IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy
Abstract
Interleukin-6 (IL-6) is critically involved in liver regeneration after partial hepatectomy (PHX). Previous reports suggest that IL-6 trans-signaling through the soluble IL-6/IL-6R complex is involved in this process. However, the long-term contribution of IL-6 trans-signaling for liver regeneration after PHX is unknown. PHX-induced generation of the soluble IL-6R by ADAM (a disintegrin and metallo) proteases enables IL-6 trans-signaling, in which IL-6 forms an agonistic complex with the soluble IL-6 receptor (sIL-6R) to activate all cells expressing the signal-transducing receptor chain glycoprotein 130 (gp130). In contrast, without activation of ADAM proteases, IL-6 in complex with membrane-bound IL-6R and gp130 activates classic signaling. Here, we describe the generation of IL-6 trans-signaling mice, which exhibit boosted IL-6 trans-signaling and abrogated classic signaling by genetic conversion of all membrane-bound IL-6R into sIL-6R proteins phenocopying hyperactivation of ADAM-mediated shedding of IL-6R as single substrate. Importantly, although IL-6R deficient mice were strongly affected by PHX, survival and regeneration of IL-6 trans-signaling mice was indistinguishable from control mice, demonstrating that IL-6 trans-signaling fully compensates for disabled classic signaling in liver regeneration after PHX. Moreover, we monitored the long-term consequences of global IL-6 signaling inhibition versus IL-6 trans-signaling selective blockade after PHX by IL-6 monoclonal antibodies and soluble glycoprotein 130 as fragment crystallizable fusion, respectively. Both global IL-6 blockade and selective inhibition of IL-6 trans-signaling results in a strong decrease of overall survival after PHX, accompanied by decreased signal transducer and activator of transcription 3 phosphorylation and proliferation of hepatocytes. Mechanistically, IL-6 trans-signaling induces hepatocyte growth factor production by hepatic stellate cells. Conclusion: IL-6 trans-signaling, but not classic signaling, controls liver regeneration following PHX.
Abbreviations
-
- 3′UTR
-
- 3′untranslated region
-
- ADAM
-
- a disintegrin and metallo
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- Fc
-
- fragment crystallizable
-
- FRT
-
- flippase recognition target
-
- GFP
-
- green fluorescent protein
-
- gp130
-
- glycoprotein 130
-
- HGF
-
- hepatocyte growth factor
-
- HSCs
-
- hepatic stellate cells
-
- IL-6
-
- interleukin 6
-
- IL-6R
-
- interleukin 6 receptor
-
- mAb
-
- monoclonal antibody
-
- ns
-
- not significant
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PHX
-
- partial hepatectomy
-
- sgp130
-
- soluble gp130
-
- sIL-6R
-
- soluble IL-6 receptor
-
- STAT
-
- signal transducer and activator of transcription
-
- TNF
-
- tumor necrosis factor
-
- wt
-
- wild type
Synergistic action of hepatocyte growth factor (HGF) and interleukin 6 (IL-6) controls the early regenerative phase after partial hepatectomy (PHX) by promoting both mitosis and survival of hepatocytes.1-5 IL-6 binds to the IL-6 receptor (IL-6R), followed by dimerization of glycoprotein 130 (gp130), leading to JAK/STAT, MAPK, and PI3K/AKT activation.6 IL-6 or IL-6R alone have no affinity toward gp130. IL-6R expression is found on few cell types, including immune cells and hepatocytes, which are directly activated by IL-6 classic signaling.7 Primarily ectodomain shedding by a disintegrin and metallo (ADAM) proteases generates the soluble IL-6R (sIL-6R).8 IL-6/sIL-6R complexes activate cells expressing only gp130, or superactivates cells expressing more gp130 than membrane-bound IL-6R.9, 10 Whereas global IL-6 signaling (classic and trans-signaling) is inhibited by neutralizing IL-6 or IL-6R antibodies, soluble gp130 (sgp130) selectively inhibits IL-6 trans-signaling.11, 12 Healthy individuals display serum levels of 250-400 ng/mL endogenous sgp130.13 Recently, we have shown that endogenous serum levels of sgp130 are not sufficient to inhibit trans-signaling but might contribute to increase the serum half-life of IL-6.7
IL-6 trans-signaling has primarily pro-inflammatory functions and inhibition of IL-6 trans-signaling by application of sgp130 as fragment crystallizable (Fc) fusion protein (sgp130Fc) resulted in suppression of chronic inflammatory diseases in preclinical settings. Recently, sgp130Fc has entered phase 2 clinical trial.13
