Quality Measures, All-Cause Mortality, and Health Care Use in a National Cohort of Veterans With Cirrhosis
Abstract
Decompensated cirrhosis is associated with high morbidity and mortality. However, no standardized quality measures (QMs) have yet been adopted widely. The Veterans Affairs (VA) Advanced Liver Disease Technical Advisory Group recently developed a set of six internal QMs to guide quality improvement efforts in cirrhosis in the domains of access to care, hepatocellular carcinoma surveillance, variceal surveillance, quality of inpatient care for upper gastrointestinal bleeding, and cirrhosis-related rehospitalizations. We aimed to (1) quantify adherence to cirrhosis QMs and (2) determine whether adherence was associated with all-cause mortality and health care use within a large national cohort of veterans with cirrhosis. We performed a retrospective study using data from the Veterans Outcomes and Costs Asociated with Liver Disease cohort of 121,129 patients newly diagnosed with cirrhosis from January 1, 2008, to December 31, 2016, at 128 VA facilities. The mean follow-up time was 2.7 years (interquartile range, 1.1-5.1 years). Adherence to outpatient access to specialty care was 71%, variceal surveillance was 32%, and early postdischarge care was 54%. In adjusted analyses, outpatient access to specialty care (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.78-0.82), hepatocellular carcinoma surveillance (HR, 0.92; 95% CI, 0.90-0.95), variceal surveillance (HR, 0.93; 95% CI, 0.89-0.99), and early postdischarge care (HR, 0.57; 95% CI, 0.54-0.60) were associated with lower all-cause mortality. Readmissions after 30 days (HR, 1.53; 1.46-1.60) and 90 days (HR, 1.88; 95% CI, 1.54-1.70) were associated with higher all-cause mortality. Higher adherence to QMs was also associated with lower inpatient health care use. Conclusion: Five of the six proposed VA cirrhosis QMs were measurable using existing data sources, associated with mortality and health care use, and may be used to guide future quality improvement efforts in cirrhosis.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- AUDIT-C
-
- Alcohol Use Disorder Identification Test
-
- CCS
-
- Clinical Classification Software
-
- CDW
-
- Corporate Data Warehouse
-
- CI
-
- confidence interval
-
- CPT
-
- current procedural terminology
-
- CT
-
- computed tomography
-
- CTP
-
- Child-Turcotte-Pugh
-
- GI
-
- gastrointestinal
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- ICD
-
- International Classification of Diseases
-
- IQR
-
- interquartile range
-
- IRR
-
- incidence rate ratio
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MRI
-
- magnetic resonance imaging
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- PPV
-
- positive predictive value
-
- PTUDS
-
- percentage of time under direct surveillance
-
- QM
-
- quality measure
-
- VA
-
- Veterans Affairs
Cirrhosis is the twelfth leading cause of death in the United States, resulting in an estimated 36,000-66,000 deaths annually,1 and the fourth leading cause of mortality among persons ages 45-54 years.2 Nearly 10% of patients admitted to the hospital with cirrhosis die during their hospitalization, and 67% experience a nonelective readmission within 1 year.3, 4 According to one analysis, the national annual direct costs of cirrhosis were $4 billion in 2002, largely driven by inpatient hospitalizations,4, 5 whereas the indirect costs from loss of productivity were $10.6 billion in 2004.6 Despite the recent advent of effective treatment for hepatitis C virus (HCV), the overall burden of cirrhosis is expected to increase due to the irreversible effects of long-standing HCV infection (including decompensated liver disease and hepatocellular carcinoma [HCC]), the persistent burden of alcoholic liver disease, and the increased prevalence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) related to the obesity epidemic.1, 7
Studies suggest there is variability in the quality of cirrhosis care and that adherence to guideline-recommended care is often inadequate for ascites, variceal bleeding, HCC, and preventive vaccinations.8-12 Due to the variation in the quality of care for cirrhosis, multiple groups have proposed identifying evidence-based cirrhosis quality measures (QMs) to guide care for all patients, regardless of their access to a liver disease specialist, and to evaluate and provide feedback on the quality of care across providers.13, 14
In 2017, the Veterans Health Administration, the largest integrated health system providing cirrhosis care in the United States, convened clinical and operations experts in cirrhosis to develop a set of guideline-based and consensus-based QMs to reduce unwarranted health care variation and improve quality of care for cirrhosis.15 The QMs address both outpatient and inpatient care of cirrhosis and include the domains of access to care, HCC surveillance, variceal surveillance, upper gastrointestinal (GI) bleeding, and inpatient health care use. However, no prior work has evaluated the relationship between adherence to these QMs and broader patient outcomes. Our objective was to quantify adherence to cirrhosis QMs and investigate whether adherence was associated with lower all-cause mortality and inpatient health care use in a large national cohort of veterans with cirrhosis.
Patients and Methods
Cohort Identification
The Veterans Outcomes and Costs Associated with Liver disease (VOCAL) cohort was derived from the Veterans Affairs (VA) Corporate Data Warehouse (CDW), which includes detailed demographic, clinical, and administrative data using validated methodology.16, 17 The main inclusion criteria for this retrospective cohort were having two outpatient or one inpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) or ICD10-CM codes for cirrhosis (ICD9-CM 571.2, 571.5, 571.6; ICD10-CM K74.*, K70.3*) from January 1, 2010, to December 31, 2016.18 The baseline period began on the date the first cirrhosis diagnosis code appeared in the medical record or on the first day of hospitalization. All patients were followed until death, transplantation, or December 31, 2017, whichever came first. Patients were only included in the cohort in each year if they had at least one primary care visit during that year and, therefore, could enter and exit the cohort based on receipt of primary care each year. The study protocol and waiver of informed consent were approved by the Institutional Review Boards in the West Haven VA, West Haven, CT and the Corporal Michael J. Crescenz VA, Philadelphia, PA.
