin A (ConA, cat# J61221), purchased from Alfa Aesar (Tewksbury, MA), was dissolved in saline and injected through the lateral tail vein at the dose of 10 mg/kg, except for the survival experiments in which 20 mg/kg was used for the WT and LXRα-KI mice, and 25 mg/kg was used for the WT and FABP-VP-LXRα mice. When necessary, GW3965 was gavaged daily at the dose of 30 mg/kg beginning 3 days before the ConA treatment and until the day of tissue harvest. For in vivo neutralization of IFN-γ, mice were injected intravenously with 200 μg InVivoMAb anti-mouse IFN-γ or the isotype control IgG (see Supporting Table S1) from Bio X Cell (West Lebanon, NH) 1 hour before the ConA injections. Mice were sacrificed at the indicated time points for serum and liver harvest. Blood was collected through cardiac puncture and subsequently centrifuged at 8,000g for 5 minutes to collect the serum. Livers were excised for histology, or they were snap-frozen on dry ice and stored at −80°C until further analysis.
Primary Lymphocyte Isolation From the Liver and Spleen
To isolate mouse hepatic mononuclear cells (MNCs), livers were perfused with 10 mL Hanks’ balanced salt solution (HBSS) containing 20 μM ethylene glycol tetraacetic acid and 1 mM HEPES (94-[2-hydroxyethyl]-1-piperazine ethanesulfonic acid). The perfused liver was cut into small pieces and then digested in Roswell Park Memorial Institute 1640 (RPMI-1640) medium containing 500 U/mL collagenase IV from Sigma-Aldrich (St. Louis, MO) for 30 minutes at 37°C. After digestion, the cell suspension was pressed through a 70-µm-cell strainer and centrifuged for 5 minutes at 50g. MNCs containing supernatants were collected, pelleted, and resuspended in 4 mL 40% (vol/vol) Percoll Plus solution from Sigma and carefully laid on 5 mL 60% (vol/vol) Percoll Plus solution followed by centrifugation at 800g for 30 minutes with no brake. The interphase that contains MNCs was washed by RPMI-1640 and resuspended in ACK Lysing Buffer from BD Biosciences (Franklin Lakes, NJ) to remove erythrocytes. The remaining cells were resuspended in HBSS with 1% fetal bovine serum (FBS) (fluorescence-activated cell sorting [FACS] buffer). To isolate lymphocytes from the spleen, the spleen tissue was excised and crushed on a 70-µm-cell strainer in RPMI-1640 medium. The resultant cell suspensions were centrifuged and the cell pellets were subjected to hemolysis to remove erythrocytes as described for the liver. The remaining spleen cells were resuspended in FACS buffer.
Flow Cytometry Analysis
Hepatic MNCs and splenic lymphocytes were subjected to cell surface or intracellular staining to determine leukocyte phenotype, activation, and immune response. The conjugated antibodies used for flow cytometric analysis are listed in Supporting Table S1. Cells were first stained for surface markers 1:200 diluted in FACS buffer, fixed and permeabilized with Intracellular Fixation & Permeabilization Buffer from eBioscience (San Diego, CA), and then the intracellular IFN-γ staining was performed. After staining, cells were washed with the FACS buffer and analyzed on Foretessa at the University of Pittsburgh Flow Cytometry Core Facility. The flow cytometry data were analyzed with the FlowJo software from Treestar (San Carlos, CA). For intracellular IFN-γ staining of primary iNKT cells to test the expression of IFN-γ following activation, cells were prestimulated with Cell Activation Cocktail (with Brefeldin A) from BioLegend (San Diego, CA) at 37°C for 6 hours.
iNKT Cell Isolation and Adoptive Transfer
The hepatic iNKT cells from 8-week-old to 10-week-old WT or LXRα-KI mice were isolated through magnetic-activated cell sorting using the NK1.1 + iNKT Cell Isolation Kit from Miltenyi Biotec (Auburn, CA) to a purity of greater than 95%. The numbers of donor-derived cells (NK1.1 + CD3+) in the liver were calculated based on flow cytometric analysis of stained cells with fluorochrome-conjugated antibodies specific for NK1.1 or CD3 (Supporting Table S1). For in vitro activation of primary iNKT cells by ConA, the supernatant was collected for cytokine evaluation after the cells were treated with 10 μg/mL ConA for 24 hours. For adoptive transfer, 1 × 106 sorted iNKT cells/per mouse were adoptively transferred into the NKT-deficient CD1d−/− mice through intrahepatic injection 1 hour before the ConA injection, and mice were sacrificed and analyzed for liver damage 24 hours after the ConA injection.
