Risk of cardiovascular disease and loss in life expectancy in NAFLD
Funding information
This research received no specific grant from any public funding agency or commercial or not-for-profit sources. H.H. was supported by grants from Region Stockholm (clinical postdoctoral appointment).
Abstract
Background and Aims
Conflicting evidence exists on cardiovascular disease (CVD) risk in patients with NAFLD, and data are lacking on whether NAFLD increases mortality after a CVD event. Moreover, life expectancy in NAFLD has not been studied. We therefore examined CVD risk and life expectancy in patients with NAFLD compared with the general population.
Approach and Results
In this nationwide population-based cohort, all patients with NAFLD diagnosis and without baseline CVD (ascertaining from the Swedish National Patient Register from 1987 to 2016, n = 10,023) were matched 10:1 on age, sex, and municipality to individuals from the general population (controls, n = 96,313). CVD diagnosis and mortality were derived from national registers. Multistate models and flexible parametric survival models were used to estimate adjusted hazard ratios (aHRs) for CVD risk and loss in life expectancy due to NAFLD. We identified 1037 (10.3%) CVD events in patients with NAFLD and 4041 (4.2%) in controls. CVD risk was 2.6-fold higher in NAFLD compared with controls (aHR = 2.61, 95% CI = 2.36–2.88) and was strongest for nonfatal CVD (aHR = 3.71, 95% CI = 3.29–4.17). After a nonfatal CVD event, the risk for all-cause mortality was similar between patients with NAFLD and controls (aHR = 0.89, 95% CI = 0.64–1.25). Life expectancy in patients with NAFLD was, on average, 2.8 years lower than controls, with the highest loss of life-years when NAFLD was diagnosed in middle age (40–60 years).
Conclusions
NAFLD was associated with a higher risk of nonfatal CVD but did not affect post-CVD mortality risk. Patients diagnosed with NAFLD have a lower life expectancy than the general population.
Abbreviations
-
- aHR
-
- adjusted HR
-
- CDR
-
- Causes of Death Register
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CVD
-
- cardiovascular disease
-
- ICD
-
- International Classification of Diseases
-
- LEL
-
- loss in expectancy of life
-
- NPR
-
- National Patient Register
-
- PY
-
- person-year
-
- T2DM
-
- type 2 diabetes mellitus
INTRODUCTION
NAFLD is the most common chronic liver disease with a global prevalence of 25% in the adult population, affecting approximately 55% of individuals with type 2 diabetes mellitus (T2DM).[1, 2] NAFLD is commonly seen as the hepatic manifestation of metabolic syndrome, encompassing well-established cardiovascular disease (CVD) risk factors.[3, 4] The accumulation of liver fat is proportional to the severity of the metabolic syndrome,[4] and the presence of both NAFLD and T2DM aggravates CVD risk compared with having T2DM alone.[5] Whether a higher risk of CVD in NAFLD is due to a shared dysmetabolic milieu or whether NAFLD independently confers an additional risk of CVD remains unclear.[6]
A comprehensive meta-analysis consisting of 34,043 individuals with NAFLD followed over a median of 6.9 years showed that NAFLD conferred a 64% higher risk of CVD after adjusting for cardiovascular risk factors.[7] In contrast, this finding has recently been questioned in a large observational study of 18 million European adults, in whom no association between NAFLD and CVD events was seen in a primary care setting.[8] In addition, results vary in fatal and nonfatal CVD,[6] in which a higher risk of CVD mortality in NAFLD was confirmed in some studies[9-12] but not in others.[13, 14] These conflicting results can be attributed to several factors, including heterogeneity in study populations, prevalence of cardiometabolic risk factors, diagnostic modality of NAFLD, and duration of the follow-up. The effect size of the association appears to depend on liver disease severity, with some studies suggesting that the positive association may be limited to patients with advanced fibrosis.[9, 11] Moreover, as the prognostic role of NAFLD may not be the same after the development of CVD, it is unclear whether NAFLD leads to an increased mortality risk for those who survive a CVD event.
The mean onset age for the first diagnosis of NAFLD is about 50 years,[15] with an increasing prevalence in a younger population.[1] Because patients diagnosed at a younger age may have a more dismal clinical profile and long-term prognosis compared with older patients, the loss in life expectancy due to NAFLD may be more evident in younger patients. However, such loss has not been determined across the lifespan.