Cirrhosis QM Assessment
The VOCAL cohort was used to assess quality of care for patients with cirrhosis. Table 1 shows a list of VA QMs with their corresponding domains and definitions. The ascertainment of each QM is described here in detail and shown in Supporting Table S1. Each QM was benchmarked against prior national estimates of the QM, and if there was a large discrepancy between the measured QM and the benchmark, two experienced adjudicators performed a medical record review to assess the diagnostic accuracy of the QM. All outpatient measures (QM1-QM3) only include veterans who had at least one outpatient primary care visit in a calendar year. Patients were censored at death or transplantation.
| Cirrhosis Quality Measure | Domain Measured | Definition | Unit of Measurement |
|---|---|---|---|
| QM1. Patients with cirrhosis should be seen by a specialist within 12 months of the cirrhosis diagnosis | Specialty care access | Veterans with at least one outpatient gastroenterology or hepatology visit within 12 months of the cirrhosis diagnosis | Patient |
| QM2. Patients with cirrhosis should receive surveillance for HCC every 6 months with ultrasound (or other liver imaging), with or without AFP | HCC surveillance | PTUDS with serial abdominal imaging (liver ultrasound, contrast-enhanced CT/MRI) within the first 2 years following the cirrhosis diagnosis | Patient |
| QM3. Patients with cirrhosis and platelet count <150,000/mm3 should receive upper GI endoscopy to assess for esophageal varices within 12 months of the initial diagnosis of cirrhosis (excluding patients on nonselective beta blockers) | Variceal surveillance | Veterans with at least one upper endoscopy within 12 months of the cirrhosis diagnosis | Patient |
| QM4. Patients who are discharged after admission for a complication of cirrhosis should be seen in clinic (either specialty or primary care) within 30 days of discharge | Specialty/primary care access | Outpatient primary care, gastroenterology, or hepatology appointment within 30 days of cirrhosis-related admission not resulting in death, transplant, discharge to a facility, or hospice | Hospital discharge |
| QM5. Patients with cirrhosis admitted to the hospital with upper GI bleeding should receive antibiotics in the hospital for ≥3 days* | Inpatient upper GI bleeding | — | |
| QM6. Percent of patients rehospitalized within 30 days of discharge (and 90 days) | Inpatient use | 30-day and 90-day cirrhosis-related readmissions not resulting in death, transplant, discharge to a facility, or hospice | Hospital discharge |
- * QM5 could not be validated; therefore, no association with clinical outcomes is reported.
QM1: Patients with cirrhosis should be seen by a specialist within 12 months of the cirrhosis diagnosis. Data on completed outpatient visits were obtained from the outpatient visit data from the CDW. Patients were classified as having seen a gastroenterology or hepatology physician or advanced practice provider (nurse practitioner, physician's assistant) within 12 months before or after the date the cirrhosis diagnosis code was first entered in the medical record. Patients coded as having seen a provider in a GI clinic or hepatology clinic at least once were classified as receiving specialty care.
QM2: Patients with cirrhosis should receive surveillance for HCC every 6 months with ultrasound (or other liver imaging), with or without alpha-fetoprotein (AFP). Imaging data were obtained from the radiology database in the CDW and supplemented from the “fee-basis” database, which contains additional data on studies performed outside of VA facilities that are paid for by VA funds; 95% of fee-basis claims are processed within 200 days.19 Imaging adherence was measured over the first 2 years of follow-up after the cirrhosis diagnosis and assessed as the percentage of time under direct surveillance (PTUDS), as reported.19 Patients were only eligible for this measure if alive for at least 2 years following the cirrhosis diagnosis. Patients were considered under direct surveillance if they had an imaging test within 7 months of a prior study (a 1-month grace period to the usual 6 months was given to account for small scheduling delays in clinical practice settings). The 7-month surveillance interval restarted on the date of reimaging if imaging was performed before the completion of the previous time interval. For example, patients who received no imaging were included in the calculation with a PTUDS of 0%, and those with two abdominal imaging studies 1 year apart would have a PTUDS of 50%.
The main analysis included outpatient or inpatient abdominal ultrasounds, contrast-enhanced computed tomography (CT) scans or magnetic resonance imaging (MRI) with gadolinium irrespective of imaging intent.19 The justification to include all studies was based on the pragmatic consideration that if a patient received adequate quality imaging for a reason other than HCC surveillance, the treating clinician would “credit them” as having received appropriate surveillance in routine clinical practice. A sensitivity analysis evaluated only outpatient studies. We did not include AFP in this calculation because it was considered optional.