DN32.D3 Cell Cultures and Transfections
The Va14+ CD1d-specific mouse iNKT hybridoma cell line DN32.D3 was a gift from Dr. Albert Bendelac at the University of Chicago. The DN32.D3 cells and primary hepatic iNKT cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 2 mM l-glutamine, 50 nM 2-mercaptoethanol (all purchased by Life Technologies). When necessary, DN32.D3 cells were treated with 200 ng/mL alpha-galactosylceramide (αGalCer) from Avanti Polar Lipids (Alabaster, AL) in plates precoated with CD1d-tetramer (from the NIH Tetramer Facility) at the indicated time points. For LXRβ knockout-down experiments, DN32.D3 cells were transfected with Lxrβ–small interfering RNA (siRNA) (5′-GCCUGGACGAUGCAGAGUA-3′) by electroporation, and 24 hours later the transfected cells were used for further drug treatment.
RNA-Sequencing Analysis
Total RNA of iNKT cells pooled from 10 mice was isolated using the RNeasy Mini kit from Qiagen (Hulsterweg, Germany). RNA-sequencing (RNA-seq) was performed by the Health Sciences Sequencing Core at the Children’s Hospital of Pittsburgh, and gene-expression profiles were generated.
Biochemical Analysis and Cytokine Measurements
Serum ALT and AST levels were quantified by using enzymatic assay kits from Stanbio Laboratory (Boerne, TX). IFN-γ levels in the supernatants of cell culture were measured by enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN).
Immunohistochemistry
The immunohistochemical staining for LXRs with patient samples was performed using monoclonal anti-LXRα antibody and anti-LXRβ antibody (Supporting Table S1), following the heat-induced antigen-retrieval procedures. The stained slides were evaluated by a pathologist in a blinded fashion and quantified using the immunohistochemistry profiler plugin in ImageJ.(20)
Histology
For histological analysis, tissues were fixed in 10% zinc formalin overnight, dehydrated, and embedded in paraffin, sectioned at 5 μm, and then processed for hematoxylin and eosin (H&E) staining. The area of hepatocellular necrosis was quantified by examining eight random fields at ×100 magnification and expressed as a percentage of necrotic area per total area examined.
Real-Time Quantitative PCR and Western Blot Analysis
Total RNA from tissues or cells was extracted with TRIzol reagent from Invitrogen (Carlsbad, CA). Complementary DNA was synthesized, and SYBR Green–based real-time PCR was performed with the ABI 7300 Real-Time PCR System. Data were normalized to the control cyclophilin by the ∆∆Ct method. PCR primer sequences are listed in Supporting Table S2. For western blot analysis, 30 μg of protein extracts was separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred onto a nitrocellulose or polyvinylidene difluoride membrane. The primary antibodies of LXRα and LXRβ used for western blot are listed in Supporting Table S1.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software from GraphPad (San Diego, CA). Values for all measurements are presented as mean ± SEM. Statistical analyses were performed using the two-tailed Student t test. The log-rank test was used for the statistical analysis of the survival rate. P values of less than 0.05 were considered statistically significant.