Although hepatocytes express relatively high levels of IL-6R, a modest increase in sIL-6R levels after PHX would enable local trans-signaling in the liver.14 Therefore, heterologous application of the trans-signaling superagonist hyper-IL-6, which is a fusion protein of IL-6 and sIL-6R,15 illuminates the maximal potential of trans-signaling in vitro and in vivo. Forced IL-6 trans-signaling by application of recombinant hyper-IL-6, but not classic signaling by IL-6, resulted in acceleration of liver regeneration after PHX.16 Ectopic expression of IL-6 and sIL-6R in double transgenic mice, but not of IL-6 alone, leads to nodular hepatocellular hyperplasia.17-19 Interestingly, IL-6/sIL-6R, but not IL-6 alone, cooperates with HGF to enhance hepatocyte proliferation, and short-time transient expression of sgp130Fc inhibits hepatocyte proliferation at early time points following PHX.20 Even though these results suggest a role for IL-6 trans-signaling in liver regeneration, long-term experiments to determine the overall consequences of IL-6 trans-signaling during liver regeneration are lacking. Increased serum levels of sIL-6R are common events in pathophysiology (e.g., following PHX), suggesting a shift from classic toward trans-signaling.14 Among others, ADAM proteases execute the stress-induced ectodomain shedding of IL-6R.8 Analysis of ADAM activity using gene-deficient ADAM10/17 mice is hampered by the complexity of the ADAM10-induced and ADAM17-induced sheddome with more than 100 different protein substrates. Moreover, the phenotype of ADAM10 and ADAM17 deficient mice is dominated by shedding deficits of a few substrates, including epidermal growth factor receptor (EGFR) ligands, Notch, and tumor necrosis factor (TNF).21 Therefore, consequences of abrogated shedding of the IL-6R could not be analyzed in ADAM10/17 deficient mice.8
Here, we generated and characterized IL-6 trans-signaling mice, which were genetically engineered to execute IL-6 trans-signaling and were indistinguishable from wild-type mice in liver regeneration after PHX.
Materials and Methods
Floxed sIL-6Rfl/fl-NEO mice were generated by ingenious targeting laboratory (www.genetargeting.com). The conditional targeting vector bearing the rearranged IL-6R exons 9 and 10 was electroporated into C57BL/6N embryonic stem cells. Targeted embryonic stem cells were microinjected into Balb/c blastocysts, and the resulting chimeras with a high percentage of black coat color were crossed to C57BL/6N mice to generate IL-6Rwt/fl-NEO. DNA from tail clippings was isolated using the DirectPCR-Tail kit with proteinase K (Peqlab, Erlangen, Germany) following the manufacturer’s instructions for genomic PCR.
Results
IL-6 Trans-signaling SIL-6R+/+ Mice Have Drastically Increased SIL-6R Levels
Here we generated transgenic soluble IL-6R+/+ (sIL-6R+/+) mice, a strategy to mimic ADAM10/17 hyperactivation for the single target protein IL-6R. sIL-6R+/+ mice reflect a situation in which only trans-signaling is active, whereas classic signaling is abrogated. sIL-6R+/+ mice exhibit amplified endogenous IL-6 trans-signaling due to an increased level of sIL-6R, which is not caused by ectodomain shedding but by Cre-mediated deletion of the genetic information coding for the transmembrane and intracellular domain of the IL-6R (Fig. 1A). In detail, exon 9 of the IL-6R gene codes for the transmembrane domain and the first part of the intracellular domain, and the last exon 10 codes for the second part of the intracellular domain followed by the 3′untranslated region (3′UTR). In the targeting vector, the 5,420-base pair (bp)-long intron 9 was deleted, resulting in functional fusion of the 100-bp and 223-bp-long coding regions of exon 9 and exon 10, respectively. Intron 9 was skipped because it would have complicated the construction of the targeting vector. The original IL-6R stop codon located in exon 10 was deleted, and a sequence coding for the 2A-peptide sequence followed by a KDEL-marked GFP was inserted. The 2A peptide from the foot-and-mouth disease virus is a self-processing sequence to achieve expression of at least two separate proteins from a single open reading frame.22 The cleavage of the IL-6R and GFP is thought to happen in a co-translational process. The remaining 2A peptide fragment at the C-terminal end of IL-6R is then cleaved off by the protease furin. In addition, the endoplasmic reticulum retention signal KDEL23 was fused at the C-terminal end of the green fluorescent protein (GFP). The E9-E10-GFP cassette was followed by the flippase recognition target (FRT)-neomycin-resistance-FRT cassette and the original 3′UTR of the IL-6R 3′UTR and ultimately flanked by loxP-sites.
