Human Immunodeficiency Virus/Hepatitis C Virus (HCV) Co-infected Patients With Cirrhosis Are No Longer at Higher Risk for Hepatocellular Carcinoma or End-Stage Liver Disease as Compared to HCV Mono-infected Patients
Abstract
It is widely accepted that human immunodeficiency virus (HIV) infection is a risk factor for increased severity of hepatitis C virus (HCV) liver disease. However, owing to better efficacy and safety of combination antiretroviral therapy (cART), and increased access to HCV therapy, whether this condition remains true is still unknown. Overall, 1,253 HCV mono-infected patients and 175 HIV/HCV co-infected patients with cirrhosis, included in two prospective French national cohorts (ANRS CO12 CirVir and CO13 HEPAVIH), were studied. Cirrhosis was compensated (Child-Pugh A), without past history of complication, and assessed on liver biopsy. Incidences of liver decompensation (LD), hepatocellular carcinoma (HCC), and death according to HIV status were calculated by a Fine-Gray model adjusted for age. Propensity score matching was also performed to minimize confounding by baseline characteristics. At baseline, HIV/HCV patients were younger (47.5 vs. 56.0 years; P < 0.001), more frequently males (77.1% vs. 62.3%; P < 0.001), and had at baseline and at end of follow-up similar rates of HCV eradication than HCV mono-infected patients. A total of 80.4% of HIV/HCV patients had an undetectable HIV viral load. After adjustment for age, 5-year cumulative incidences of HCC and decompensation were similar in HIV/HCV and HCV patients (8.5% vs. 13.2%, P = 0.12 and 12.8% vs. 15.6%, P = 0.40, respectively). Overall mortality adjusted for age was higher in HIV/HCV co-infected patients (subhazard ratio [SHR] = 1.88; 95% confidence interval [CI], 1.15-3.06; P = 0.011). Factors associated with LD and HCC were age, absence of sustained virological response, and severity of cirrhosis, but not HIV status. Using a propensity score matching 95 patients of each group according to baseline features, similar results were observed. Conclusion: In HCV-infected patients with cirrhosis, HIV co-infection was no longer associated with higher risks of HCC and hepatic decompensation. Increased mortality, however, persisted, attributed to extrahepatic conditions.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- ANRS
-
- French Agency for Research on AIDS and Viral Hepatitis
-
- anti-HCV
-
- antibodies against hepatitis C virus
-
- cART
-
- combination antiretroviral therapy
-
- CI
-
- confidence interval
-
- DAAs
-
- direct acting antivirals
-
- GGT
-
- gamma-glutamyl transferase
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HD
-
- hepatic decompensation
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- LD
-
- liver decompensation
-
- LT
-
- liver transplantation
-
- SHR
-
- sub-distribution hazard ratio
-
- SVR
-
- sustained virological response
-
- US
-
- ultrasound
Co-infection with hepatitis C virus (HCV) is a particular area of concern in patients infected with human immunodeficiency virus (HIV). Global prevalence of HCV has been estimated at around 6.2% in this population, with much higher prevalence in some risk groups such as intravenous drug users and men who have sex with men (MSM).1
In the period before combination antiretroviral therapy (cART), it was widely observed that HIV/HCV co-infected patients suffered from faster progression of liver fibrosis compared to HCV mono-infected patients, resulting in increased rates of cirrhosis and decompensation of liver disease.2, 3 Specific antibodies against hepatitis C virus (anti-HCV) immune responses are decreased and the viral replication of HCV is generally higher in HIV/HCV co-infected subjects than in mono-infected subjects.4 Chronic immune activation and inflammation, attributed both to HIV and bacterial translocation,5 mitochondrial toxicity, and steatosis triggered by some nucleoside reverse transcriptase inhibitors (NRTIs), such as stavudine and didanosine,6, 7 have also contributed in the past to more-severe intrahepatic damage in HIV/HCV co-infected than in mono-infected subjects.8
In 2001, in a meta-analysis performed by Graham et al., the respective progression of fibrosis to cirrhosis and end-stage liver disease was 2- and 6-fold higher in co-infected patients compared to mono-infected patients.9 These findings were confirmed in the recent meta-analysis by Deng et al. including 16,750 patients from 29 trials, who found an overall odds ratio (OR) of 5.45 (95% confidence interval [CI] = 2.54-11.71) for decompensated liver disease for HIV/HCV co-infected compared to HCV-infected patients. In an analysis of subgroups assessing survival, the researchers found also a substantially increased risk of death, 3.60 (95% CI = 3.12-4.15).10
However, all these studies, which were performed before early access to cART, had significant heterogeneity of outcomes, different numbers of patients in cohorts, bias in the fibrosis staging of patients relying or not on a liver biopsy, bias in the effect of HCV infection duration on progression to severe liver disease, as well as publication bias.