Results
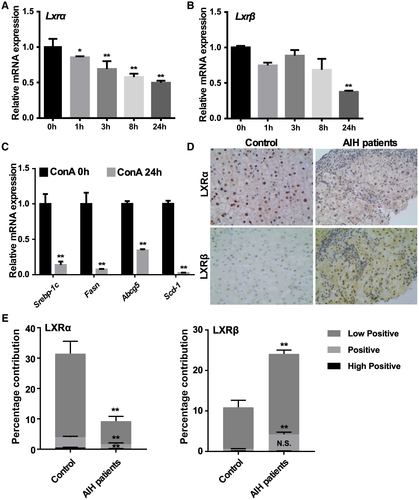
The Hepatic Expression and Activity of LXRs Are Down-regulated in ConA-Induced Mouse Model of Hepatitis and in Patients With AIH
Because LXRα has been reported to display an abnormal expression in several liver diseases, including nonalcoholic fatty liver disease, and hepatitis B and C virus–associated hepatic steatosis,(21, 22) we initially wondered whether the expression of LXRs is also affected in the ConA model of hepatitis. In this experiment, female WT mice were subjected to tail-vein injection of ConA (10 mg/kg) before sacrificing and liver tissue harvest at different time points. Treatment with ConA decreased the hepatic messenger RNA (mRNA) expression of Lxrα (Fig. 1A) and Lxrβ (Fig. 1B) in a time-dependent manner. At 24 hours, the hepatic expression of LXR target genes, including Srebp-1c (sterol regulatory element-binding protein 1c), Fasn (fatty acid synthase), Scd-1 (stearoyl-CoA desaturase-1) and Abcg5 (ATP-binding cassette gene), was markedly down-regulated (Fig. 1C). The down-regulation of LXRα was also observed in the livers of patients with AIH. LXRα was abundantly expressed in the control livers, but its expression was markedly decreased in the AIH livers as shown by immunohistochemistry (Fig. 1D) and its quantification (Fig. 1E). Interestingly, LXRβ was only weakly expressed in the control livers, whereas its expression was increased in the AIH livers (Fig. 1D,E). In male mice, ConA treatment led to a down-regulation of Lxrα and some of the LXR target genes, while up-regulating the expression of Lxrβ as in the patients with AIH (Supporting Fig. S1). The dynamic expression and activity of LXRs suggested that they might be involved in immune-mediated hepatitis.
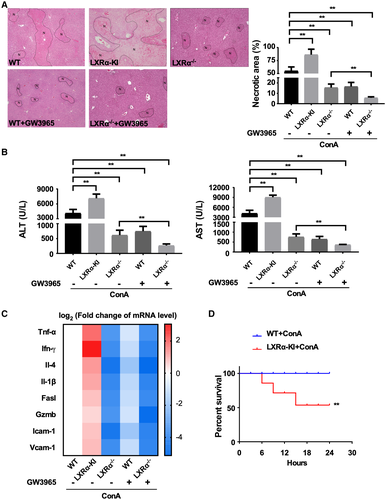
Whole-Body Activation of LXRα Sensitizes Mice to, Whereas LXRα Ablation Protects Mice From, ConA-Induced Hepatitis
We then used LXR gain-of-function and loss-of-function models to investigate the role of LXRs in ConA-induced hepatitis. The gain-of-function models include the VP-LXRα whole-body knock-in (LXRα-KI) mice, in which the constitutively activated LXRα was knocked into the mouse Lxrα gene locus, as well as WT and LXRα−/− mice treated with the synthetic LXR agonist GW3965. In the saline-treated groups, neither the LXRα gene knock-in or knockout nor the GW3965 treatment affected the serum levels of serum ALT and AST (Supporting Fig. S2). The ConA-treated WT female mice showed expected liver toxicity, as evidenced by liver necrosis (Fig. 2A), markedly increased serum levels of ALT and AST (Fig. 2B), and increased expression of a panel of pro-inflammatory cytokine genes (Tnf-α [tumor necrosis factor alpha], Ifn-γ, Il-4, and Il-1β) and genes encoding vascular adhesion molecule 1 (Vcam1), intercellular adhesion molecule 1 (Icam1), Fas ligand (Fasl), and granzyme B (Gzmb) (Fig. 2C). Knowing the reported anti-inflammatory activity of LXRs, we were surprised to find the female LXRα-KI mice exhibited heightened sensitivity to ConA (Fig. 2A-C). In contrast, the female LXRα−/− mice were attenuated from ConA-induced liver toxicity (Fig. 2A-C). In the pharmacological models, treatment of female WT mice with GW3965 showed an overall protective effect (Fig. 2A-C). Interestingly, the treatment of GW3965 in female LXRα−/− mice provided additional protection (Fig. 2A-C), which could be due to the activation of LXRβ in this genotype. The efficiency of GW3965 in activating LXR was confirmed by the induction of hepatic expression of LXR target genes (Supporting Fig. S3). To further demonstrate the hyper-susceptibility as a result of LXRα activation, a high dose of ConA (20 mg/kg) was administrated to the LXRα-KI mice. As shown in Fig. 2D, while all of the female WT mice survived, nearly 50% of LXRα-KI mice died within 24 hours after the ConA injection. The sensitization of LXRα-KI mice and resistance of LXRα−/− mice to ConA-induced liver injury were also observed in male mice (Supporting Fig. S4), suggesting that the phenotype was not sex-specific. Together, our results suggest that activation of LXRα sensitized the mice to ConA-induced hepatitis.