Therefore, this study aimed to (1) assess the association between NAFLD and CVD outcomes compared with the general population and establish whether the association differs in fatal and nonfatal CVD; (2) examine whether cirrhosis could amplify the risk of CVD; and (3) evaluate life expectancy of patients with NAFLD relative to the general population.
METHODS
Study population
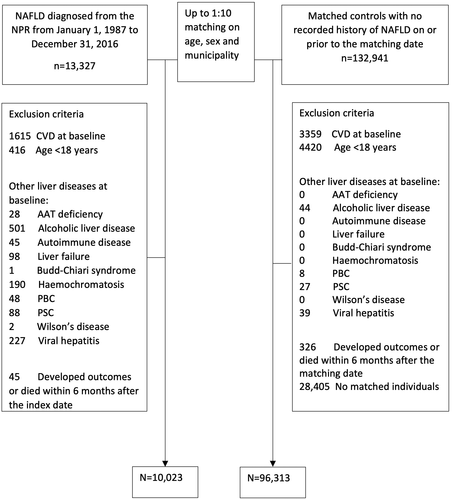
We conducted a cohort study using retrospective data of all patients diagnosed with NAFLD from January 1, 1987, to December 31, 2016, through the Swedish National Patient Register (NPR). The NPR contains data from inpatient care with national coverage from 1987. Since 2001, the NPR also includes visits in specialized outpatient care from private and public caregivers. The validity of the NPR is high, with positive predictive values ranging from 85% to 95%.[16, 17] We used International Classification of Diseases (ICD) codes to define the presence of NAFLD (571.8 in ICD-9; K75.8 and K76.0 in ICD-10) and cirrhosis (see Table S1 for the definition of cirrhosis). The date of the first NAFLD diagnosis was defined as the index date, and age at diagnosis was obtained for all patients.
We excluded individuals <18 years old, those with recorded CVD on or before the index date, and those with any diagnosis of liver disease other than NAFLD on or before the index date (see Table S1 for the definitions of CVD and other liver diseases). For each patient with NAFLD, up to 10 individuals from the general population (controls) were randomly selected from the Total Population Register[18] and were matched to a patient with NAFLD on age, sex, and municipality. Individuals were excluded if they developed CVD outcomes or died within 6 months from the index date. We also excluded controls with a diagnosis of NAFLD before baseline. Otherwise, inclusion and exclusion criteria were identical between the two study groups. Figure 1 shows a flowchart of participant inclusion and exclusion criteria. Patients and controls who obtained a diagnosis of any liver disease other than NAFLD during follow-up were censored at that time in accordance with recent expert consensus guidance.[19]
Outcomes
We used the NPR and Causes of Death Register (CDR) to identify outcomes. Vital status was defined through the CDR, which contains information on the date of death and underlying causes of death for all deceased inhabitants in Sweden, even if the death occurred abroad.[20] CVD was defined as the first acute ischemic heart disease event (410–413 in ICD-9; I20–I24 in ICD-10) or hemorrhagic or ischemic stroke (431 and 433 in ICD-9; I61, I61, I63, and I64 in ICD-10) in the NPR and CDR. We further categorized CVD into nonfatal and fatal events. Nonfatal CVD was defined as the first record of CVD without mortality; fatal CVD was defined as CVD mortality regardless of whether a patient had previously experienced a nonfatal CVD event. This definition means that patients could have both nonfatal and fatal CVD events in that they could survive a nonfatal CVD first and die from CVD afterward.
Covariates
Demographic parameters were age, sex, and municipality at the index date. Data on relevant metabolic comorbidities (T2DM diagnosis, dyslipidemia, obesity, and hypertension) recorded on or before the index date were collected from the NPR. In addition, chronic obstructive pulmonary disease (COPD), as a surrogate for smoking status, was defined as patients aged ≥45 years and with a COPD recorded on or before the index date from the NPR. The ICD codes used to define these comorbidities are given in Table S1.