QM3: Patients with cirrhosis and platelet count <150,000/mm3 should receive upper GI endoscopy to assess for esophageal varices within 12 months of the initial diagnosis of cirrhosis (excluding patients on nonselective beta blockers). Data for upper endoscopy were obtained from outpatient data and supplemented with fee-basis data to capture studies performed outside of the VA (using current procedural terminology [CPT] codes 43191-43232, 43235-43259).13, 14, 19 Procedures for upper endoscopy with stent placement, foreign body extraction, balloon dilation, and guidewire insertion were specifically excluded.13, 14 Patients were considered eligible for upper endoscopy if they were not prescribed propranolol, nadolol, or carvedilol within ±90 days of the cirrhosis diagnosis (because these patients were assumed to have appropriate primary variceal prophylaxis). Patients were considered adherent with upper endoscopy if it was performed within 12 months before or after the first cirrhosis diagnosis code in the medical record.
QM4: Patients who are discharged after admission for a complication of cirrhosis should be seen in clinic (either specialty or primary care) within 30 days of discharge. Inpatient admission data were obtained, which included all admission and discharge diagnoses, admission and discharge dates, and discharge disposition (home, rehabilitation, hospice, etc.). Cirrhosis-related admissions were identified using ICD-9-CM and ICD-10-CM codes for common liver-related complications such as ascites, variceal bleeding, spontaneous bacterial peritonitis, and hepatorenal syndrome (detailed categorization of liver-related admissions is provided in Supporting Table S2). The diagnosis codes were additionally supplemented with Clinical Classification Software (CCS), a diagnosis categorization scheme which collapses ICD-9-CM and ICD-10-CM codes into fewer clinically relevant categories and has been widely used to investigate inpatient health care use.10, 20, 21 CCS categories related to bacterial infections, electrolyte disturbances, GI bleeding, liver cancer, biliary disorders, and alcohol-related disorders were used to more broadly capture potential cirrhosis-related admissions. Patients were considered adherent if they had a postdischarge visit to primary care, gastroenterology, or hepatology providers (physicians or advance practice practitioners) within 30 days of discharge, obtained from the outpatient data from the CDW as for QM1. Using CPT codes for elective procedures within CCS, all elective admissions were excluded from the analysis. Discharges that resulted in death within 30 days or hospice care within 30 days or were to locations other than home were likewise excluded, which accounted for 1.8% of admissions.
QM5: Patients with cirrhosis admitted to the hospital with upper GI bleeding should receive antibiotics in the hospital for ≥3 days. Variceal and nonvariceal upper GI bleeding were ascertained using ICD-9-CM and ICD-10-CM codes linked to inpatient admissions (Supporting Table S3). Antibiotic prescriptions were obtained from the inpatient pharmacy barcoded medication administration table, which includes all inpatient oral and intravenous medications. Patients were considered adherent if they received any antibiotic with gram-negative coverage that could serve as effective prophylaxis for spontaneous bacterial peritonitis for 3 or more days during the hospitalization (see Supporting Table S4).
After initial descriptive analysis, the calculated adherence was only 10%, raising concern for misclassification bias. A medical record review was performed by two hepatologists (M.S. and D.E.K.) on 280 inpatient admission records to independently assess the performance characteristics of ICD9-CM and ICD-10-CM codes for upper GI bleeding such as sensitivity, specificity, positive predictive values (PPVs), and area under the receiver operator curve. CPT codes for upper endoscopy and data on packed red blood cell transfusions were added to test whether this would improve the PPV. A medical record review showed that the PPV for upper GI bleed using ICD-9-CM/ICD-10-CM codes was only 46%. This increased to 61% and 75% when adding packed red blood cell transfusions and packed red blood cell transfusions with inpatient esophagogastroduodenoscopy (EGD), respectively; however, at the higher PPV, sensitivity for capturing GI bleed was only 2.3% (Supporting Table S5). Multivariable models for mortality were, therefore, not performed due to insufficient diagnostic accuracy of this measure using administrative data.
QM6: Percent of patients rehospitalized within 30 days of discharge (and 90 days) should be quantified. Readmissions data for cirrhosis-related admissions were obtained using the same methodology as for QM4. All rehospitalizations that occurred within 30 days (or 90 days) of discharge for any reason were counted as a readmission. Admissions that were elective or that resulted in death, hospice care, or nonhome discharge were excluded from the QM adherence calculation.
Outcomes
We assessed the relationship between each quality metric and all-cause mortality at the patient level. All-cause mortality was evaluated using the VA Vital Status Master File, which is highly sensitive for capturing death.22, 23 Detailed information on the index date and mortality definition for each measure is described in Supporting Table S1. For several of the QMs we also assessed its relationship with health care use, defined as the number of cirrhosis-related hospitalizations within 2 years of the QM index date. Cirrhosis-related hospitalizations were defined using the same methodology as for QM1 and QM4.