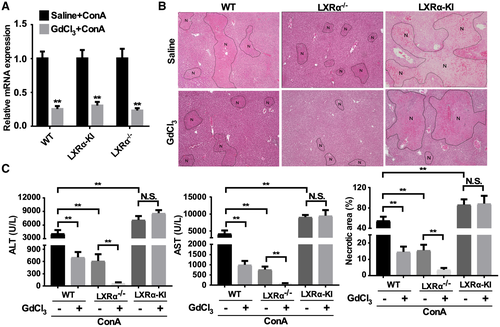
Kupffer Cells Are Not Required for the Sensitizing Effect of LXRα Activation on ConA-Induced Hepatitis
It has been reported that KCs can be activated by ConA to produce pro-inflammatory cytokines and promote liver injury.(23, 24) Meanwhile, activation of LXRs have been shown to suppress the production of inflammatory cytokines by KCs.(25-27) We therefore wanted to determine whether KCs are involved in the effect of LXRα activation or ablation on ConA-induced hepatitis. Treatment with GdCl3 was used to deplete KCs,(23) and the depletion efficiency was confirmed by the decreased hepatic expression of KC marker gene F4/80 (Fig. 3A). In WT mice, pretreatment with GdCl3 decreased ConA-induced liver necrosis (Fig. 3B), serum levels of ALT and AST (Fig. 3C), consistent with the crucial roles of KCs in ConA-induced hepatitis.(23) KC depletion can further protect the LXRα−/− mice from ConA-induced hepatitis (Fig. 3B,C). In contrast, KC depletion had little effect on the sensitization of the LXRα-KI mice (Fig. 3B,C), suggesting that KCs were not required for the sensitizing effect of the LXRα-KI allele.
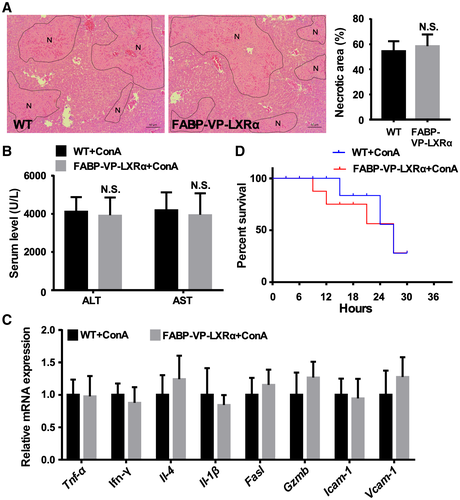
Hepatocyte-Specific Activation of LXRα Does Not Exacerbate ConA-Induced Hepatitis
The LXRα-KI mice bear whole-body or systemic activation of LXRα, as the VP-LXRα transgene was knocked into the mouse Lxrα gene locus. We then used the FABP-VP-LXRα transgenic mice to determine whether hepatocyte-specific activation of LXRα contributed to the hypersusceptibility to ConA-induced hepatitis. The FABP-VP-LXRα transgenic mice express VP-LXRα exclusively in the hepatocytes under the control of the FABP gene promoter, as we have previously described.(19) To our surprise, transgenic activation of LXRα in the hepatocytes had little effect on ConA-induced hepatitis, because the FABP-VP-LXRα transgenic mice exhibited a sensitivity similar to the WT mice, as shown by histology (Fig. 4A), measurement of the serum levels of ALT and AST (Fig. 4B), and expression of pro-inflammatory genes (Fig. 4C). The survival of FABP-VP-LXRα transgenic mice in response to a high dose of ConA (25 mg/kg) was also not different from their WT counterparts (Fig. 4D). Together, these results suggest that hepatocyte activation of LXRα does not appear to contribute to the sensitization to ConA.