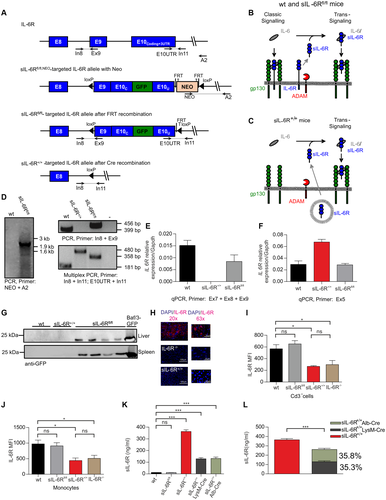
In the founder transgenic mice, the FRT-neomycin-resistance-FRT cassette was deleted by crossing to Flp-recombinase expressing mice, referred to as homozygous sIL-6Rfl/fl mice. We expected that the modified IL-6R protein in IL-6Rfl/fl mice would be produced with comparable efficiency compared with the wild-type IL-6R protein and result in IL-6 classic and trans-signaling comparable to wild-type mice (Fig. 1B). Cre-mediated recombination in the germline resulted in homozygous IL-6R+/+ mice carrying the deletion of the fused exons 9 and 10 on both alleles (Fig. 1A). As a consequence, the translated IL-6R in Cre-recombined sIL-6R+/+ mice lack the trans-membrane and intracellular domains and will be directly secreted as soluble IL-6R (Fig. 1C, Supporting Fig. S1A,B). Due to the lack of membrane-bound IL-6R, sIL-6R+/+ mice will selectively execute only trans-signaling. Introduction of the E9-E10-GFP cassette in sIL-6Rfl/fl mice and deletion of the E9-E10-GFP cassette in sIL-6R+/+ mice was confirmed by genomic PCR (Fig. 1D). Next, we quantified the IL-6R mRNA level in wild-type, sIL-6Rfl/fl, and sIL-6R+/+ mice. Whereas the IL-6R mRNA level was comparable in wild-type and sIL-6Rfl/fl mice, a significantly increased sIL-6R mRNA level was detected in sIL-6R+/+ mice, suggesting that genetic rearrangement in sIL-6Rfl/fl mice did not influence overall stability of the IL-6R mRNA but the deletion of most of the 3′UTR in sIL-6R+/+ mice enhanced mRNA stability (Fig. 1E,F). Unfortunately, we were not able to detect GFP fluorescence in hepatocytes and immune cells from sIL-6Rfl/fl mice, albeit western blotting of liver and spleen tissue clearly detected GFP proteins in sIL-6Rfl/fl mice, but not in wild-type and sIL-6R+/+ mice (Fig. 1G). Next, we performed immunohistochemistry of the IL-6R on liver samples from wild-type, IL-6R deficient, and sIL-6R+/+ mice. We were able to detect IL-6R in cells of wild-type mice but not in IL-6R deficient mice, demonstrating that the staining was specific. Interestingly, staining for sIL-6R in sIL-6R+/+ mice was also absent, indicating that all membrane-bound IL-6R was converted in sIL-6R in sIL-6R+/+ mice and rapidly secreted (Fig. 1H). Moreover, staining of the IL-6R in wild-type mice suggests that IL-6R molecules were found primarily in intracellular compartments and not on the cell surface (Fig. 1H). Flow cytometry analysis of IL-6R on CD3+ T cells and monocytes demonstrated that wild-type and sIL-6Rfl/fl mice had comparable levels of IL-6R on the cell surface, demonstrating that genetic rearrangement of IL-6R in sIL-6Rfl/fl mice did not interfere with expression (Fig. 1I,J, Supporting Fig. S2). Importantly, IL-6R-/- and sIL-6R+/+ mice lacked IL-6R cell surface expression, again supporting our conclusion that all membrane-bound IL-6R was converted into sIL-6R after Cre-recombination in sIL-6R+/+ mice (Fig. 1I,J, Supporting Fig. S2). Next, determination of the soluble IL-6R levels in the serum by enzyme-linked immunosorbent assay (ELISA) showed that wild-type and sIL-6Rfl/fl mice had almost identical sIL-6R serum levels of approximately 11 ng/mL. Interestingly, sIL-6R+/+ mice had about 33-fold increased sIL-6R levels of approximately 363 ng/mL (Fig. 1K). The increased sIL-6R level might at least to some degree also be caused by increased IL-6R mRNA levels in sIL-6R+/+ mice. A previous report demonstrated that serum sIL-6R was originating almost completely from hepatocytes and neutrophils/macrophages, because Alb-Cre-recombined IL-6R-/- mice had 67.95% and LysMCre-recombined IL-6R-/- mice displayed 39.95% of the sIL-6R levels of wild-type mice (Supporting Table S1).24 Here, we also crossed our sIL-6Rfl/fl mice with the highly effective Alb-Cre and LysM-Cre lines (Supporting Fig. S3A,B).25-28 Homozygous sIL-6R+/+ LysM-Cre and sIL-6R+/+Alb-Cre mice had about 129 ng/mL and 128 ng/mL sIL-6R in the serum, respectively (Fig. 1L). The sum of these sIL-6R serum levels was about 257 ng/mL, representing only about 70.8% of the sIL-6R levels detected in sIL-6R+/+ mice (Fig. 1L, Supporting Table S1). These data suggest that although the native sIL-6R in wild-type mice were derived primarily from hepatocytes and monocytes/neutrophils, other cells also expressed appreciable amounts of IL-6R, even though they did not contribute to sIL-6R level in wild-type mice.