Early initiation of cART was recommended for all HIV-infected patients11 and withdrawal of didanosine and stavudine (mitochondrial toxicity) from the NRTI, armamentarium, which has markedly changed the treatment landscape. It has been shown that higher CD4+ T-cell recovery protected HIV co-infected individuals from HCV-related clinical events, and that lower HIV RNA and higher CD4+ T-cell recovery appeared to protect against Fibrosis-4 transitions to worse stages.12, 13 Overall survival of HIV/HCV co-infected patients has markedly increased and HCV morbidity has been reduced, even in co-infected patients with established cirrhosis.
In this context of early cART and of availability of directly active anti-HCV drugs, the aim of this study was to assess the impact of HIV infection on the incidence of hepatic events and death in patients with HCV-related compensated cirrhosis. For this purpose, we combined and analyzed the data from two prospective French nationwide multicenter cohorts sponsored by the French Agency for Research on AIDS and Viral Hepatitis (ANRS) in order to capture the entire spectrum of these events and study them in a competing risk framework through different therapeutic eras.
Patients and Methods
Patients and Definition of Baseline
Patients were recruited from two French national prospective cohorts of the ANRS.
CirVir-ANRS CO12 is a cohort of patients with compensated biopsy-proven cirrhosis attributed to viral B or C hepatitis, recruited in 35 clinical centers specialized in hepatology from March 2006 until June 2012.14 Hepavih-ANRS CO13 is a cohort of HIV/HCV co-infected patients recruited from 2005 to 2016 from 27 clinical centers specialized in management of HIV infection.15 In both cohorts, patients with cirrhosis have follow-up every 6 months for hepatocellular carcinoma (HCC) surveillance whereas patients without cirrhosis have yearly follow-up. Both protocols were approved by the Ethics Committee and conformed to ethical guidelines of the 1975 Declaration of Helsinki. All patients gave written informed consent to participate in the cohorts. The full CirVir and Hepavih protocols are available on the ANRS website (http://anrs.fr).
Inclusion criteria in this substudy were as follows: age older than 18 years; histologically proven cirrhosis, whatever the time of biopsy; negative hepatitis B surface antigen; absence of previous complications of cirrhosis (particularly ascites, gastrointestinal hemorrhage, or HCC); patients in Child-Pugh class A stage; and absence of severe uncontrolled extrahepatic disease resulting in estimated life expectancy of less than 1 year. Patients without liver biopsy and those with an F3/F4 Metavir score from the Hepavih cohort on their liver biopsy were excluded from the analysis.
For patients from the Cirvir cohort, baseline corresponded with date of inclusion. For patients from Hepavih eligible for this substudy, baseline was defined by the date of inclusion in this cohort if the cirrhosis was histologically proven before inclusion, otherwise by the date of liver biopsy performed during follow-up.
Follow-Up and Definitions of Endpoints
Patients were seen by physicians every 6 months, and the usual clinical and biological data were recorded. Examination by Doppler ultrasound (US) was performed every 6 months. In cases of focal liver lesion detected by US, a diagnostic procedure using contrast-enhanced imaging (computed tomography scan or magnetic resonance imaging), serum alpha-fetoprotein (AFP) assay, and/or guided biopsy was performed according to 2005 American Association for the Study of Liver Diseases (AASLD) guidelines.16 HCC diagnosis was thus established either by histological examination performed by an experienced pathologist or by using probabilistic noninvasive criteria (mainly dynamic imaging showing early arterial hypervascularization and portal washout) according to the different periods of time (before and after 2011). When HCC diagnosis was established, treatment was determined using a multidisciplinary approach according to AASLD guidelines for HCC.16
Patient follow-up was maintained from the date of cohort inclusion until December 2015 or until the date of event, meaning that they were censored at the earliest at the: date of last follow-up, date of liver transplantation (LT), and date of liver-related or non-liver-related death in accord with the studied event. All events occurring during follow-up, liver-related or not, were recorded based on information obtained from medical files of patients from each center. In particular, all incidental cases of HCC and all episodes of liver decompensation (LD) encompassing ascites, hepatic encephalopathy, and gastrointestinal bleeding were described, as well as their severity, management according to international recommendations,16 and outcome. Causes of death were assessed by an independent validation committee. Sustained virological response (SVR) following an anti-HCV regimen was defined by undetectable HCV RNA 12 weeks after treatment cessation.
Statistical Analyses
Continuous variables were described by means ± SD or medians (interquartile range; IQR) and categorical data by numbers (percentages). Baseline characteristics were compared between the two groups of patients according to HIV coinfection status using the Student t test or Mann-Whitney U test for continuous variables. Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate.