Covariates
Patient-level covariates included demographics (age, sex, race) and percent service connectedness, which is a measure of VA benefits coverage; patients with >50% service connectedness generally have lower copayments. Distance between the VA facility where the patient was receiving care and the nearest VA transplant center was asessed using facility zip codes. Obesity was defined as body mass index >30 kg/m2 at least once during the baseline period. Diabetes was defined using a previously validated VA algorithm.24 Alcohol use was ascertained using the Alcohol Use Disorders Identification Test (AUDIT-C), which is annually administered in VA primary care clinics; a score of 5 or greater for men and 4 or greater for women was considered to indicate significant alcohol use.25 Data were also obtained regarding the etiology of liver disease, Model for End-Stage Liver Disease (MELD) score, and Child-Turcotte-Pugh (CTP) score using previously validated methodology.7, 26, 27 Comorbidity adjustment was performed with the cirrhosis comorbidity index, which includes ICD9/10 diagnosis codes for acute myocardial infarction, peripheral arterial disease, epilepsy, abuse of a substance other than alcohol, heart failure, cancer, and chronic kidney disease.28 VA facility characteristics included geographic region, academic affiliation, rurality, and whether the facilty had a high number of outpatient cirrhosis-related visits per year (volume). High volume was defined as being above the 50th percentile of outpatient visits. Rurality codes were derived from the US Department of Agriculture's 2013 Rural–Urban Continuum Codes.29 All patient-level covariates were measured at baseline (the time of cirrhosis diagnosis) with the exception of MELD and CTP scores, which were used as time-updating covariates in cases where the exposures were time-updating (see details below, Statistical Analysis).
Statistical Analysis
Multivariable Models for All-Cause Mortality
For QM1 (specialty care access within 12 months of the cirrhosis diagnosis) and QM3 (receipt of upper endoscopy within 12 months of the cirrhosis diagnosis), we used Cox proportional hazards models censored at 2 years (or for death or liver transplantation). Receipt of the QM was specified as a time-updating exposure to address possible immortal time bias.30 For example, patients not receiving specialty care were analyzed as unexposed until the specialty care appointment occurred. Secondary analyses for QM1 and QM3 excluded patients who died within 30 days of the cirrhosis diagnosis, to account for possible late referral bias.31 For QM2 (HCC surveillance with abdominal imaging), we used a Cox model with the exposure being PTUDS categorized as a dichotomous variable (≥50% of time under surveillance versus <50%) as the primary exposure. The PTUDS threshold was chosen for ease of measurement and interpretation.19 For QM4 (postdischarge specialty/primary care access) and QM6 (30-day and 90-day rehospitalizations), Cox models were fit with hospital discharge as the unit of analysis. Details regarding analyses for each QM are in Supporting Table S1.
All models were adjusted for patient and facility-level variables (listed in covariates) and for each individual VA facility. Robust standard errors were calculated, accounting for correlation of observations within patients. Models with time-updating exposures (imaging studies, hospitalizations) were fit with time-updating MELD and CTP scores; values closest to the exposure date were used. Analyses for all QMs were repeated fitting multilevel mixed-effects survival models with a Weibull distribution (using the Stata mestreg command) to account for cluster-specific random effects.
Multivariable Models for the Number of Cirrhosis-Related Hospitalizations
The associations between adherence to QM1-QM4 and health care use, defined as the number of cirrhosis-related admissions within 2 years of cohort entry (QM1-QM3) or for 2 years after the index cirrhosis-related hospitalization (QM4), were evaluated with negative binomial regression models, which are used to count outcomes in instances of overdispersion (in this case due to a large proportion of patients who were never hospitalized). The covariate adjustment approach was similar to models for all-cause mortality. The primary analyses excluded patients who died within a 2-year period; secondary analyses were conducted including all patients.
Missing Values
Missing laboratory values were imputed using chained regression equations with the mice package, R programming environment, using age, race, body mass index, diabetes, liver disease etiology, alcohol, liver decompensation diagnosis codes, and future laboratory values.32 Missing AUDIT-C values (15%) were analyzed as 0 (no alcohol abuse)33; in sensitivity analyses regression models for all QMs and outcomes were fit with missing AUDIT-C as a separate category. All other analyses were conducted using Stata 15.0 (StataCorp, College Station, TX).
Results
The baseline characteristics of the 121,129 patients who were diagnosed with cirrhosis from 2010 to 2016 and had at least one primary care appointment in that calendar year are shown in Table 2. The mean age was 62.1 years (standard deviation, 8.7). Greater than half of the patients were white, 15.4% were black, and 9.1% were Hispanic. About one third of patients were ≥50% service-connected, indicating a higher degree of VA benefits coverage. At baseline, 51.5% of the cohort had diabetes, and 67.6% had obesity. Greater than half of the cohort had a past reported history of substance abuse, and 43.6% had prior alcohol abuse at baseline. The most common cirrhosis etiologies were HCV and/or alcohol, together accounting for 72.3% of cases and 18.1% had NAFLD/NASH. The mean MELD score at cohort entry was 6 (interquartile range [IQR], 6-12); 64.3% were CTP-A, 29.8% were CTP-B, and 5.9% were CTP-C. The median follow-up time was 2.7 years (IQR, 1.1-5.1), the median time to death was 1.8 years (IQR, 0.6-3.6), and the median time to transplantation was 2.0 years (IQR, 0.9-3.7). The median time to decompensation was 1.3 years (IQR, 0.3-2.9). Nearly half of the patients died during follow-up, 12.0% developed HCC, and about half developed hepatic decompensation.
