Transcriptomic Traces of Adult Human Liver Progenitor Cells
Advances in single-cell sequencing technology have facilitated the precise study of gene expression patterns in the mouse liver,1-4 revealing a previously unknown degree of heterogeneity within hepatocyte, cholangiocyte, endothelial cell, and immune cell compartments. Yet, translating lessons learned from the mouse liver to the human liver remains difficult, a situation underscored by the increasing incidence of liver disease in the United States5 and exacerbated by our limited understanding of the cellular composition of the human liver. For instance, the ability to unambiguously identify adult human liver stem cells or progenitor cells—their more restricted progeny—would profoundly affect the direction of translational research, yet the existence and characteristics of such cells remain hotly contested. In a recent study published in Nature, Aizarani and colleagues addressed this problem by interrogating the adult human liver environment on a single-cell scale.6 In the second such study of its kind,7 Aizarani and colleagues used fresh and frozen cell preparations from 9 donors to map the gene expression of all major cell types in the human liver onto an interactive cell atlas. Furthermore, the authors characterized the zonation profiles of hepatocytes and endothelial cells, described heterogeneous gene expression patterns within immune cell compartments, identified in the EPCAM–positive cell compartment putative bipotential progenitor cells (i.e., cells that can give rise to both cholangiocytes and hepatocytes), and compared the gene expression profiles of perturbed cell states to their reference atlas. These observations will serve to more accurately inform future studies of human liver diseases, particularly those focused on the characterization and validation of adult liver stem/progenitor cells.
Aizarani and colleagues initially focused their investigation on zonation in hepatocyte and liver sinusoidal endothelial cell (LSEC) populations. By ordering hepatocytes and LSECs by diffusion pseudo-time in order to reflect transcript zonation as the major variable, the authors found that 41% of hepatocyte genes (compared to 50% in mouse1) and 67% of endothelial cell genes (compared to 35% in mouse2) were zonated. Pathway enrichment analysis revealed shared functions between midzonal hepatocytes and central and midzonal LSECs, e.g., uptake of ligands by scavenger receptors, which may indicate a functionally cooperative relationship between the two cell types across the liver lobule. Surprisingly, only 68% of hepatocyte genes and 60% of endothelial cell genes were found to be similarly zonated between mouse and human, which highlights the potential limitations of murine liver disease models.
The authors then shifted focus to immune cell populations and defined inflammatory and immunoregulatory subpopulations of Kupffer cells, in agreement with a previous study.7 In addition, they described the gene signatures of circulating and resident populations of B cells, defined by MS4A1 and CD37 expression and MZB1, DERL3, SSR4, and IGHG4 expression, respectively. Finally, their analysis uncovered CD56-positive and negative subpopulations of natural killer and natural killer T cells.
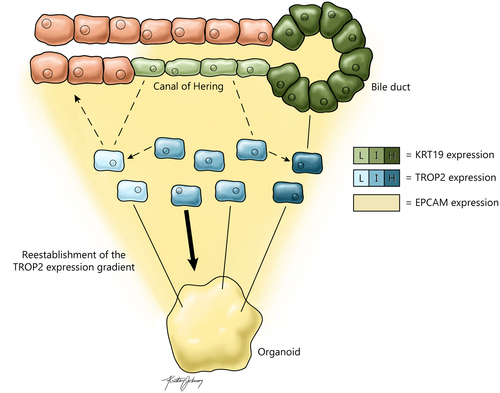
Perhaps this study’s most interesting findings speak to the potential existence of “genuine” adult liver progenitor cells under homeostatic conditions. The authors focused their search on EPCAM-positive cells because in previous studies EPCAM has been implicated as a marker expressed by human liver stem cells. To achieve analytical specificity, single-cell data from biliary cell populations in the initial pan-liver analysis were reanalyzed using RaceID3, a clustering algorithm specializing in identification of rare cell types. This led the authors to discover a gradient of transcriptional heterogeneity ranging from a hepatocyte-biased population enriched for ASGR1 expression to a fully differentiated cholangiocyte population enriched for KRT19 and CFTR expression. The authors supplemented this analysis using StemID2 and FateID algorithms, which are designed to reconstruct cell lineages and infer lineage relationships. This approach revealed a population of cells in the center of the transcriptional gradient with the dual potential to become hepatocytes or remain as biliary cells and further differentiate into mature cholangiocytes. Specifically, genes operating in multiple signaling pathways such as