Predictors associated with outcomes were assessed using uni- and multivariate Cox proportional regression models. All features were assessed according to their baseline levels, with the exception of HIV treatments (cART) and SVR. These variables were included as time-dependent covariates in all models because treatments may start during follow-up and SVR may occur at different time points. Fixed cART values were used for patients receiving cART at baseline (cART = 1) and those who had never received cART (cART = 0). Patients without cART at baseline who received cART during follow-up had their cART status switched from 0 to 1 at the start of treatment. Similarly, fixed SVR values were used for patients who had never experienced SVR (SVR = 0) and for those with SVR at the time of their inclusion (SVR = 1). Non-SVR patients at inclusion who achieved SVR during follow-up had their status switched from non-SVR to SVR; this took into account the end of treatment, which led to undetectable HCV RNA as the time point for setting SVR values from 0 to 1. No reinfection or relapse, as defined by detectable HCV RNA in a patient who had previously achieved SVR, was observed during the follow-up. Variables in the multivariate models were defined after backward step-wise elimination of nonsignificant variables at a P level <0.05.
Age-adjusted cumulative incidence curves according to HIV status were built for HCC, LD (ascites, digestive bleeding and encephalopathy), overall mortality, and liver- and non-liver-related mortality at the mean age of the entire population (56.9 years), with estimates of incidence determined using a Fine-Gray competing risk regression model computing age-adjusted subhazard ratios (SHRs) along with their 95% CIs. Competing events were defined as follows: overall death for HCC and liver decompensation, LT for overall mortality, non-liver-related death for liver-related mortality, and liver-related death for non-liver-related mortality. As a sensitivity analysis, age-adjusted cumulative incidence of all events was also computed using a Cox proportional hazard regression model to compare results from competing risk analysis.
Crude cumulative incidence of overall, liver-related, and non-liver-related death were determined by calculating the cumulative incidence function, and the influence of HIV status on these outcomes was evaluated by a Gray test and a Fine-Gray model.
To take into account the differences between HIV/HCV co-infected patients and HCV mono-infected patients at baseline, a propensity-score–matching analysis was performed. This propensity score was built using a logistic regression model. The model included all the covariables for which a significant difference (P < 0.05) was found between the two groups: age, sex, bilirubin, alanine aminotransferase (ALT), body mass index (BMI), mode of contamination, HCV RNA, tobacco consumption, alcohol consumption, diabetes, and HCV genotype. Pairs of patients with and without HIV co-infection were created (1:1 matching) based on the closest logit of propensity scores and a caliper width of a 0.2 SD of the logit of this propensity score. It allowed matching 95 pairs of patients with similar characteristics. In this subpopulation, incidence curves of occurrence of HCC, liver failure, and overall and specific (liver- and non-liver-related) survival curves were constructed using the Kaplan-Meier method, according to HIV status. Effect of HIV co-infection on complications in this subpopulation was assessed by a Cox regression model, without and with adjustment on virological response, accounting for the clustered structure of the data between HCV and HIV/HCV patients.
Results are expressed as the risk of events for HIV/HCV co-infected patients compared to HCV mono-infected patients.
All statistical analyses were performed using Stata software (version 13.0; StataCorp LP, College Station, TX). A P value ≤0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients
A total of 1,671 patients with cirrhosis and 142 HIV/HCV co-infected patients with cirrhosis were extracted from both cohorts and considered in the present analyses (see flow-chart, Fig. 1). Among the 1,671 patients from the CirVir cohort, 365 were excluded because of HBV co-infection, lack of data on HIV co-infection, or duplicate with HIV/HCV cohort. Among the 142 patients from the Hepavih cohort, 20 were excluded because of absence of liver biopsy with Metavir grade F4, HBV co-infection, or past history of liver complication before liver biopsy procedure (HCC, LD, or LT). Consequently, final analyses were performed on 1,458 patients, comprising 1,253 HCV mono-infected patients with cirrhosis and 175 HIV/HCV co-infected patients with cirrhosis, including 53 HIV/HCV patients from the CirVir cohort (Fig. 1).
At baseline (Table 1), HIV/HCV patients were younger (P < 0.001), were more frequently males (P < 0.001), and had more frequently HCV genotype 3 (P = 0.006) and consumption of alcohol and tobacco (P < 0.001) than HCV mono-infected patients. Overall, 92.3% of HIV/HCV patients were on cART and 80.4% had an undetectable HIV. Both groups had similar rates of HCV eradication at baseline (16.6% vs. 19.9% in HCV mono-infected patients; P = 0.30). At the end of follow-up, the rate of patients with SVR did not differ either between both groups (47.0 % vs. 52.0% in HCV mono-infected patients; P = 0.22; Table 1), as well as the percentage of patients treated and cured with direct-acting antiviral (DAA) therapy (17 of 77 [22.1%] vs. 167 of 637 [26.2%]; P = 0.49).