Statistical analysis
At baseline, demographic and clinical parameters were compared using the chi-square tests for categorical variables and Mann Whitney U tests for continuous variables. Patients and controls were followed from the index date to the date of CVD diagnosis of interest, emigration, death, or end of follow-up (December 31, 2016), whichever came first. For all outcomes, we calculated incidence rates (IRs) per 1000 person-years (PYs). Stratified Cox proportional hazard models were used to estimate HRs and 95% CIs for incident fatal and nonfatal CVD events, using follow-up time in years as the underlying timescale. Two models were considered: Model 1 was conditioned on the matching variables only (i.e., age, sex, municipality), while model 2 additionally adjusted for the baseline presence of relevant metabolic comorbidities (T2DM, obesity, dyslipidemia, and hypertension) and COPD. The proportional hazards assumption was tested using Schoenfeld residuals. Violations of proportionality were observed for hypertension; the model was therefore modified to stratify by hypertension. In the NAFLD-only cohort, Cox regression models were repeated to calculate HRs for the association between cirrhosis and CVD outcomes. We first treated prevalent cirrhosis as exposure, and in a separate analysis, cirrhosis (both prevalent and incident) was regarded as a time-varying variable. In sensitivity analyses, we calculated E-values to evaluate the robustness of the results to the potential unmeasured confounding.[21] Briefly, this estimates the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away the found association. A large E-value implies that considerable unmeasured confounding would be needed to negate an effect estimate.
We then applied cumulative incidence functions to compute the cumulative incidences of all CVDs (nonfatal and fatal) by NAFLD and controls nonparametrically (by integrating the product of the overall survival function and the cause-specific hazard) in the presence of competing risk of non-CVD death.[22] The analysis was repeated by cirrhosis status at baseline in the NAFLD cohort.
To investigate the impact of NAFLD on the clinical course of CVD, we constructed a multistate model using transition pathways to assess multiple outcomes.[22, 23] All individuals started in an initial CVD-free state, from which they could transit to (1) incident nonfatal CVD or (2) all-cause death (competing event). The transition from incident CVD to death was also possible. The multistate model allows simultaneous and transition-specific estimation of the risk of NAFLD on (1) incident nonfatal CVD, (2) overall mortality in those without a CVD event, and (3) overall mortality in those with a nonfatal CVD. Adjusted HR (aHR) and 95% CIs of NAFLD on each transition were estimated from separate Cox regression models conditional on matching variables (age, sex, and municipality) and adjusting for a set of confounders.
In addition, we calculated loss in expectancy of life (LEL), defined as the difference between life expectancy in NAFLD and that in the general population. We only used patients diagnosed from 2001 and onward for this analysis, given that the NPR started to cover outpatient services in 2001.[16] Because we expected hospitalized patients to have a worse prognosis, we also performed a sensitivity analysis to examine life expectancy in patients with NAFLD diagnosed separately during inpatient and outpatient care. Because not all patients can be followed until death, extrapolation of survival function beyond available data is commonly used to estimate life expectancy.[24] We used flexible parametric models adapted for relative survival (with 4 degrees of freedom for the baseline rate and 3 degrees for the time-dependent effect) to estimate LEL. For degrees of freedom selection, the Akaike information criteria were used.[25, 26] Expected survival rates of the Swedish population were retrieved from the Human Mortality Database project matched to the patients with NAFLD on age, sex, and calendar year at diagnosis.[27] Therefore, for this comparison, we did not use the matched controls. LEL can be estimated as the difference in the mean observed survival time in patients with NAFLD and the mean expected survival in the general population, assuming that the life expectancy in the general population is what the NAFLD cohort would have experienced had they been NAFLD-free. Because the changes in LEL by calendar year of diagnosis was not our main interest, we calculated LEL by age groups and sex without considering the impact of calendar year on survival.
All reported p values were two-sided, and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata SE 16.0 (StataCorp LP, College Station, Texas, USA).
The study was approved by the Regional Ethics Review Board in Stockholm (dnr 2017/1019-31/1). Because this study included analyses of deidentified data, written consent from participants was not required.