| Variable | n = 121,129 |
|---|---|
| Age, mean (SD) | 62.1 (8.7) |
| Male, n (%) | 118,318 (2.4) |
| Race, n (%) | |
| White | 68,286 (56.4) |
| Black | 16,689 (15.4) |
| Hispanic | 11,064 (9.1) |
| Asian/Pacific Islander/Alaskan | 2,961 (2.4) |
| Unknown/other | 20,170 (16.7) |
| Service connected, n (%) | |
| 50%-100% | 35,944 (29.7) |
| 0%-50% | 30,339 (25.0) |
| Other | 54,887 (45.3) |
| Comorbidities | |
| Diabetes, n (%) | 62,401 (51.5) |
| Obesity (body mass index >30), n (%) | 81,880 (67.6) |
| Substance abuse history,* n (%) | 76,031 (62.8) |
| Alcohol misuse (AUDIT-C),† n (%) | 52,792 (43.6) |
| Cirrhosis comorbidity index* n (%) | |
| 0 | 73,165 (60.4) |
| 1 + 0 | 25,363 (20.9) |
| 1 + 1 | 8,203 (88.1) |
| 3 + 0 | 4,122 (3.4) |
| 3 + 1 | 9,953 (8.2) |
| 5 + 0 | 17 (0.01) |
| 5 + 1 | 347 (0.29) |
| Liver disease etiology, n (%) | |
| HCV | 18,454 (15.2) |
| HCV and alcohol | 29,858 (24.6) |
| Alcohol | 39,428 (32.5) |
| NAFLD | 21,873 (18.1) |
| Hepatitis B virus | 2,931 (2.4) |
| Cryptogenic | 4,918 (4.1) |
| Primary biliary cholangitis | 1,372 (1.1) |
| Primary sclerosing cholangitis | 649 (0.5) |
| Hemochromatosis | 1,332 (1.1) |
| Autoimmune | 355 (0.3) |
| MELD score, median (IQR) | 6 (6-12) |
| CTP score, n (%) | |
| A (5-6) | 77,966 (64.3) |
| B (7-9) | 36,069 (29.8) |
| C (10-15) | 7,135 (5.9) |
| Follow-up time (years), median (IQR) | 2.7 (1.1-5.1) |
| Died during follow-up, n (%) | 58,006 (47.9) |
| Time to death (years), median (IQR) | 1.8 (0.6-3.6) |
| Transplanted during follow-up, n (%) | 2,975 (2.5) |
| Time to transplantation (years), median (IQR) | 2.0 (0.9-3.7) |
| Developed hepatic decompensation,‡ n (%) | 12,992 (16.7) |
| Time to hepatic decompensation (years), median (IQR) | 1.3 (0.3-2.9) |
- * Comorbidity index includes acute myocardial infarction, peripheral arterial disease, epilepsy, substance abuse other than alcoholism, heart failure, cancer, and chronic kidney disease.38
- † Threshold score for identifying alcohol use disorder: ≥4 for men and ≥3 for women.
- ‡ Hepatic decompensation was defined as ascites, spontaneous bacterial peritonitis, or variceal hemorrhage. Hepatic decompensation calculated among patients with CPT-A cirrhosis (n = 77,966). Missing laboratory values were imputed using chained equations, mice package in R. Missing AUDIT-C values were coded as 0 (no alcohol abuse).
Facility characteristics of the study cohort (based on the first facility where patients received the incident cirrhosis diagnosis code) are described in Table 3. A total of 84.3% of patients received care at urban facilities, and 57.7% received care at academically affiliated facilities. Only 17.6% of veterans with cirrhosis received care at a VA facility within a 200-mile radius of a VA transplant center, 75.7% of patients were at high-volume facilities.
| Variable, n (%) | |
|---|---|
| Region | |
| Northeast | 17,036 (14.1) |
| Southeast | 29,072 (24.0) |
| Midsouth | 27,805 (23.0) |
| Central | 18,867 (15.6) |
| West | 28,349 (23.4) |
| Rurality | |
| Metropolitan | 102,121 (84.3) |
| Nonmetropolitan, 20,000 or more | 5,943 (4.9) |
| Nonmetropolitan up to 19,999 | 13,065 (10.8) |
| Academic VA facility | 69,930 (57.7) |
| Facility <200 miles from a VA transplant center | 21,352 (17.6) |
| High-volume facility | 90,434 (75.7) |
- Characteristics reported for the VA facility with the incident cirrhosis diagnosis. High-volume facility defined as having greater than the median number of annual outpatient specialty care cirrhosis visits (median number of cirrhosis visits = 616; 1,809 patients at seven facilities had no specialty care visits).
Table 4 shows the percent adherence to QM1-QM4 as well as 30-day and 90-day readmission rates. Among the 121,129 patients newly diagnosed with cirrhosis, 70.5% were seen by a specialist within 12 months of the cirrhosis diagnosis (QM1). For QM2, among 50,434 eligible patients, the median PTUDS for HCC was 37.2%, with 42.0% of the cohort having PTUDS ≥50% in the 2 years after cirrhosis diagnosis. Among the 50,079 patients with platelet counts <150,000 who were not taking a prophylactic beta blocker (QM3), 31.9% underwent upper endoscopy for variceal surveillance. Among the 46,821 eligible cirrhosis-related hospitalizations, 53.6% had early outpatient follow-up visits with primary care or gastroenterology/hepatology within 30 days of hospital discharge (QM4). The percentages of cirrhosis-related hospitalizations with 30-day and 90-day readmissions (QM6) were 34.0% and 53.4%, respectively.