| Baseline characteristics* | No. of Patients |
Whole Population (n =1,428) |
HIV/HCV (n = 175) |
HCV (n = 1,253) |
P Value† |
|---|---|---|---|---|---|
| Male sex | 1,428 | 916 (64.2) | 135 (77.1) | 781 (62.3) | <0.001 |
| Age (years) | 1,428 | 54.3 [47.9-63.5] | 47.3 [43.8-50.1] | 56.0 [49.4-64.8] | <0.001 |
| Method of HCV transmission | 1,403 | <0.001 | |||
| IV drug use | 456 (32.5) | 122 (79.2) | 334 (26.7) | ||
| Transfusion | 443 (31.6) | 10 (6.5) | 433 (34.7) | ||
| Sexual partner | 10 (0.7) | 5 (3.3) | 5 (0.4) | ||
| Other | 231 (16.5) | 3 (1.9) | 228 (18.3) | ||
| Not determined | 263 (18.7) | 14 (9.1) | 249 (29.9) | ||
| HCV genotype | 1,353 | 0.006 | |||
| 1 | 913 (67.5) | 101 (57.8) | 812 (68.6) | ||
| 2 | 71 (5.3) | 5 (3.0) | 66 (5.6) | ||
| 3 | 225 (16.6) | 43 (25.4) | 182 (15.4) | ||
| 4 | 121 (8.9) | 19 (11.2) | 102 (8.6) | ||
| 5 or 6 | 23 (1.7) | 1 (0.6) | 22 (1.9) | ||
| Diabetes | 1,428 | 257 (18.0) | 10 (5.7) | 247 (19.7) | <0.001 |
| Arterial hypertension | 1,412 | 395 (28.0) | 36 (22.6) | 359 (26.7) | 0.11 |
| BMI (kg/m2) | 1,264 | 25.5 [22.6-28.6] | 21.7 [20.2-24.2] | 25.9 [23.2-29.0] | <0.001 |
| IV drug use | 1,390 | <0.001 | |||
| Past | 461 (33.2) | 141 (81.5) | 320 (26.3) | ||
| Ongoing | 6 (0.4) | 2 (1.2) | 4 (0.3) | ||
| Illicit drug use at baseline | 1,394 | 0.13 | |||
| No | 1,383 (99.2) | 162 (98.2) | 1,221 (99.4) | ||
| Yes | 11 (0.8) | 3 (1.8) | 8 (0.6) | ||
| Tobacco consumption | 1,326 | <0.001 | |||
| Past | 295 (22.3) | 32 (19.8) | 263 (22.6) | ||
| Ongoing | 532 (40.1) | 113 (69.7) | 419 (36.0) | ||
| Alcohol intake | 1,303 | <0.001 | |||
| Past | 310 (23.8) | 59 (36.6) | 251 (22.0) | ||
| Ongoing | 358 (27.5) | 65 (40.4) | 293 (25.6) | ||
| Negative HCV viral load | 1,426 | 409 (28.7) | 36 (20.6) | 373 (29.8) | 0.011 |
| HCV SVR at baseline | 1,426 | 278 (19.5) | 29 (16.6) | 249 (19.9) | 0.30 |
| HCV SVR at end of FU | 1,388 | 714 (51.4) | 77 (47.0) | 637 (52.0) | 0.22 |
| Past or ongoing anti-HCV treatment use | 1,427 | 1,319 (92.4) | 145 (82.9) | 1,174 (93.8) | <0.001 |
| cART at baseline | 169 | — | 156 (92.3) | — | — |
| Negative HIV viral load | 163 | — | 131 (80.4) | — | — |
| CD4 (cell/mm3) | 151 | — | 419 [253-604] | — | — |
| Platelet count (103/mm3) | 1,398 | 137.0 [97.0-183.0] | 143.0 [100.0-178.0] | 136.5 [96.0-183.0] | 0.79 |
| Platelet count <100 (103/mm3) | 1,398 | 373 (26.7) | 40 (24.1) | 333 (27.0) | 0.53 |
| Prothrombin time | 1,372 | 89.0 [80.0-98.0] | 90.5 [81.0-99.0] | 89.0 [79.0-98.0] | 0.24 |
| Creatinin (µmol/L) | 1,411 | 71.0 [61.9-81.0] | 71.0 [63.0-81.0] | 71.0 [61.9-80.4] | 0.35 |
| GFR | 1,411 | 97.4 [82.1-113.6] | 105.3 [83.2-124.0] | 96.2 [82.0-113.0] | 0.004 |
| AFP | 1,398 | 6.0 [3.6-11.0] | 5.2 [3.1-10.7] | 6.0 [3.7-11.0] | 0.34 |
| AST (IU/L) | 1,420 | 57.0 [35.0-92.0] | 53.0 [36.0-86.0] | 58.0 [35.0-93.0] | 0.48 |
| ALT (IU/L) | 1,422 | 63.0 [35.0-106.0] | 59.0 [32.0-90.0] | 63.0 [35.0-109.0] | 0.045 |
| GGT (IU/L) | 1,422 | 85.0 [47.0-160.0] | 86.0 [54.0-169.0] | 85.0 [45.0-159.0] | 0.29 |
| Serum albumin (g/L) | 1,407 | 41.6 [38.0-44.7] | 41.3 [38.6-44.0] | 41.8 [38.0-44.9] | 0.62 |
| Serum albumin ≤35 g/L | 1,406 | 145 (10.3) | 18 (11.3) | 127 (10.2) | 0.68 |
| Total bilirubin (µmol/L) | 1,421 | 12.0 [8.0-17.0] | 13.0 [9.0-22.0] | 12.0 [8.0-16.0] | 0.002 |
| Total bilirubin >17 µmol/L | 1,421 | 337 (23.7) | 60 (35.5) | 277 (22.1) | <0.001 |
- Abbreviations: IV, intravenous; FU, follow-up; GFR, glomerular filtration rate estimated by the MDRD (Modification of the Diet in Renal Disease) formula; AST, aspartate aminotransferase.