RESULTS
After exclusions, the cohort included 10,023 patients with NAFLD and 96,313 matched controls (Figure 1). Among all patients with NAFLD, 3728 (37.2%) had their first diagnosis of NAFLD during inpatient care, and 6295 (62.8%) were diagnosed during outpatient visits. The median (interquartile range [IQR]) age at diagnosis was 54 years (22) in patients with NAFLD and 53 (23) in controls, with a slight male predominance (52%). At baseline, patients with NAFLD were more likely to have metabolic comorbidities (T2DM, dyslipidemia, obesity, hypertension) and COPD compared with controls (p < 0.001 for all; Table 1). Of note, 8772 (87.5%) patients with NAFLD had their first diagnosis since January 1, 2001. The median (IQR) follow-up time for NAFLD patients was 5 (8) years and for controls 6 (9) years.
| Total population (n = 106,336) | NAFLD cohort (n = 10,023) | Matched controls (n = 96,313) | p value | |
|---|---|---|---|---|
| Age (years) | 53 (23) | 54 (22) | 53 (23) | <0.001 |
| Sex (female) | 50,828 (47.8) | 4790 (47.8) | 46,037 (47.8) | 0.878 |
| Follow-up (years) | 6 (9) | 5 (8) | 6 (9) | <0.001 |
| Cirrhosis | 263 (0.25) | 263 (2.62) | 0 (0) | — |
| Type 2 diabetes | 1781 (1.7) | 1654 (16.6) | 127 (0.13) | <0.001 |
| Dyslipidemia | 836 (0.79) | 805 (8.0) | 31(0.03) | <0.001 |
| Obesity | 1095 (1.0) | 1073 (10.7) | 22 (0.02) | <0.001 |
| Hypertension | 2638 (2.5) | 2378 (23.8) | 260 (0.27) | <0.001 |
| COPD | 922 (0.87) | 261 (2.6) | 661 (0.69) | <0.001 |
Note
- Data are presented as number (%) or median (interquartile range).
Impact of NAFLD on CVD events
A total of 1037 (10.3%, IR = 16.5/1000 PYs) CVD events were seen in patients with NAFLD and 4041 (4.2%, IR = 5.4/1000 PYs) in matched controls, translating to an aHR of 2.61 (95% CI = 2.36–2.88) of developing CVD. In patients with NAFLD, 843 (8.4%) developed nonfatal CVD and 320 (3.2%) fatal CVD events. In controls, 2204 (2.3%) developed nonfatal CVD and 2192 (2.3%) fatal CVD events. Patients with NAFLD had a higher risk of nonfatal CVD (aHR 3.71, 95% CI = 3.29–4.17), but no statistically significant increase in risk was found for fatal CVD (aHR 1.20, 95% CI = 0.98–1.42) (Table 2).
| A. Total population | All CVD | Nonfatal CVD | Fatal CVD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | HRa (95% CI) | aHRb (95% CI) | Casese (%) | HRa (95% CI) | aHRb (95% CI) | Casese (%) | HRa (95% CI) | aHRb (95% CI) | |
| Controls | 4041 (4.2) | Reference | Reference | 2204 (2.3) | Reference | Reference | 2192 (2.3) | Reference | Reference |
| NAFLD | 1037 (10.3) | 3.74 (3.47–4.03) | 2.61 (2.36–2.88) | 843 (8.4) | 5.37 (4.93–5.86) | 3.71 (3.29–4.17) | 320 (3.2) | 1.81 (1.61–2.05) | 1.20 (0.98–1.42) |
| Cirrhosis | 28 (10.6) | 7.92 (4.71–13.3) | 2.56 (1.31–5.01) | 21 (8.0) | 8.68 (4.73–15.9) | 2.21 (1.02–5.12) | 7 (2.7) | 2.72 (1.22–6.56) | 1.24 (0.45–3.38) |
| B. Within NAFLD | HRc | aHRd | HRc | aHRd | HRc | aHRd | |||
|---|---|---|---|---|---|---|---|---|---|
| Cirrhosis (no) | 1009 (10.3) | Reference | Reference | 822 (8.4) | Reference | Reference | 313 (3.2) | Reference | Reference |
| Cirrhosis (yes) | 28 (10.6) | 1.44 (1.02–2.00) | 1.24 (0.85–1.82) | 21 (8.0) | 1.30 (0.87–1.93) | 1.17 (0.78–1.73) | 7 (2.7) | 1.14 (0.60–2.14) | 0.79 (0.31–1.98) |
- Abbreviation: aHR, adjusted HR.