| Cirrhosis QM | Unit of Analysis (n) | QM Adherence |
|---|---|---|
| QM1. Seen by a specialist within 12 months of cirrhosis diagnosis,* percent adherent | Patient (n = 121,129) | 70.5% |
| QM2. HCC surveillance—PTUDS ≥50%* | Patient (n = 50,434) | 42.0% |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on prophylactic beta blocker, percent adherent | Patient (n = 50,079) | 31.9% |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related VA hospitalization,†, ‡ percent adherent | Discharge (n = 46,821) | 53.6% |
| QM5. Percent hospitalizations for upper GI bleed should be receiving antibiotics for at least 3 days§ | Discharge (n = 15,105) | — |
| QM6a. Percent of 30-day of cirrhosis-related VA hospital readmissions with 1 year of index admission,†, ‡ | Discharge (n = 46,821) | 34.0% |
| QM6b. Percent of 90-day of cirrhosis-related VA hospital readmissions within 1 year of index admission,†, ‡ | Discharge (n = 46,821) | 53.4% |
- * Measure includes patients with at least one primary care appointment in a calendar year.
- † Measure excludes patients with elective admissions, in-hospital death, nonhome discharge, and hospice discharge.
- ‡ Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse.
- § Measure not found not be valid after medical record review.
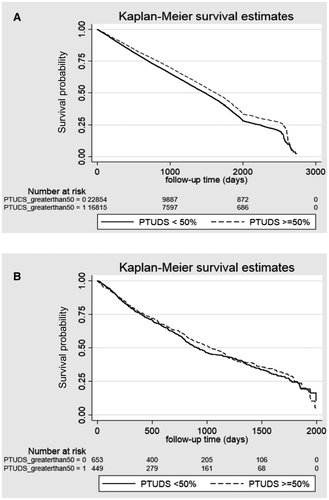
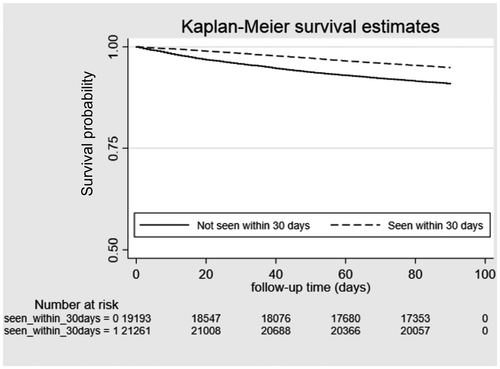
Unadjusted Kaplan-Meier curves for QMs 2 and 4 are shown in Figs. 1 and 2; unadjusted and adjusted results of multivariable models for with all-cause mortality are presented in Table 5. In analyses adjusted for patient demographics, liver disease etiology and severity, medical comorbidity, and facility-level characteristics, higher adherence to QM1 (gastroenterology/hepatology appointment within 12 months of the cirrhosis diagnosis [hazard ratio {HR}, 0.80; 95% confidence interval {CI}, 0.78-0.82]) and QM3 (variceal surveillance within 3 months of the cirrhosis diagnosis in the presence of portal hypertension [HR, 0.93; 95% CI, 0.89-0.99]) was associated with lower 2-year mortality. After excluding patients who died within 30 days of the cirrhosis diagnosis, the adjusted associations remained significant (QM1: HR, 0.90; 95% CI, 0.87-0.93; QM3: HR, 0.85; 95% CI, 0.82-0.90). With regard to QM2 (HCC surveillance with liver ultrasound or contrast-enhanced CT/MRI), PTUDS ≥50% with any of these imaging studies was associated with lower mortality (HR, 0.92; 95% CI, 0.90-0.95). Figure 1 shows the unadjusted Kaplan-Meier curves for the association between PTUDS and all-cause mortality stratified by CTP classes A and B (Fig. 1A) versus CTP-C cirrhosis (Fig. 1B). The unadjusted association between PTUDS and lower mortality was limited to CTP classes A and B and was not significant for CTP class C. Receipt of a specialty or primary care appointment within 30 days of hospital discharge (QM4) was associated with lower mortality (HR, 0.57; 95% CI, 0.54-0.60), whereas readmissions (QM6) within 30 days (HR, 1.53; 95% CI, 1.46-1.60) and 90 days (HR, 1.62; 95% CI, 1.54-1.70) were associated with higher mortality.
| Practice QM | Outcome | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|
| QM1. Seen by a specialist within 12 months of cirrhosis diagnosis* | 2-year mortality | 0.63 (0.61-0.64) | 0.80 (0.78-0.82) |
| QM2. HCC surveillance—PTUDS ≥50% versus <50%† | Overall mortality|| | 0.89 (0.87-0.92) | 0.92 (0.90-0.95) |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on prophylactic beta blocker, percent adherent* | 2-year mortality | 0.82 (0.78-0.86) | 0.93 (0.89-0.99) |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related hospitalization‡, § | 90-day mortality | 0.67 (0.65-0.70) | 0.57 (0.54-0.60) |
| QM6a. Readmission within 30 days of cirrhosis-related hospitalization‡, § | 2-year mortality | 1.70 (1.62-1.78) | 1.53 (1.46-1.60) |
| QM6b. Readmission within 90 days of cirrhosis-related hospitalization‡, § | 2-year mortality | 1.88 (1.80-1.97) | 1.62 (1.54-1.70) |
- All models were adjusted for patient factors: age, sex, race, service connectedness, patient distance to VA transplant center, diabetes, substance abuse, alcohol use, etiology of liver disease, cirrhosis comorbidity index, MELD score, CTP score, and VA facility factors (academic facility, volume of outpatient cirrhosis visits, VA region, rurality).