HES1, SFRP5, FGFR2, and FGFR3 were up-regulated in this bipotential population of EPCAM-positive cells. Importantly, the authors observed that TROP2 (TACSTD2) gene expression negatively correlates with hepatocyte bias and positively correlates with cholangiocyte bias, particularly in mature cells that highly express the genes KRT19 and CFTR (Fig. 1). The bipotential cells express intermediate levels of TROP2 and can be teased out by cell sorting. This spotlight on TROP2 is reinforced by another recent investigation by Segal and colleagues who similarly identified TROP2 as a marker of mature human cholangiocytes.8 Segal and colleagues also found TROP2 to be a marker of bipotential EPCAM-positive cells in the adult, but not fetal, human liver, which they described as hepatobiliary hybrid progenitors. In agreement with these results, Aizarani and colleagues sorted EPCAM-positive TROP2-intermediate cells and found that, when cultured, they demonstrated greater organoid-forming capacity than both TROP2-low and TROP2-high EPCAM-positive subsets. These results suggest that the putative bipotential progenitor cells identified by the authors constitute a subpopulation of biliary cells. In parallel, studies have shown that Trop2 is also expressed in the biliary cell compartment in mice following toxin-induced liver injury yet is undetected in biliary cells of healthy mice.3
Finally, Aizarani and colleagues used their freshly minted liver cell atlas as a reference for analyzing perturbed cellular states. Comparison of dissociated hepatocellular carcinoma tissue from 3 patients to normal human liver cells revealed that cancer cells experience a loss of metabolic gene expression, e.g., CYP2E1, CYP2C8, and CPS1, and an induction of WNT and Hedgehog signaling pathways, a profile also observed in organoids derived from EPCAM-positive TROP2-intermediate cells. In addition, the authors investigated changes in gene expression profiles in human hepatocytes and endothelial cells transplanted into mice with a gene defect causing liver failure. Although the fundamental gene expression profiles of both cell types were unchanged, e.g., ALB and PCK1 expression in hepatocytes and CLEC4G, PECAM1, and CD34 expression in endothelial cells, WNT and Hedgehog signaling pathways and cell cycle genes were up-regulated, suggesting that rare highly proliferative subpopulations engrafted and expanded following transplantation. An alternative interpretation, at least for hepatocytes, is that the gene expression profile of the recovered cells is a direct result of the cells responding to growth signals elicited by liver failure in the recipient mice.
Overall, this study is the most comprehensive single-cell gene profiling of the human liver to date, thus providing a powerful resource to the biomedical research community. Despite a broad experimental scope, Aizarani and colleagues were able to reproduce key findings from complementary studies,7, 8 lending credence to their in silico approach to lineage reconstruction. However, ambiguity remains surrounding the definition of TROP2-intermediate liver progenitor cells. Intriguingly, the authors demonstrate differences in KRT19 expression between EPCAM-positive cells in liver tissue sections, with KRT19-low/negative cells shown to be located in the canal of Hering—long assumed to be the location of adult liver stem cells—and KRT19-high cells constituting the mature bile duct (Fig. 1). In parallel, transcriptomic analysis suggests an overlap between KRT19-low/negative and TROP2-intermediate cells. Extending these findings to include documentation of in situ TROP2 expression would have helped to substantiate the idea that TROP2-intermediate liver progenitor cells are KRT19-low/negative biliary cells residing in the canal of Hering. Furthermore, the bipotentiality of these progenitor cells, as determined by cell fate-prediction algorithms, remains to be validated in vivo. For instance, the authors demonstrate that the gradient of TROP2 expression is reestablished in organoids derived exclusively from TROP2-intermediate EPCAM-positive cells (Fig. 1); however, their single-cell data suggest substantial differences between gene expression in these organoids and the EPCAM-positive cells isolated from human tissue. These examples illustrate the need for future in vivo studies, particularly an in situ tissue analysis informed by the authors’ single-cell data, and a demonstration of the proposed liver progenitor cells’ lineage relationship to hepatocytes and mature cholangiocytes.