- a Conditioned on the matching variables (age, sex, municipality).
- b Conditioned on the matching variables (age, sex, municipality), hypertension, and adjusted for type 2 diabetes, dyslipidemia, obesity, and COPD status.
- c Adjusted for age and sex.
- d Adjusted for age, sex, type 2 diabetes, dyslipidemia, hypertension, obesity, and COPD status.
- e Individuals could have both nonfatal and fatal CVD; therefore, the number of nonfatal and fatal CVD events does not sum up all CVD events.
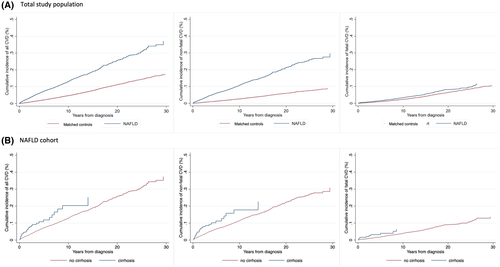
Cumulative incidences for CVD outcomes increased steadily during the follow-up (Figure 2). Patients with NAFLD, in comparison to matched controls, had a higher cumulative incidence of all CVD and nonfatal CVD events. For example, the 10-year cumulative incidence of all CVD events was 13.1% in patients with NAFLD and 4.6% in controls. The corresponding results for nonfatal CVD events were 10.7% in NAFLD and 2.7% in controls. However, the cumulative incidence of fatal CVD events was similar over the follow-up period with a 10-year cumulative incidence of 3.3% in patients with NAFLD and 2.3% in controls (Figure 2A).
Impact of cirrhosis on CVD events
Patients with NAFLD and cirrhosis at baseline had higher rates of all CVD events in the first model (HR 7.92, 95% CI = 4.71–13.3), although this estimate was lower after adjustments for CVD risk factors (aHR 2.56, 95% CI = 1.31–5.01). A similar trend was found for nonfatal and fatal events (Table 2). Similarly to all patients with NAFLD, no increased risk of fatal CVD was observed. In the NAFLD-only group we did not note an increased CVD rate in patients with cirrhosis at baseline (aHR 1.24, 95% CI = 0.85–1.82) (Table 2). Comparable results were observed when cirrhosis was modeled as a time-varying exposure (aHR 0.92, 95% CI = 0.58–1.16). The cumulative incidences of CVD outcomes were less differentiated between cirrhosis and noncirrhosis at baseline (Figure 2B), primarily because of a high competing risk of non-CVD death in patients with cirrhosis during follow-up (Figure S1).
Impact of NAFLD on mortality after a CVD event
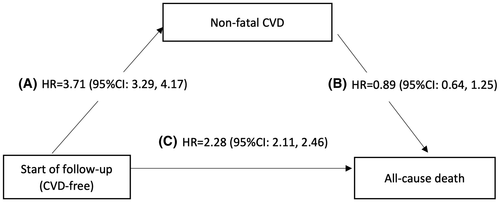
During follow-up, 3034 individuals developed incident nonfatal CVD (IR = 13.2/1000 PYs in patients with NAFLD and 2.9/1000 PYs in controls), and 1208 died afterward (IR = 70.4/1000 in patients with NAFLD and 77.4/1000 PYs in controls). The median survival time from nonfatal CVD diagnosis was 10 years (9) in patients with NAFLD and 12 years (9) in controls. Meanwhile, 8984 individuals died without experiencing any CVD event (IR = 21.5/1000 PYs in patients with NAFLD and 10.2/1000 PYs in controls). Figure 3 describes the impact of NAFLD on nonfatal CVD and death in a multistate model. Although a higher risk for nonfatal CVD events was observed in NAFLD, we did not observe an increase in mortality after such an event in patients with NAFLD compared to controls (aHR 0.89, 95% CI = 0.64–1.25). The impact of NAFLD on all-cause mortality without a prior CVD event was more than 2-fold higher compared with controls (aHR 2.28, 95% CI = 2.11–2.46).