- * Measure includes only routine users of VA healthcare defined as at least one outpatient primary care appointment in the calendar year that the measure was assessed.
- † A 30-day grace period was given for surveillance, studies considered adherent if 7 months apart.
- ‡ Measure excludes patients with elective admissions, in-hospital death, non-home discharge, and hospice discharge.
- § Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse).
- || Mortality was measured until end of follow-up.
Results of the multivariable models for associations between inpatient health care use and the number of cirrhosis-related hospitalizations within 2 years are shown in Table 6. Gastroenterology/hepatology appointment within 12 months of the cirrhosis diagnosis (QM1) and variceal surveillance (QM3) was associated with higher 2-year inpatient use (QM1: incidence rate ratio [IRR], 1.24; 95% CI, 1.18-1.32; QM2: IRR, 1.44; 95% CI, 1.33-1.56). HCC surveillance (QM2) was associated with lower inpatient use (IRR, 0.85; 95% CI, 0.80-0.90). Receipt of a specialty or primary care appointment within 30 days of hospital discharge (QM4) was not associated with the number of subsequent cirrhosis-related readmissions within a 2-year period (IRR, 0.97; 95% CI, 0.97-1.02) or the odds of readmission (30-day readmission: odds ratio [OR], 1.04; 95% CI, 0.98-1.12, P = 0.20; 90-day readmission: OR, 0.98; 95% CI, 0.93-1.04, P = 0.55). Results were similar when including patients who died within 2 years of cohort entry.
| Practice QM | Cirrhosis-Related Admissions Unadjusted IRR (95% CI) | Cirrhosis-Related Admissions Adjusted IRR (95% CI) |
|---|---|---|
| QM1. Gastroenterology/hepatology appointment within 12 months of cirrhosis diagnosis* | 1.16 (1.11-1.22) | 1.24 (1.18-1.32) |
| QM2. HCC surveillance—PTUDS ≥50% versus <50%* | 0.81 (0.77-0.86) | 0.85 (0.80-0.90) |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on prophylactic beta blocker | 1.36 (1.25-1.49) | 1.44 (1.33-1.56) |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related hospitalization†, ‡ | 0.96 (0.92-1.00)§ | 0.97 (0.94-1.02)|| |
- All models were adjusted for patient factors: age, sex, race, service connectedness, patient distance to VA transplant center, diabetes, substance abuse, alcohol use, etiology of liver disease, cirrhosis comorbidity index, MELD score, Child Turcotte Pugh Score, VA facility factors (academic facility, volume of outpatient cirrhosis visits, VA region, rurality).
- * Measure includes patients with at least one primary care appointment in a calendar year.
- † Measure excludes patients with elective admissions, in-hospital death, nonhome discharge, and hospice discharge.
- ‡ Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse.
- § P = 0.066.
- || P = 0.226.
Sensitivity Analyses
In secondary analyses for mortality fitting multivariable mixed-effects models to account for cluster-specific random effects (e.g., VA facilities for all measures and individual patients for hospitalization-related measures), effect estimates were similar to our primary analyses (Supporting Table S6). Additional detailed descriptive data regarding adherence to cirrhosis QMs and clinical outcomes stratified by age, liver disease severity, liver disease etiology, and geographic region are presented in Supporting Tables S7-S10.
Discussion
In this study of the proposed cirrhosis QMs among a large national cohort of veterans with cirrhosis, we found that five the six proposed VA QMs generally had higher adherence than reported in non-VA settings. We also found that adherence to the QMs was generally associated with better patient outcomes. Specifically, after adjusting for multiple patient-level and facility-level characteristics, we found lower mortality among patients with access to liver disease specialists within 1 year of the cirrhosis diagnosis (20% lower 2-year mortality), with EGD surveillance for patients with portal hypertension (7% lower mortality 2-year mortality), and with early postdischarge care after a cirrhosis-related hospitalization with a primary or specialty care provider (43% lower 90-day mortality).