Hepatic iNKT Cells Are Required for Sensitizing the LXRα-KI Mice to ConA-Induced Hepatitis
Besides KCs and hepatocytes, T cells and NKT cells have also been reported to play crucial roles in ConA-induced hepatitis.(4) To determine whether T cells and NKT cells play a role in the sensitizing effect of the LXRα-KI allele, flow cytometric analysis was performed to evaluate the CD4+ T cells, CD8+ T cells, and iNKT cells in the liver. The IFNγ+ cells are gated as depicted in Supporting Fig. S5. In vehicle-treated mice, there were no significant differences in the frequencies of IFNγ+ CD4, CD8, and iNKT cells between WT mice and LXRα-KI mice (Supporting Fig. S5). Following the ConA challenge, the frequency of activated IFNγ+ iNKT cells in the livers of LXRα-KI mice was significantly higher than that in WT mice, but no changes in the frequency of IFNγ+ CD4+ and IFNγ+ CD8+ T cells were observed (Fig. 5A). Production of IFNγ is a signature response of iNKT cells following activation. Based on these results, we speculated that increased activation of hepatic iNKT cells in LXRα-KI mice may have contributed to the hypersusceptibility to ConA in this genotype.
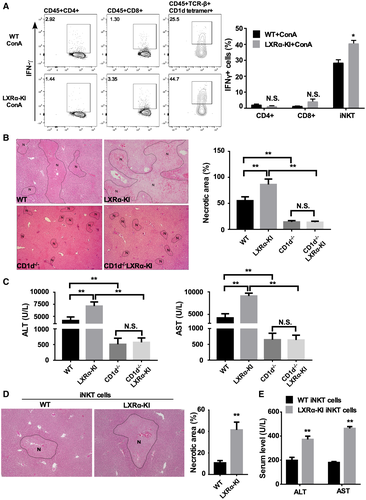
To further define the role of iNKT cells in the hypersusceptibility of LXRα-KI mice to ConA in vivo, we bred the LXRα-KI allele into the NKT cell–deficient CD1d−/− mice.(28) Compared with WT mice, the CD1d−/− mice were protected from ConA toxicity, as shown by histology (Fig. 5B) and measurement of the serum levels of ALT and AST (Fig. 5C), consistent with the notion that NKT cells play a crucial role in ConA-induced hepatitis.(8) Moreover, loss of NKT cells abolished the sensitizing effect of LXRα-KI (Fig. 5B,C), suggesting that NKT cells are required for the sensitizing effect of the LXRα-KI allele.
To provide direct evidence for the contribution of LXRα activation in hepatic iNKT cells, we tested whether adoptive transfer of iNKT cells derived from LXRα-KI mice was sufficient to render CD1d−/− mice susceptible to ConA-induced hepatitis. iNKT cells isolated from the WT or LXRα-KI mice were injected into the liver of CD1d−/− mice before the mice were challenged with ConA, as previously described.(29) Our results show that intrahepatic injection of iNKT cells isolated from LXRα-KI mice increase ConA-induced liver necrosis (Fig. 5D) and elevate the serum levels ALT and AST (Fig. 5E). Overall, these results suggest that hepatic iNKT cells are necessary and sufficient to sensitize LXRα-KI mice to ConA-induced hepatitis.
Production of IFN-γ Is Required for the Sensitization of LXRα-KI Mice to ConA-Induced Hepatitis
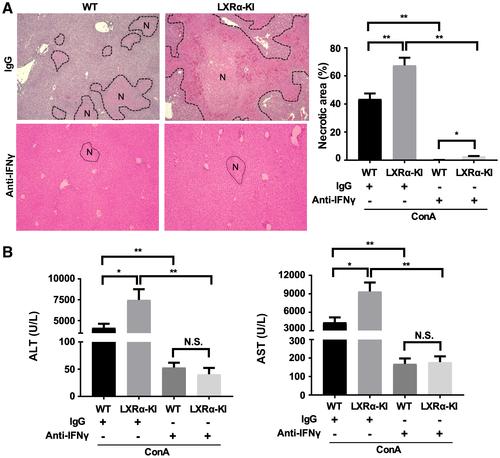
NKT cells are responsible for the production of several key pro-inflammatory cytokines in the ConA model, including IFN-γ,(30) which has been reported to be a molecular target of LXR.(31) To determine whether the secretion of IFN-γ is necessary for the hypersusceptibility of LXRα-KI mice, we treated WT or LXRα-KI mice with an IFN-γ neutralizing antibody or a control IgG 1 hour before subjecting them to the ConA challenge. Treatment with the anti-IFN-γ antibody prevented ConA-induced hepatitis in WT mice. Moreover, the anti-IFN-γ antibody abolished the sensitizing effect of the LXRα-KI allele, as shown by histology (Fig. 6A) and measurement of the serum levels of ALT and AST (Fig. 6B).