Life expectancy in NAFLD compared with the general population
Figure 4 displays life expectancy estimates after a NAFLD diagnosis stratified by sex. On average, life expectancy was 2.8 (95% CI = 0.4–5.6) years lower in patients with NAFLD than the general population: 2.4 (95% CI = 0.4–4.5) years for females and 3.1 (95% CI 0.4–6.6) years for males (Table 3). Patients diagnosed with NAFLD in middle age had, on average, higher life expectancy loss than both younger and older patients. For example, males had 4.9 (95% CI = 1.3–8.4) years and females 3.8 (95% CI = 0.4–7.0) years lower life expectancy compared with the general population when NAFLD was diagnosed between 40 and 60 years of age. However, when a diagnosis of NAFLD was made at ≥80 years of age, life expectancy loss was only 0.3 years lower, regardless of sex.
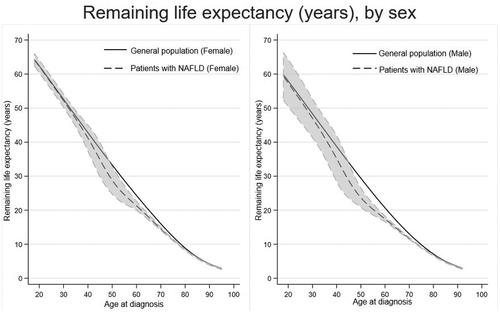
| Loss in life expectancy (95%CI) | Observed mean survival (95% CI) | Expected mean survival | |
|---|---|---|---|
| Total population | 2.8 (0.4, 5.6) | 27.8 (24.9, 30.1) | 30.6 |
| Female | 2.4 (0.4, 4.5) | 27.1 (24.9, 29.1) | 29.5 |
| Male | 3.1 (0.4, 6.6) | 28.5 (24.9, 32.0) | 31.6 |
| Age groups, years | |||
| 18–40 | 1.3 (−3.5, 6.2) | 47.2 (42.4, 52.0) | 48.5 |
| 40–60 | 4.3 (0.8, 7.7) | 26.6 (23.1, 30.0) | 30.9 |
| 60–80 | 2.0 (1.4, 2.6) | 15.6 (14.9, 16.2) | 17.6 |
| ≥80 | 0.3 (0.1, 0.5) | 6.4 (6.1, 6.6) | 6.7 |
| Female | |||
| 18–40 | 0.6 (−1.6, 2.9) | 50.7 (48.4, 53.0) | 51.3 |
| 40–60 | 3.8 (0.4, 7.0) | 28.1 (24.8, 31.4) | 31.9 |
| 60–80 | 1.9 (1.3, 2.7) | 16.9 (16.2, 17.6) | 18.8 |
| ≥80 | 0.3 (0.1, 0.5) | 6.7 (6.6, 7.0) | 7.0 |
| Male | |||
| 20–40 | 1.7 (−4.3, 7.8) | 45.4 (39.3, 51.5) | 47.1 |
| 40–60 | 4.9 (1.3, 8.4) | 24.2 (21.6, 28.7) | 30.1 |
| 60–80 | 2.0 (1.5, 2.5) | 13.7 (13.2, 14.2) | 15.7 |
| ≥80 | 0.3 (0.1, 0.4) | 5.6 (5.4, 5.8) | 5.9 |
LEL was highly affected in patients who received their first NAFLD diagnosis in the inpatient register, with an average loss of 7.9 years (95% CI = 5.8–9.9) for all: 7.4 (95% CI = 5.4–9.4) in males and 8.3 (95% CI = 6.2–10.4) in females. For patients who received their first NAFLD diagnosis in the outpatient register, LEL was 2.0 years (95% CI = 0.7–3.5) for all: 2.4 (95% CI = 0.8–4.1) in males and 1.8 (95% CI = 0.7–2.9) in females (Figure S2A,B).
Sensitivity analyses using E-values suggested that the observed associations were robust to the unmeasured confounding for the CVD outcomes (Table S2). For example, the E-value for the association of NAFLD and all CVD risk was 4.56, meaning that an unmeasured confounder would need to have a minimal relative risk of 4.56 with both NAFLD and CVD to explain the found association. Weaker unmeasured confounding could not do so, although the confidence interval could be toward the null, with a smaller risk ratio of 4.16. Additionally, we found that the incidence rate of CVD and mortality in women with NAFLD is higher than that in referent women. The differences in CVD and mortality between those with and without NAFLD were larger among women than men (Table S3).