- * Except SVR at end of follow-up.
- † HIV/HCV versus HCV.
- Statistically significant P values are in bold.
Incidences of Liver-Related Events (HCC, LD)
At the end of follow-up in December 2015, median follow-up was 55.7 and 59.3 months in HIV/HCV co-infected and HCV mono-infected patients, respectively, and did not differ between both groups (P = 0.10).
During this time frame, 192 (13.4%) patients (179 HCV, 14.3%; 13 HIV/HCV, 7.4%) developed an HCC and 225 (15.4%) (206 HCV, 16.4%; 19 HIV/HCV, 10.9%) developed at least one episode of LD (Tables 2 and 3). Median delay for occurrence of an HCC was 33.2 months and did not differ between HIV/HCV co-infected and HCV mono-infected patients (36.6 vs. 32.4 months, respectively; P = 0.90). However, HCC presentation at diagnosis was more severe in HIV/HCV co-infected patients as reflected by the larger size of the main tumor (P = 0.023) and the higher frequency of tumoral portal thrombosis (P = 0.011) than in HCV mono-infected patients. This was observed despite a similar delay since the last normal liver US echography in both groups (6.3 [IQR, 5.6-8.2] vs. 6.5 months [IQR, 5.6-9.2]; P =0.66) and a time ≥7 months since last imaging examination before HCC diagnosis in 30.8% and 40.2%, respectively (P = 0.50; Table 2). Moreover, HIV/HCV patients were younger than HCV mono-infected patients at the time they developed HCC (median, 52.9 vs. 61.8 years). Although the percentages of patients outside Milan were not significantly different between the two groups, a univariate analysis of the predictors for presenting HCC outside Milan showed that only a younger age (OR, 0.96; 95% CI, 0.92-0.99; P = 0.026) was associated with this outcome.
| Characteristics |
Whole Population (n = 1,428) |
HIV/HCV (n = 175) |
HCV (n = 1,253) |
P Value |
|---|---|---|---|---|
| No. of HCC patients | 192 (13.4) | 13 (7.4) | 179 (14.3) | 0.013 |
| Tumor type | 0.19 | |||
| 1 nodule | 111 (63.4) | 6 (54.5) | 105 (64.0) | |
| 2 or 3 nodules | 45 (25.7) | 2 (18.2) | 43 (26.2) | |
| >3 nodules | 12 (6.9) | 2 (18.2) | 10 (6.1) | |
| Infiltrating | 7 (4.0) | 1 (9.1) | 6 (3.7) | |
| MD | 17 | 2 | 15 | |
| Diameter of largest nodule (mm) | 0.023 | |||
| ≤20 | 88 (55.3) | 5 (45.4) | 83 (56.1) | |
| 21-30 | 35 (22.0) | 0 | 35 (23.6) | |
| 31-50 | 20 (12.6) | 4 (36.4) | 16 (10.8) | |
| >50 | 16 (10.1) | 2 (18.2) | 14 (9.5) | |
| MD | 33 | 2 | 31 | |
| Portal thrombosis | 17 (10.2) | 4 (40.0) | 13 (8.3) | 0.011 |
| MD | 25 | 3 | 22 | |
| Within Milan criteria | 135 (79.0) | 7 (63.6) | 128 (80.0) | 0.25 |
| 1 nodule ≤50 mm | 102 | 6 | 96 | |
| 2 or 3 nodules ≤30 mm | 33 | 1 | 32 | |
| Outside Milan criteria | 36 (21.0) | 4 (36.4) | 32 (20.0) | |
| MD | 21 | 2 | 19 | |
| AFP level at HCC diagnosis (ng/mL) | ||||
| Median [Q1-Q3] | 17.6 [7.0-90.2] | 39.9 [28.4-84.0] | 15.1 [6.4-90.2] | 0.080 |
| >200 ng/mL | 20 (14.2) | 2 (22.2) | 18 (13.9) | 0.62 |
| MD | 53 | 4 | 49 | |
|
Time of last imaging examination before HCC diagnosis (months) |
6.5 [5.6-9.0] | 6.3 [5.6-8.2] | 6.5 [5.6-9.2] | 0.66 |
|
Time of last imaging examination before HCC diagnosis ≥7 months |
76 (39.6) | 4 (30.8) | 72 (40.2) | 0.50 |
- Statistically significant P values are in bold.