SIL-6R in SIL-6R+/+ Mice Mediated IL-6 Signals Through Trans-signaling
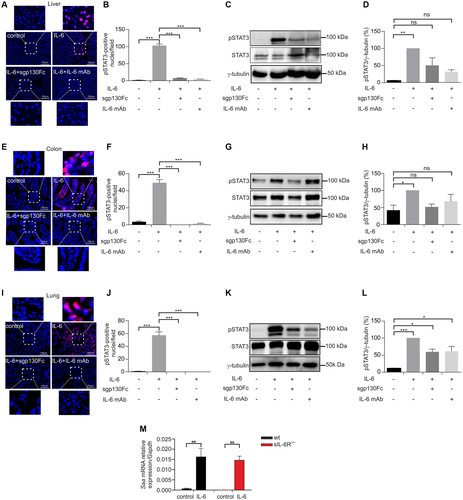
Injection of recombinant IL-6 in wild-type mice showed that the colon, and to a lesser extent also the liver and lung, are targeted by IL-6 trans-signaling.11 Our sIL-6R+/+ mice have a 33-fold higher sIL-6R serum level compared with wild-type mice (Fig. 1K), suggesting that sIL-6R+/+ mice efficiently and specifically execute trans-signaling. To test this, sIL-6R+/+ mice were injected with 5 µg recombinant IL-6, and phosphorylation of signal transducer and activator of transcription 3 (pSTAT3) was analyzed in the colon, liver, and lung. IL-6 induced sustained STAT3 phosphorylation in these organs as demonstrated by immunohistochemical staining and western blotting compared with control mice (Fig. 2A-L, Supporting Fig. S4A-C). Moreover, mice were co-injected with 50 µg sgp130Fc or 250 µg neutralizing IL-6 monoclonal antibody (mAb) to block IL-6 signaling. Co-injection of IL-6 with sgp130Fc or IL-6 mAb significantly blocked pSTAT3 in colon, liver, and lung (Fig. 2A-L, Supporting Fig. S4A-C). To analyze whether the acute phase response differs between wild-type and sIL-6R+/+ trans-signaling mice, we injected per mouse 5 µg IL-6 and analyzed the expression of the acute phase protein serum amyloid A2 (Saa2) by quantitative real-time PCR. As shown in Fig. 2M, induction of Saa2 expression in wild-type and sIL-6R+/+ trans-signaling mice was almost identical, suggesting that at least in the liver, IL-6 trans-signaling can completely compensate for the loss of IL-6 classic signaling. Our results demonstrate that the sIL-6R in sIL-6R+/+ mice is biologically active and transmits IL-6 signals through trans-signaling, thereby indicating that forced IL-6 trans-signaling can activate all cells of the organism. Thus, endogenous IL-6 trans-signaling is a very efficient cellular activation mode, which is specifically triggered during pathophysiological conditions.
SIL-6R+/+ Mice Fully Compensated Disabled IL-6 Classic Signaling by IL-6 Trans-signaling During Liver Regeneration After PHX
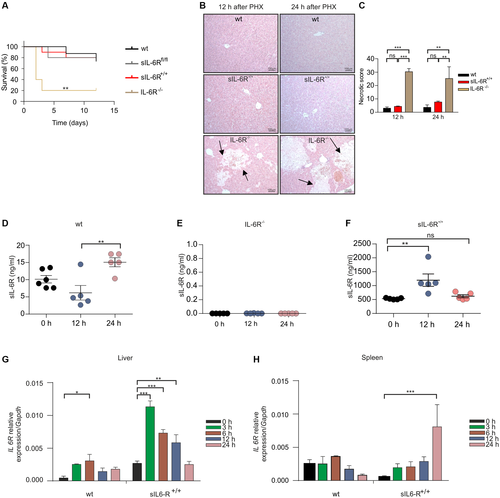
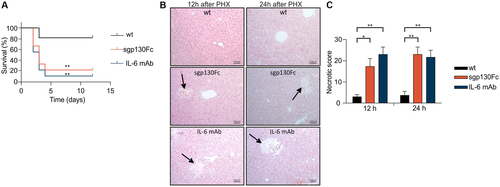
PHX was performed in wild-type, sIL-6Rfl/fl, sIL-6R+/+, and IL-6R-/- mice to analyze whether IL-6 trans-signaling is sufficient for normal liver regeneration. Overall survival rate of wild-type and sIL-6Rfl/fl mice 12 days after PHX was about 80%, whereas IL-6R deficient mice had an overall survival rate of only 20%. Survival of sIL-6R+/+ trans-signaling mice after PHX was identical to wild-type mice and sIL-6Rfl/fl mice (Fig. 3A). Moreover, large necrotic areas were only found in the liver of IL-6R-/- mice but not in wild-type, sIL-6Rfl/fl, and sIL-6R+/+ mice (Fig. 3B). Consequently, the necrotic score was increased in IL-6R-/- mice in comparison to wild-type, sIL-6Rfl/fl, and sIL-6R+/+ mice (Fig. 3C). There was, however, only a trend for increased alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels 12 and 24 hours following PHX in IL-6R-/- mice compared with wild-type, sIL-6Rfl/fl, and sIL-6R+/+ mice (Supporting Fig. S5A,B). Liver weight to body weight ratio 12, 24, and 168 hours after PHX was not different between wild-type and sIL-6R+/+ mice (Supporting Fig. S6A). Next, we analyzed sIL-6R levels following PHX in wild-type, IL-6R-/-, and sIL-6R+/+ mice. As reported previously14 and supported by our data, sIL-6R level in wild-type mice increased 1.5 fold from 11 ng/mL to 15 ng/mL 24 hours after PHX (Fig. 3D), whereas no sIL-6R was detectable in IL-6R-/- mice at any time after PHX (Fig. 3E). Importantly, sIL-6R+/+ mice also showed a 3.2-fold increase of sIL-6R from 372 ng/mL to 1,200 and 620 ng/mL 12 hours and 24 hours after PHX (Fig. 3F). As shown previously, the sIL-6R in sIL-6R+/+ mice was not generated by ADAM-mediated shedding, but by direct secretion of sIL-6R. Therefore, we analyzed mRNA levels of IL-6R following PHX in wild-type and sIL-6R+/+ mice in liver and spleen by quantitative real-time PCR. IL-6R mRNA levels were significantly increased in livers of wild-type mice and sIL-6R+/+ mice by a factor of 6.6 and 5.5, respectively (Fig. 3G), suggesting that transcriptional activation of IL-6R is the main driving force of sIL-6R generation in sIL-6R+/+ mice following PHX. Increased transcription likely contributes to increased sIL-6R level in wild-type mice following PHX; however, ectodomain shedding is the final trigger for sIL-6R generation. IL-6R mRNA levels were not increased in the spleen of wild-type mice and in sIL-6R+/+ mice only after 24 hours (Fig. 3H). Because in wild-type mice the membrane-bound IL-6R is converted into sIL-6R by ectodomain shedding, production of sIL-6R will be delayed in comparison to direct secretion of sIL-6R in sIL-6R+/+ mice, which might be the reason for the faster increase of the sIL-6R protein level in sIL-6R+/+ mice compared with wild-type mice (Fig. 3D,F).
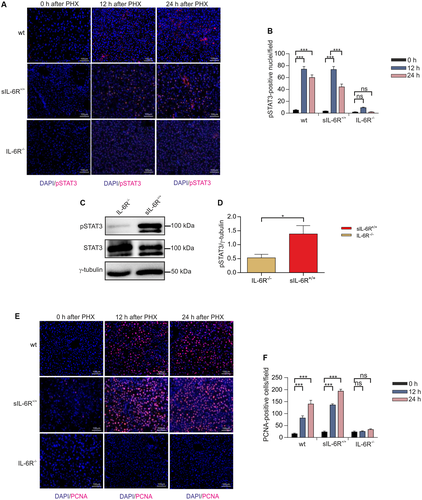
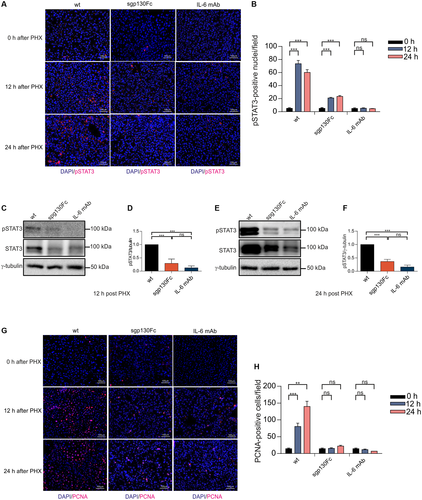
Next, we analyzed STAT3 phosphorylation in the liver of wild-type, IL-6R-/-, and sIL-6R+/+ 12 hours and 24 hours following PHX. STAT3 phosphorylation was comparable in wild-type and sIL-6R+/+ mice as determined by immunohistochemistry (Fig. 4A). Importantly, IL-6R-/- mice had significantly reduced pSTAT3 levels in the liver 12 hours and 24 hours following PHX (Fig. 4A-D, Supporting Fig. S7). Proliferating cell nuclear antigen (PCNA) is required for DNA synthesis during replication and hepatocyte proliferation.28 Immunohistochemical staining of PCNA in liver sections 24 hours after PHX revealed a reduction of about 76.4% of PCNA-positive cells in IL-6R-/- mice compared with wild-type mice, whereas PCNA levels of wild-type and IL-6R+/+ mice were comparable (Fig. 4E,F).
Taken together, our data demonstrate that IL-6 trans-signaling can fully compensate for the loss of classic signaling to ensure liver regeneration following PHX.