DISCUSSION
Several observations can be made from this large nationwide cohort study set in a secondary or tertiary setting. First, we found an elevated risk of nonfatal CVD events in patients with NAFLD compared with matched controls. Second, patients with cirrhosis had a higher CVD risk than controls, but not compared to patients with noncirrhotic NAFLD. Third, while NAFLD was associated with increased overall mortality, no increased mortality was observed in patients with NAFLD with incident CVD compared to matched controls who had also experienced a nonfatal CVD event. Finally, the overall loss of life expectancy in patients with NAFLD was about 3 years, which was affected by age and clinical setting at diagnosis. LEL was highest in hospitalized patients and when the diagnosis of NAFLD was made at middle age, whereas no apparent loss in life expectancy was observed for those aged ≥80.
A growing body of evidence shows that NAFLD is independently associated with an increased risk of CVD events, and such an association has been consistently replicated across different populations.[7, 10-13, 28, 29] A comprehensive meta-analysis by Mantovani et al. provided substantive evidence for a significant association between NAFLD and CVD risk, and the risk increases with the severity of NAFLD.[29] Here, we observed an HR of 2.6 for all CVD outcomes, which is slightly higher than the pooled estimates of 1.8 from this meta-analysis. This discrepancy is probably secondary to the choice and definitions of confounders, the method of NAFLD diagnosis, and vast differences in the comparison group. We also observed that the risk was somewhat higher in patients with cirrhosis than controls, confirming previous studies.[12, 29, 30] Of note, we found that female sex might no longer be protective against CVD in women with NAFLD, which was in line with previous finding by Allen et al.[31] It appears that relative to men, women are less susceptible to NAFLD, but once NAFLD is established,[31] the protection of female sex against CVD disappears.
Despite the convincing evidence for higher risk of nonfatal CVD events related to NAFLD, disagreement primarily falls within the published risk estimates for fatal CVD events.[9-14, 29, 30] Our results of no elevated risk of CVD mortality in patients with NAFLD compared to matched controls might in part be driven by the large proportion of patients with NAFLD (98%) having a more benign (i.e., noncirrhotic) NAFLD, as severe forms of NAFLD, rather than simple steatosis, have been reported to increase the risk of CVD mortality.[9, 11, 23] This view is in line with a recent study using histological data reporting that NAFLD severity was linked to a higher CVD risk.[10] Additionally, we did not observe an increased risk of CVD in patients with cirrhosis when restricting the analysis in patients with NAFLD. As indicated by the supplementary analysis showing 40% of patients with cirrhosis died from non-CVD events (e.g., liver-related death) during the follow-up, a high competing risk of non-CVD mortality might have diluted our estimates due to selective survival.
Because of the high risk of nonfatal CVD events in NAFLD, we extended the observation for those who survived with incident CVD and observed that there was no increased overall mortality risk after a CVD event in NAFLD than the controls who also survived with a CVD event. Such an association has not been previously studied. This finding suggests that incident CVD overtakes the influence of NAFLD on mortality and that the impact of NAFLD on mortality after a CVD event is negligible. Collectively, our results suggest that patients with NAFLD are at a higher risk of CVD, but once CVD manifests, the role of NAFLD on mortality is limited. Hence, making a NAFLD diagnosis after a CVD event might be unnecessary, although this needs to be confirmed in studies with more granular data, as an alternate explanation could be that a high proportion of controls might have developed NAFLD before their CVD event.