- Abbreviation: MD, missing data.
After adjustment for age, HCC 5-year cumulative incidence was similar in HIV/HCV patients and in HCV mono-infected patients (8.5% vs. 13.2%; SHR = 0.63; 95% CI, 0.35-1.13; P = 0.12; Fig. 2A).
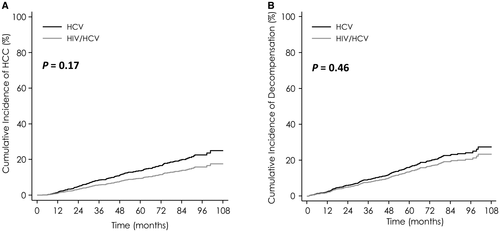
Median delay for occurrence of LD was 32.8 months and did not differ between HIV/HCV co-infected and HCV mono-infected patients (30.1 vs. 35.5 months, respectively; P = 0.17; Fig. 2B).
After adjustment for age, 5-year cumulative incidence of LD was similar in HIV/HCV patients and in HCV mono-infected patients (12.8% for HIV/HCV vs. 15.6% for HCV; SHR = 0.81; 95% CI, 0.49-1.33; P = 0.40).
In addition, cumulative incidences of HCC and LD assessed by the Kaplan-Meier method and Cox regression gave similar results.
Supporting Tables S1 and S2 summarize results of univariate analyses. In multivariate models (Table 4), HIV/HCV co-infection with or without cART intake was not associated with a higher risk of liver complications. Meanwhile, absence of SVR, severity of disease as assessed by platelet count <100,000/mm3 or albumin ≤35 g/L, and elevated gamma-glutamyl transferase (GGT) were significantly associated with occurrence of liver complications.
Mortality and Causes of Death
One hundred eighty-nine patients died during the follow-up (23 HIV-HCV co-infected patients [13.1%] vs. 166 HCV mono-infected [13.2%]).
Median delay for occurrence of a death was 46.3 months.
Overall causes of death were HCC or cholangiocarcinoma (suppress CCA; n = 48; 25.4%), other liver-related disease (n = 47; 24.9%), non-liver-related death (n = 97; 39.2%), and unknown (n = 20; 10.6%; Table 3).
| Main Events Occurring During Follow-up |
Whole Population (n = 1,428) |
HIV/HCV (n = 175) |
HCV (n = 1,253) |
P Value |
|---|---|---|---|---|
| HCC* | 192 (13.4) | 13 (7.4) | 179 (14.3) | 0.17 § |
| LD* | 225 (15.8) | 19 (10.9) | 206 (16.4) | 0.46 § |
| Digestive hemorrhage | 66 (4.6) | 3 (1.7) | 63 (5.0) | 0.095 § |
| Ascites | 182 (12.8) | 18 (10.3) | 164 (13.1) | 0.87 § |
| Encephalopathy | 63 (4.4) | 3 (1.7) | 60 (4.8) | 0.27 § |
| Liver transplantation | 43 (3.0) | 5 (2.9) | 38 (3.0) | 0.45 § |
| Death* | 189 (13.2) | 23 (13.1) | 166 (13.2) | 0.011 § |
| Causes of death† | 0.19‖ | |||
| Hepatic cause | 95/189 (50.3) | 8/23 (34.7) | 87/166 (52.4) | 0.78¶ |
| HCC | 45/189 (23.8) | 3/23 (13.0) | 42/166 (25.3) | |
| Cholangiocarcinoma | 3/189 (1.6) | 0 | 3/166 (1.8) | |
| Other hepatic cause | 47/189 (24.9) | 5/23 (21.7) | 42/166 (25.3) | |
| Extrahepatic cause | 74/189 (39.1) | 11/23 (47.9) | 63/166 (38.0) | 0.53# |
| Bacterial infection (infected ascites excluded) | 23/189 (12.2) | 4/23 (17.4) | 20/166 (12.1) | |
| Nonliver cancer | 18/189 (9.5) | 1/23 (4.3) | 17/166 (10.2) | |
| Cardiovascular event | 10/189 (5.3) | 1/23 (4.3) | 9/166 (5.4) | |
| Other nonliver event‡ | 23/189 (12.2) | 5/23 (21.7) | 17/166 (10.2) | |
| Missing or unknown cause | 20/189 (10.6) | 4/23 (17.4) | 16/166 (9.6) |
- * Number of patients (%) developing the event in each group.
- † Number of patients (%) who died from the studied cause.
- ‡ Causes of death for the 5 HIV/HCV patients: 1 lactic acidosis, 1 multifocal leucoencephalitis, 1 acute alcoholism, 1 HIV encephalitis, and 1 candida pneumonia.