Selective Inhibition of IL-6 Trans-signaling Prevents Liver Regeneration Following PHX
Discrimination between classic and trans-signaling is also possible by comparing the functional outcome of classic and trans-signaling blockade by IL-6 and/or IL-6R antibodies with the specific blockade of trans-signaling by sgp130Fc. Therefore, we injected a high dose of monoclonal antibodies directed against IL-6 (250 µg/mouse) and a low dose of sgp130Fc (50 µg/mouse) into wild-type mice, to independently characterize the contribution of classic and trans-signaling to liver regeneration. A total of 50 µg/mouse (1.25 mg/kg) sgp130Fc was chosen because previous studies showed selective inhibition of trans-signaling for this dosage, whereas lower amounts had no effect.29, 30 Abrogation of IL-6 classic and trans-signaling in wild-type mice treated with neutralizing IL-6 mAb (250 µg/mouse) every 2 days following PHX resulted in an overall survival of 10% after 12 days (Fig. 5A), which is in good agreement with the survival of IL-6R-/- mice after PHX (Fig. 3A). Importantly, selective inhibition of IL-6 trans-signaling by repetitive intraperitoneal injection of a low dose of 50 µg/mouse sgp130Fc (1.25 mg/kg) resulted in a comparably low survival rate as observed for mice treated with 250 µg/mouse IL-6 mAb (6.25 mg/kg) after PHX (Fig. 5A). In contrast, an even lower dose of 10 µg/mouse sgp130Fc (0.25 mg/kg) had no effect on survival (Supporting Fig. S8). Histological examination of the overall liver damage 12 hours and 24 hours after PHX showed larger necrotic areas in mice treated with IL-6 mAb and sgp130Fc (1.25 mg/kg) compared with control mice (Fig. 5B). Consequently, the necrotic score was increased in IL-6 mAb and sgp130Fc treated mice in comparison to control mice (Fig. 5C). There was, however, just a trend for increased ALT/AST level 12 hours and 24 hours after PHX in IL-6 mAb treated mice compared with wild-type and sgp130Fc treated mice (Supporting Fig. S5A,B). Liver weight to body weight ratio 12 hours and 24 hours after PHX was not different between experimental groups (Supporting Fig. S6B). Due to the low survival rate of IL-6 mAb-treated and sgp130Fc-treated mice liver weight to body weight ratio at a later time point was not determined. Next, we analyzed STAT3 phosphorylation in the liver following PHX in mice treated with IL-6 mAb, sgp130Fc, or left untreated. STAT3 phosphorylation in the liver was decreased after global blockade of IL-6 signaling for 12 hours and 24 hours compared with control mice, as determined by immunohistochemistry (Fig. 6A,B) and western blotting (Fig. 6C-F, Supporting Fig. S9A,B). Importantly, selective inhibition of IL-6 trans-signaling also prevented phosphorylation of STAT3 in the liver 12 hours and 24 hours after PHX (Fig. 6A-F, Supporting Fig. S9A,B). Immunohistochemical staining of PCNA in liver sections 24 hours following PHX demonstrated a 94.1% reduction of PCNA-positive cells after global blockade of IL-6 signaling and a 85.4% reduction after selective inhibition of IL-6 trans-signaling compared with control mice (Fig. 6G,H). Taken together, our data showed that IL-6 trans-signaling is the main pathway controlling liver regeneration following PHX.
IL-6 Trans-signaling Induces HGF Expression in Mice Following PHX and in Hepatic Stellate Cells
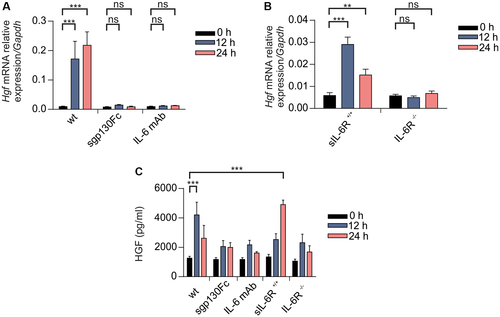
HGF is important for induction of hepatocyte proliferation26 and was up-regulated following PHX in wild-type mice in the liver at the mRNA level (Fig. 7A). Importantly, inhibition of classic and trans-signaling by IL-6 mAb or trans-signaling by sgp130Fc prevented up-regulation of Hgf on mRNA level in wild-type mice following PHX (Fig. 7A). Up-regulation of Hgf was, however, abrogated in IL-6R-/- mice (Fig. 7A,B), but not in sIL-6R+/+ mice (Fig. 7A,B). Next, we determined HGF level in the liver lysate of mice following PHX. Whereas wild-type and sIL-6R+/+ mice had significantly increased HGF-level 12 hours and 24 hours following PHX, the IL-6 mAb-treated, sgp130Fc-treated, and IL-6R-/- mice failed to show up-regulation of HGF (Fig. 7C). These data indicate that HGF up-regulation following PHX is dependent on IL-6 trans-signaling.