Beyond lower life expectancy in NAFLD, we also found that higher loss of life expectancy was attributed primarily to patients diagnosed in middle age, and such loss appeared to be less evident in those diagnosed at a younger or older age. Data from the nationwide study in the United States found a 4-fold mortality in those diagnosed at age 45–54 years, whereas mortality risk was similar between participants without NAFLD and those diagnosed at 55–80 years.[32] Patients with NAFLD diagnosed at middle age may have more cardiovascular risk factors compared with their non-NAFLD counterparts. On the other hand, because cardiovascular comorbidities are common in older adults, those who develop NAFLD at old age are similar to reference individuals, leading to a lower relative increase in mortality risk attributable to NAFLD. This notion has been supported by Kagansky et al., who demonstrated that no differences in metabolic impairments were found between octogenarians with NAFLD and those without, claiming that NAFLD is a benign condition in old age.[33] Another notable finding is that we did not see a big difference in younger age (i.e., 18–40 years). This relative lack of difference might be due to this group’s relatively low background mortality rate (3.1% for NAFLD vs. 0.9% for controls) and the low number of younger individuals with NAFLD. Thus, this finding should be interpreted cautiously. As expected in the analyses stratified by diagnosis from the inpatient and outpatient registers, the peak in LEL primarily arises from hospitalized patients with a NAFLD diagnosis at middle age, suggesting that this group of patients could benefit the most from intensive treatment of NAFLD to improve survival. Nevertheless, we also observed a lower life expectancy of about 2 years in patients with NAFLD diagnosed as outpatients.
Both strengths and limitations can be linked to the use of national, population-based registers using historical data in this study. Given the high validity of the NPR for acute CVD events and the high coverage rate from the CDR, we have a high capture rate of the outcomes studied. Selection bias is minimal in that reporting to the Swedish registers is mandatory, and health care in Sweden is run by public institutions. However, primary care does not report to the NPR, explaining why such patients are omitted. Therefore, generalization of our data should primarily be restricted to patients diagnosed in secondary or tertiary care. A major limitation is the potential misclassification of NAFLD. As NAFLD is often asymptomatic, it is underdiagnosed and its prevalence is likely to be underestimated. Due to the high prevalence of NAFLD in the general population, there are potentially misclassified cases in the control group, resulting in falsely low estimates of the outcomes studied. However, such misclassification would dilute our estimates toward the null, which is why the effect of NAFLD on the outcomes might be higher than reported here. As reflected by the low prevalence of metabolic comorbidities in the controls, another pitfall concerns the diagnosis of the disease ascertained from the NPR instead of from primary care. The prevalence of these metabolic comorbidities is likely to be underdiagnosed. The estimates therefore could represent a group of patients with a more severe form of NAFLD; however, only 2% had a diagnosis of cirrhosis at baseline. Another limitation is that although we attempted to control relevant confounders, we could not control other potential residual confounders (e.g., cholesterol values or lifestyle factors) due to such data being unavailable in the used registers. However, our E-value analyses suggests that an unmeasured confounder would need to give a very high increased in risk (>4.6) for CVD, which may be unlikely.
Despite these caveats, our findings have important clinical implications for careful monitoring of CVD in patients with NAFLD. Patients with NAFLD should be closely assessed for CVD risk factors and offered early intervention to reduce CVD risk. Our results also show that it may not be necessary to actively investigate NAFLD in patients with a CVD event. In addition, because patients diagnosed at middle age have the highest LEL, the screening, diagnosis, and clinical management for this patient group would appear to be especially important. Conversely, diagnosing NAFLD in older patients (≥80 years) may lack clinical significance.
To conclude, in this population-based cohort of individuals with NAFLD without a previous history of CVD, we found an increased risk of cardiovascular outcomes compared with the general population. This increased risk of cardiovascular events was primarily for nonfatal CVD. After a CVD event, patients with NAFLD had a similar mortality risk to controls with a CVD event. Patients with NAFLD have, on average, a 3-year lower life expectancy than the general population. However, this lower life expectancy is influenced by age at diagnosis, with a greater loss of life at middle age and no apparent loss in life years when the diagnosis occurred at an older age.
CONFLICT OF INTEREST
Nothing to report.
AUTHOR CONTRIBUTIONS
Study concept and design: Ying Shang, Patrik Nasr, Linnea Widman, and Hannes Hagström. Data acquisition: Linnea Widman and Hannes Hagström. Statistical analysis: Ying Shang and Linnea Widman. Data interpretation: Ying Shang, Patrik Nasr, Linnea Widman, and Hannes Hagström. Manuscript draft: Ying Shang. Critical revision: Ying Shang, Patrik Nasr, Linnea Widman, and Hannes Hagström. Guarantor of the manuscript: Ying Shang and Hannes Hagström.