- § P value from Cox regression model adjusted for age.
- ‖ Comparison of the distribution of causes of death between the two groups (hepatic vs. extrahepatic cause).
- ¶ Comparison of the distribution of hepatic causes between the two groups.
- # Comparison of the distribution of extrahepatic causes between the two groups.
- Statistically significant P values are in bold.
| Features |
HCC (n = 1,175) |
Liver Decompensation (n = 1,276) |
Overall Death (n = 1,240) |
|||
|---|---|---|---|---|---|---|
| HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | |
| Group* | 0.14 | 0.50 | 0.031 | |||
| HIV/HCV – cART+ | 0.57 [0.28; 1.16] | 0.12 | 0.92 [0.52; 1.64] | 0.78 | 1.75 [0.99; 3.10] | 0.055 |
| HIV/HCV – cART– | 3.37 [0.45; 25.20] | 0.24 | 3.15 [0.43; 23.08] | 0.26 | 7.43 [0.99; 55.28] | 0.050 |
| HCV | Ref | Ref | Ref | |||
| Age | 1.03 [1.01; 1.05] | <0.001 | 1.01 [1.00; 1.03] | 0.042 | 1.03 [1.02; 1.05] | <0.001 |
| SVR* | 0.48 [0.31; 0.73] | 0.001 | 0.40 [0.26; 0.62] | <0.001 | 0.39 [0.24; 0.64] | <0.001 |
| Albumin ≤35 g/L | 2.10 [1.48; 2.99] | <0.001 | 2.50 [1.69; 3.69] | <0.001 | ||
| Platelets (103/mm3) | 0.038 | <0.001 | <0.001 | |||
| <100 | 1.69 [1.12; 2.55] | 0.012 | 2.87 [1.95; 4.22] | < 0.001 | 1.77 [1.20; 2.62] | 0.004 |
| [100; 150] | 1.46 [0.99; 2.16] | 0.057 | 1.27 [0.84; 1.91] | 0.25 | 0.90 [0.59; 1.38] | 0.64 |
| >150 | Ref | Ref | Ref | |||
| GGT (IU/L) | 0.002 | <0.001 | 0.005 | |||
| ≤N | Ref | Ref | Ref | |||
| [N; 2N] | 2.16 [1.26; 3.70] | 0.005 | 1.75 [1.04; 2.96] | 0.035 | 2.46 [1.43; 4.24] | 0.001 |
| >2N | 2.48 [1.49; 4.14] | <0.001 | 2.49 [1.53; 4.04] | <0.001 | 2.10 [1.24; 3.55] | 0.006 |
| Alcohol intake | 0.004 | |||||
| Never | Ref | |||||
| Past | 1.84 [1.25; 2.71] | 0.002 | ||||
| Ongoing | 1.00 [0.67; 1.49] | 0.99 | ||||
| AFP >7 ng/mL | 1.42 [1.03; 1.97] | 0.035 | 1.33 [0.98; 1.81] | 0.067 | 1.49 [1.04; 2.13] | 0.029 |
| Prothrombin time ≤80% | 1.40 [1.00; 1.95] | 0.047 | 1.33 [0.98; 1.80] | 0.068 | ||
| Total bilirubin >17 µmol/L | 1.30 [0.96; 1.77] | 0.088 | ||||
| Diabetes | 1.44 [1.05; 1.98] | 0.023 | 1.37 [0.95; 1.98] | 0.089 | ||
| HCV genotype | 0.15 | |||||
| 1 | Ref | |||||
| 2 | 1.51 [0.80; 2.85] | 0.20 | ||||
| 3 | 1.18 [0.73; 1.90] | 0.49 | ||||
| 4 | 0.43 [0.19; 1.00] | 0.050 | ||||
| 5 or 6 | 0.50 [0.07; 3.65] | 0.50 | ||||
- * Included as a time-dependent covariate.
- Statistically significant P values are in bold.
Overall crude mortality rate was similar in both groups (12.9% for HIV/HCV vs. 10.8% HCV at 5 years) with an SHR of 1.11 (95% CI, 0.72-1.72; P = 0.64) as well as crude liver-related and non-liver-related mortality rates (liver-related, 5.1% for HIV/HCV vs. 5.4% for HCV at 5 years, P = 0.36; non-liver-related, 6.5% for HIC/HCV vs. 4.7% for HCV at 5 years, P = 0.32).
However, HIV/HCV co-infected patients died at a younger age—10 years in median—than HCV mono-infected patients (47.3 vs. 56 years; P < 0.001).
After adjustment for age, HIV/HCV co-infected patients presented a higher risk of overall mortality (SHR = 1.88; 95% CI, 1.15-3.06; P = 0.011; Fig. 3A) and non-liver-related mortality (SHR = 2.44; 95% CI, 1.18-5.07; P = 0.016; Fig. 3C), compared to HCV mono-infected patients.