LXRs Are Expressed and Functional in iNKT Cells, and Activation of LXRα Sensitizes iNKT Cells to Activation
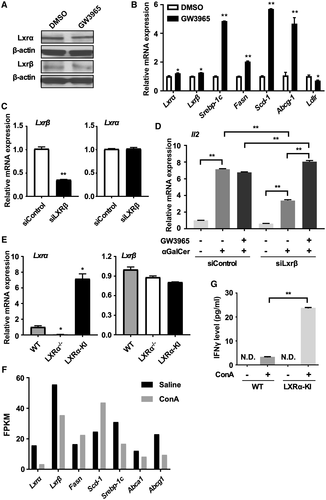
To confirm that LXRs are expressed and functional in iNKT cells, we measured the expression of LXRs and their target genes in both the iNKT hybridoma cell line DN32.D3 and mouse primary iNKT cells. Both LXRα and LXRβ were abundantly expressed in DN32.D3 cells. After a 24-hour treatment with GW3965, the protein (Fig. 7A) and mRNA (Fig. 7B) expression of LXRs were unchanged in DN32.D3 cells, whereas the expression of LXR target genes was up-regulated (Fig. 7B), suggesting that LXRs are functional in these cells. To specifically activate LXRα, we used a LXRβ targeting siRNA (siLXRβ) to knock down the expression of LXRβ in DN32.D3 cells before treating the cells with GW3965. The efficiency of LXRβ knockdown was verified by real-time PCR (Fig. 7C). DN32.D3 cells can be activated by αGalcer, which was evidenced by the elevation of Il-2 gene expression (Fig. 7D). The induction of Il-2 by αGalcer was further enhanced by the treatment of GW3965 in LXRβ knockdown cells, while there was no change in the siControl cells after the GW3965 treatment (Fig. 7D), indicating that activation of LXRα can sensitize DN32.D3 cells to αGalcer activation. When primary hepatic iNKT cells were isolated from WT, LXRα−/− and LXRα-KI mice, we found that the expression of LXRα was detected in WT and LXRα-KI mice, but was undetectable in LXRα−/− mice (Fig. 7E), whereas the expression of LXRβ was similarly detected in all three genotypes of iNKT cells (Fig. 7E). RNA-seq analysis on primary iNKT cells isolated from WT mice and treated with vehicle or ConA in vitro showed that the expression of Lxrα, Lxrβ, and some of the LXR target genes were down-regulated by ConA (Fig. 7F), consistent with the pattern of mice in vivo. At the functional level and following the stimulation by ConA, the production of IFN-γ by the LXRα-KI iNKT cells was higher than that by the WT iNKT cells (Fig. 7G). These results suggest that activation of LXRα sensitized iNKT cells to activation.
Discussion
AIH is a significant disease whose etiology remains to be clearly defined. The standard clinical management of AIH is glucocorticoid therapy, but a small percentage of patients with AIH fail to respond to standard treatment and may quickly develop liver fibrosis and cirrhosis.(32) A better understanding of the pathophysiology of AIH will help to develop novel therapies for this disease. In this study, we showed how the gain-of-function LXRα-KI mice and loss-of-function LXRα−/− mice exhibited hypersensitivity and resistance to ConA-induced hepatitis, respectively. The resistance of LXRα−/− mice to ConA-induced hepatitis was consistent with a recent report.(27) The protective phenotype of LXRα−/− mice suggested that the down-regulation of LXRα in the livers of ConA-treated mice and patients with AIH may represent a protective response.