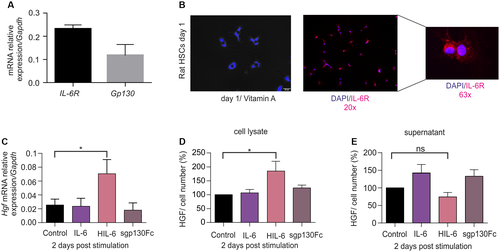
Hepatic stellate cells (HSCs) are a known source of HGF following PHX.31 To answer the question of whether IL-6 classic and/or trans-signaling trigger expression of HGF, rat HSCs were analyzed. Interestingly, HSCs express IL-6R and Gp130 on the mRNA level as determined by quantitative real-time PCR (Fig. 8A). Expression of IL-6R protein was verified by immunohistochemical staining (Fig. 8B), and again IL-6R was found primarily in intracellular compartments and only sparingly on the cell membrane. Despite the expression of IL-6R, only the stimulation of HSCs with hyper-IL-6 but not with IL-6 or sgp130Fc, resulted in increased of Hgf mRNA level (Fig. 8C). Even though increased HGF protein level found only in lysates of hyper-IL-6 stimulated HSCs (Fig. 8D), release of HGF was not stimulated by IL-6 trans-signaling in this in vitro setting (Fig. 8E). Our data show that IL-6 trans-signaling induced HGF production in HSCs, but suggested that in addition to IL-6 trans-signaling, additional factors might be needed to induce release of HGF from HSCs.
Discussion
We present three major findings in this study that define the role of IL-6 signaling in the process of liver regeneration. First, our data suggest that IL-6 trans-signaling is the main mechanism of IL-6 action in liver regeneration following PHX in mice. Second, the trans-signaling sIL-6R+/+ mice represent a genetic strategy to phenocopy substrate-selective hyperactivation of ectodomain shedding that is executed primarily by ADAM proteases.8 Third, using our trans-signaling sIL-6R+/+ mice, we independently demonstrate that IL-6 trans-signaling controls liver regeneration, and show that classic signaling is absolutely dispensable for this process in this setting. Exemplified on the basis of liver regeneration, the trans-signaling sIL-6R+/+ mouse model is a valuable tool to study IL-6 trans-signaling under physiological and nonphysiological conditions, including acute and chronic inflammation and cancer development. This strategy might also serve as a blueprint to study increased release of other ADAM substrates. Of note, it is not possible to study most substrates in ADAM overexpressing mice, because the phenotypes in these mice would be dominated by only a subset of dominating substrates, including EGFR ligands, NOTCH, and TNF. Our strategy also complements existing models, including mice with noncleavable ADAM-substrates, as shown for L-selectin, which is an elegant way to analyze diminished ADAM activity for a selected substrate.32
The genetic conversion of a transmembrane protein into a soluble protein to phenocopy single-substrate ADAM activation has to be carefully designed, because deletion of exons/introns might also interfere with the generation and stability of the respective mRNA. Importantly, genetic fusion of exon 9 and 10 and introduction of a 2A-GFP cassette did not influence endogenous IL-6R mRNA and protein production. However, partial deletion of the 3′UTR of the IL-6R by Cre-mediated recombination resulted in increased sIL-6R-level, suggesting that negative regulatory elements are located in the IL-6R 3′UTR. Moreover, in the case of IL-6R, the genetically engineered soluble IL-6R was terminated by a stop codon in the former intron 8, which becomes part of the sIL-6R mRNA after Cre-mediated recombination. As a consequence, the sIL-6R in IL-6R+/+ mice contained the three additional C-terminal amino acids (Gly, Lys, and Arg) after the last original amino acid Leu352, coded by the last codon of exon 8 and located close to the C-terminal end of the stalk region of the translated IL-6R. Based on structural evidence, it is highly unlikely that these three additional C-terminal amino acids will influence the function of the soluble IL-6R, as the IL-6 binding domains of the IL-6R are not altered.33 Moreover, the naturally occurring and biologically active human sIL-6R, which is produced by differential splicing, contains 10 additional C-terminal amino acids (GSRRRGSCGL) that are not present in the membrane-bound IL-6R.34 These additional amino acids showed no effect on the binding affinity of IL-6 to the alternatively spliced sIL-6R-protein.35 In mice, the situation is much less complicated, because the murine IL-6R is not differentially spliced and the sIL-6R is only produced by ectodomain shedding.36 Importantly, our experiments showed that the sIL-6R in trans-signaling sIL-6R+/+ mice was biologically active, because injection of IL-6 efficiently induced trans-signaling in sIL-6R+/+ mice.