It is not surprising that timely access to specialty care was associated with lower mortality as multiple previous studies, including those by our team, have shown higher receipt of guideline-recommended care for hepatitis B virus, HCC, variceal surveillance, and management of ascites among patients who were seen by a gastroenterologist or hepatologist.8, 10, 11, 19, 34 Although our estimate of 71% access to specialty care within 1 year of the cirrhosis diagnosis is higher than previously reported, the relationship with mortality is nearly identical to a recent smaller study by Mellinger et al., which showed that access to subspecialty care for cirrhosis among veterans within the VA's Midwestern US network was associated with a 19% lower mortality.31 The reasons for a higher proportion of receiving specialty care may be due to our cohort having a new cirrhosis diagnosis prompting a specialty consult and the fact that we credited patients with a specialist visit even if this occurred within 1 year prior to the cirrhosis diagnosis. Furthermore, a recent single-center study reported that hepatology telemedicine consultation among 513 patients with liver disease was associated with improved survival.35 With regard to the QM of variceal surveillance for patients with portal hypertension (not taking a prophylactic beta blocker), our estimated adherence was 32%, which is within range of the previously reported data among small patient samples.10
Adherence to QM2, which was defined by HCC surveillance at 6-month intervals (as recommended by the American Association for the Study of Liver Diseases [AASLD]) with a 1-month grace period, was 37% when measured as the PTUDS, with 42% of patients having PTUDS ≥50%; on average, 60% of the patients had one abdominal imaging study per year. Our estimates are generally higher than those noted in a systematic review of nine US HCC surveillance observational studies, where the pooled adherence was 19%. However, adherence ranged from 11% to 78%. The variation might be explained by the heterogeneity of the studies, including that the definition of HCC surveillance varied from one-time to biannual imaging with or without AFP and the fact that we restricted our sample to individuals who survived at least 2 years after the diagnosis.36
In the analysis of HCC surveillance on overall mortality, we noted that PTUDS was associated with lower mortality. Our results need to be interpreted in context of the fact that the majority of patients with cirrhosis do not die of HCC but rather die of other cirrhosis-related or unrelated complications. Previous retrospective studies have generally shown a mortality benefit with greater HCC surveillance; however, the greatest benefits of surveillance were apparent among patients with well-compensated liver disease.37 Similar findings were noted in this study; in unadjusted analyses, lower mortality was apparent for patients with CTP-A/B cirrhosis but not with CTP-C (although the sample size was lower for CTP-C patients, increasing the potential for type II error). Despite these noted associations between higher HCC surveillance and lower mortality, the mechanism for these findings is not well understood. For example, a recent methodologically rigorous matched case-controlled VA study showed no association between HCC surveillance and HCC-related mortality.38 Despite the lack of association between surveillance and early cancer detection in other studies, effective HCC surveillance is likely a surrogate of health care quality, whether through health care engagement, improved access, or provider vigilance. It has a plausible role in identifying early-stage HCC and, as such, is a reasonable quality indicator.
We also confirmed that early postdischarge care after a cirrhosis-related admission was associated with lower mortality, whereas greater inpatient use was associated with higher mortality.9 The adherence to early postdischarge follow-up was only 52%. Given its strong association with lower mortality, this QM is a highly viable target for local as well as national quality improvement efforts in cirrhosis. We found that 30-day and 90-day readmissions were associated with higher mortality, representing a measure associated with higher risks of a poor outcome. Although we do not make specific recommendations regarding readmission-rate benchmarks, identifying patients with 30-day and 90-day readmissions may help guide hospital quality improvement efforts, whether by focusing on preventable readmissions or identifying patients in need of social services, transplantation referral, or palliative care. Future in-depth investigations of determining readmission targets and mitigating potentially preventable readmissions (e.g., routine paracentesis or hepatic encephalopathy) should be conducted.
The data in this study are an important early step toward establishing baseline rates of adherence to the proposed QMs and informing ongoing efforts to improve quality of care in cirrhosis. As an integrated system of care, the VA has proactively funded and spearheaded quality improvement efforts in advanced liver disease. For example, the HIV, Hepatitis, & Related Conditions (HHRC) Programs within the Office of Specialty Care Services have established Hepatic Innovation Teams within each Veteran Integrated Service Network to improve care in viral hepatitis, human immunodeficiency virus, and cirrhosis. In 2017, the HHRC created the Advanced Liver Disease Technical Advisory Group and, in addition to identifying cirrhosis QMs, developed a working prototype of a cirrhosis dashboard based on the VA electronic heath record, which facilitates population management within each VA facility and allows for assessment of adherence to cirrhosis quality measures.15 Investigators at Michael E. DeBakey VA Medical Center in Houston, Texas, recently developed and implemented a population-based cirrhosis identification and management system, which allowed for identification and linkage to care of 30% of patients with cirrhosis who were previously lost to follow-up.39 The AASLD Practice Metrics Committee has also focused on developing evidence-based cirrhosis quality, which includes important clinical and patient-reported outcomes.40 Future studies will need to investigate how these QMs and electronic heath record–based tools can be leveraged in routine clinical care as well as the barriers and facilitators to their adoption.
We must acknowledge several study limitations. This was a retrospective cohort study with the potential for misclassification bias as cirrhosis was captured using diagnosis codes, which may have underestimated its true prevalence. While our multivariable models robustly adjust for the measurable patient and facility-level characteristics, the remaining differences in mortality could nonetheless be explained by other unmeasured patient and health-system factors. We did not specifically assess VA facility variation in care, although we adjusted for hospitals and hospital characteristics; this requires further in-depth investigation. Future studies using mixed methodology (including patient and provider interviews) should focus on reasons for low and high adherence that cannot be obtained using administrative data. In addition, studies in the veteran population may not be readily generalizable due to the predominantly male population, the single integrated system of care, and the fact that recent internal VA initiatives have been targeting population-based cirrhosis management and quality improvement. Our figures likely underestimate readmissions as these could have occurred at non-VA hospitals. Finally, this study excluded transplant recipients and did not evaluate referral for transplantation as a quality measure.
While further work is needed to test these quality measures in VA and non-VA populations, this study sheds important light on the quality of care provided to veterans with cirrhosis, highlights areas for improvement within the VA, and, perhaps most importantly, provides some evidence that these quality measures capture important information about the quality of care among patients with cirrhosis. Future studies should investigate how earlier referral for either transplantation, active HCC therapy, or palliative care when appropriate should be instituted. Additionally, prospective implementation studies of how quality improvement can best be integrated into clinical practice are needed. The VA, as an integrated national system of health care and an early adopter of telemedicine technology to improve access to care, is well poised to lead these efforts.