The sensitizing effect of LXRα activation on ConA-induced and T-cell-mediated hepatitis was a surprise, considering that activation of LXR has been suggested to attenuate immune-related diseases, such as glomerulonephritis, lupus-like autoimmunity,(12) and autoimmune uveitis.(13) Intriguingly, several independent studies showed that treatment with the LXR agonist GW3965 exacerbated collagen-induced rheumatoid arthritis in a LXR-dependent manner.(14, 15) It appeared that the effect of LXR on immune-related diseases is disease-specific. A major limitation of the previous reports is that the cell type(s) responsible for the effect of LXR on immune-related diseases were not defined. The cell type–specific effects of LXR on immune and/or nonimmune cells may have explained the disease-specific effect of LXR on immune-related diseases. In the current study and through the use of iNKT-deficient mice, we clearly showed that the sensitizing effect of LXRα activation was iNKT cell–dependent.
To determine the sufficiency of LXRα activation in iNKT cells in the sensitization, we transferred iNKT cells isolated from WT or LXRα-KI mice to CD1d−/− mice. This strategy allowed a specific LXRα activation in iNKT cells and excluded the effect of other cell types. Our results showed that adoptive transfer of LXRα-KI iNKT cells was sufficient to sensitize CD1d−/− mice to ConA-induced hepatitis. However, we cannot exclude the role of other cell types in the sensitization of LXRα-KI mice in vivo.
Besides demonstrating that iNKT cells are required for the sensitizing effect of LXRα activation on ConA-induced hepatitis, we provided direct evidence that LXRs are expressed and functional in NKT cells. Activation of LXRα sensitized the activation of both the primary iNKT cells and the DN32.D3 NKT hybridoma cells. Because hepatic NKT cells are known to play hepato-detrimental roles in ConA-induced liver injury,(4-8) our results suggested that the LXR-responsive activation of iNKT cells is a plausible mechanism by which LXRα activation sensitizes mice to ConA-induced hepatitis.
In addition to AIH, immune-mediated liver injury is also involved in the pathogenesis of a wide range of liver diseases, such as alcoholic hepatitis, nonalcoholic steatohepatitis, viral hepatitis, and drug-induced liver injury.(33-35) A recent study has demonstrated that LXRα regulates hepatic immune function when the mice were challenged with a high-fat and high-cholesterol diet (HFCD).(36) Specifically, the authors reported that iNKT cells are functionally impaired in the liver of HFCD-fed LXRα−/− mice, which is associated with the attenuation of ConA-induced liver injury in HFCD-fed LXRα−/− mice. The iNKT cell phenotype in the HFCD-fed LXRα−/− mice and its interpretation were consistent with our conclusion that activation of LXRα in the LXRα-KI mice sensitized iNKT cell activation, leading to their heightened sensitivity to ConA-induced hepatitis.
We showed that IFN-γ was induced in ConA-treated LXRα-KI mice, and IFN-γ was required for the sensitizing phenotype. IFN-γ is a pivotal cytokine secreted by NKT cells, and is also one of the key cytokines in the development of ConA-induced AIH. A previous study reported that IFN-γ is a direct target gene of LXR, and the transactivation of IFN-γ by LXR was mediated by a LXR response element in the IFN-γ gene promoter,(31) providing a plausible mechanism by which LXR activation induces the expression and production of IFN-γ by iNKT cells.
In addition to LXRα, LXRβ is also an important LXR isoform that is ubiquitously expressed. By treating the mice with GW3965, both LXR isoforms can be activated. In our ConA model, we found that treatment with GW3965 can further protect the LXRα−/− mice (Fig. 2), suggesting that activation of the remaining LXRβ in LXRα−/− mice may have played a protective role. In WT mice, the treatment of GW3965 showed an overall protective effect, which may have also contributed by the activation of LXRβ. However, future studies using specific LXRβ gain-of-function and loss-of-function models are necessary to define the role of LXRβ in AIH.
In summary, our results have uncovered a function of LXRα in ConA-induced AIH. The sensitizing effect of LXRα activation depends on iNKT cell activation and production of IFN-γ. Our results suggest that LXRα plays an important role in the development of AIH.
Acknowledgment
We thank Dr. Albert Bendelac (University of Chicago) for his gift of the DN32.D3 cell line. W.X. was supported in part by the Koslow Endowed Professorship from the University of Pittsburgh School of Pharmacy.