In wild-type mice, sIL-6R serum levels are in the range of 6-15 ng and may rise 2-fold to 3-fold under stress conditions.20, 37, 38 Previously, it was calculated that all circulating sIL-6R in wild-type mice is derived from neutrophils/macrophages and hepatocytes, suggesting that these cells are also the main producer of membrane-bound IL-6R.24 However, here we show by using LysM- and Alb-Cre-recombined sIL-6R+/+ mice that only about 70.8% of all IL-6R is produced by neutrophils/macrophages and hepatocytes, indicating that the remaining 29.2% must be expressed by other cell types (Fig. 1J). Even though neutrophils/macrophages and hepatocytes generate more than 90% of the naturally formed sIL-6R in the serum, our data indicate that they express proportionally less membrane-bound IL-6R. It might just be that neutrophils/macrophages and hepatocytes release proportionally higher amounts of sIL-6R compared with other cells that also express IL-6R. Neutrophils are exceptionally efficient sIL-6R releasers. They have a very short life span before they rapidly undergo apoptosis. Apoptosis, however, is an efficient trigger of ADAM17-mediated ectodomain shedding of the IL-6R.36
Many studies highlight the critical role of IL-6 in liver regeneration after PHX, with the consistent finding that IL-6 deficient mice show impaired liver regeneration based on reduced proliferation of hepatocytes accompanied by a high mortality rate of 40%-80% versus 10% in wild-type mice.1, 2, 16, 20, 39 After PHX, gut-derived factors including lipopolysaccharide activate liver-resident Kupffer cells to secrete IL-6 in a TNFα-dependent manner.4 The parallel increase of sIL-6R after PHX20 opens a functional window for IL-6 trans-signaling. Because hepatocytes express much more gp130 than IL-6R, the increased presence of IL-6 and sIL-6R will result in more gp130 activation and a stronger IL-6 signal. This is accompanied by the longer duration of the IL-6 signal when mediated through trans-signaling, because of the slower internalization of IL-6/sIL-6R complexes compared with IL-6/membrane-bound IL-6R complexes.40 Our previous study using hydrodynamic injection of a sgp130Fc expression plasmid was designed to study the first 48 hours of liver regeneration after PHX.20 Although it lacks a proper anti-IL-6 control, it already suggested a positive role for trans-signaling.20 The data presented here suggest that blocking of IL-6 trans-signaling is as detrimental for liver regeneration after PHX as blocking of global IL-6 signaling. Nevertheless, all in vivo data with the trans-signaling inhibitor sgp130Fc have to be interpreted with caution, because we previously showed in vitro that high concentrations of sgp130Fc also cross-inhibit classic signaling at molar ratios for sIL-6R/IL-6 larger than 1, which are typically found in the serum of healthy and moderately inflamed mice.41 Importantly, recent studies defined the minimal effective dose of sgp130Fc in vivo, clearly differentiating between classic and trans-signaling effects in a sepsis and a bacterial infection model.29, 30 Here we used 1.25 mg sgp130Fc/kg body weight, which is at the lower border of this minimal effective dose, to ensure inhibition of trans-signaling, but largely to exclude cross-inhibition of classic signaling.
Previously, failure of liver regeneration after PHX was shown in IL-6 antibody-treated42 and IL-6 deficient mice.1 Consistently IL-6R deficient mice were compromised in liver regeneration following PHX in our setting.1 This is noteworthy, because in a murine skin wound-healing model, only IL-6 but not IL-6R deficient mice showed delayed healing,24 which might be explained by the ability of the IL-6R to bind also to at least two other cytokines of the IL-6 family, CNTF and p28.43-45 Therefore, our working hypothesis was that after PHX, IL-6R deficient mice might also behave like wild-type mice and not like IL-6 deficient mice, which also warrants the requirement to include treatment with IL-6R mAbs as additional control in PHX. Importantly, IL-6R deficient mice showed the same phenotype after PHX as IL-6 deficient mice, exhibiting reduced STAT3 phosphorylation and hepatocyte proliferation as determined by reduced expression of PCNA.
HGF is produced by HSCs and plays an important role in the induction of hepatocyte proliferation following PHX.27 Although HSCs express Gp130 and IL-6R, production of HGF was stimulated only through IL-6 trans-signaling. The reason for this phenomenon is unknown, but immunohistochemical staining of IL-6R in HSCs revealed that most IL-6R proteins are present in intracellular compartments, which might prevent efficient induction of classic signaling.
In summary, our data show that IL-6 trans-signaling is the main driver of liver regeneration following PHX. Although hepatocytes express membrane-bound IL-6R and are therefore also a target for classic signaling, the signal induced through the membrane-bound IL-6R on hepatocytes alone is not sufficient to induce a proliferative response. In wild-type mice, the combined injection of IL-6 plus sIL-6R, but not of IL-6 alone, accelerates liver regeneration after PHX.16 Our study now provides functional proof that IL-6 trans-signaling has not only the potential to accelerate liver regeneration, but it is critically involved in normal liver regeneration, which might have implications for the use of anti-IL-6/IL-6R therapeutics in the clinic and for the forthcoming clinical evaluation of sgp130Fc.
Acknowledgments
We gratefully acknowledge the technical support from Sebastian Fey. We appreciate the animal facility of the Heinrich-Heine University of Düsseldorf and the Zett operation team for their great support. We thank Erika Engelowski, Dr. Chris Heifeng Xu, Dr. Alexander Lang, and Dr. Saeideh Nakhaei-Rad for their great scientific support for this study.