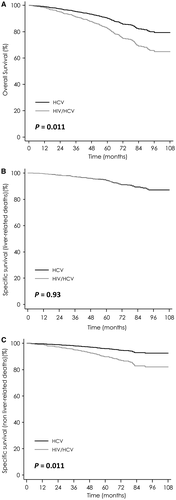
Similar findings were obtained by the Kaplan-Meier method and Cox regression models.
Results of univariate analyses for overall mortality are reported in Supporting Table S3. In multivariate models (Table 4), HIV/HCV co-infection was associated with a higher risk of overall death. The other factors associated with overall death were absence of SVR, age, and severity of disease as assessed by platelets count <100,000/mm3 and albumin ≤35 g/L (Table 4).
A sensitivity analysis for the predictors of HCC, LD, and mortality was performed with exclusion of the few patients not receiving cART at baseline. The corresponding results are presented in Supporting Tables S4-S7.
Use of a Propensity Score
In order to take into account the differences between HCV mono-infected patients and HIV-HCV co-infected patients at baseline, we used a propensity score constructed from the regression coefficients of all the variables included in the model.
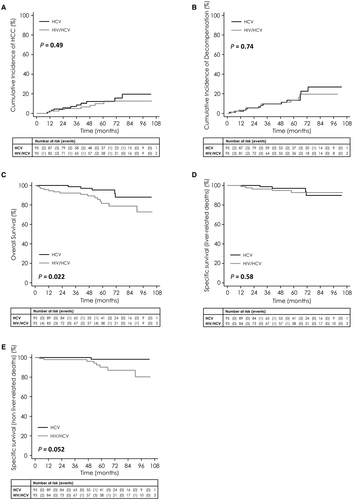
This propensity score enabled the matching of 95 patients from each group with the same score and similar baseline characteristics (Supporting Table S8). Similar results were observed in this subpopulation as in the entire study population. Five-year incidence of HCC and of hepatic decompensation did not differ significantly between HIV/HCV and HCV mono-infected patients (hazard ratio [HR] = 0.73; P = 0.47 and HR = 0.88; P = 0.74, respectively; Fig. 4A,B). Overall survival remained shorter in HIV/HCV co-infected patients than in HCV mono-infected patients (HR = 3.08; P = 0.011; Fig. 4C). Adjusted for SVR status (HR = 0.20; P = 0.043), the effect of HIV co-infection on overall survival persisted (HR = 3.02; P = 0.015).
Discussion
Based on the analysis of two French nationwide prospective cohorts, we showed that despite having an increased overall mortality, HIV/HCV co-infected patients had a similar risk of developing LD and HCC compared to HCV mono-infected patients. These results, somewhat unexpected, were validated by a sensitivity analysis using a propensity score and several competing risk analyses taking into account age, non-liver related deaths, LT, and antiretroviral therapy.
The main factors that impacted the probabilities of LD and of HCC were absence of HCV clearance, severity of cirrhosis, and older age, but not HIV status.
These results contrast with the usual reported course of HIV/HCV co-infected patients9, 10 and merit raising several hypotheses.
The first potential explanation is the consequence of the beneficial effect of cART on correction of immunosuppression. Indeed, all HIV-infected patients are now treated with cART early, and the vast majority of them have an undetectable viral load and have gained a satisfactory CD4 restoration. These two factors have been shown to protect against occurrence of HCV-related events and fibrosis aggravation.12, 13
The second factor likely to explain why HIV/HCV co-infected patients with cirrhosis are no longer at increased risk of liver-related events is the availability of antiretroviral drugs providing improved liver safety. In early cART combinations, the nucleoside analogs, didanosine, azidothymidine, and stavudine, were the pillars of antiretroviral therapy. They were associated with a risk of mitochondrial cytopathy, and for didanosine, with the occurrence of rare, but severe, cases of liver nodular hyperplasia.8, 9, 17 Since 2006, these drugs have progressively been abandoned and replaced in this class of antiretrovirals by tenofovir and abacavir, which have no known impact on the liver.
The last explanation that can be raised is that the patients with more-severe conditions died, such as those with persistent intravenous drug use and those with excessive alcohol consumption. But there is no reason why these populations should not also be represented in the HCV mono-infected group. It is noteworthy that HCV infection occurred at a younger age in the HIV/HCV co-infected group, in which the median age at baseline was 10 years younger than in the HIV/HCV co-infected group (47.3 vs. 56 years; P < 0.001).
The fact that incidence of liver complications has become similar in both populations is a major result.
Another interesting finding is the suggestion of a more-aggressive presentation of HCC in HIV-infected patients once diagnosed compared to mono-infected patients, as reflected by larger size of the main HCC nodule and the higher frequency of tumoral portal thrombosis in HIV-HCV co-infected patients. This was observed even though HIV/HCV patients were younger than HCV mono-infected patients at baseline at the time they developed HCC (median of 52.9 years for the 13 patients who developed HCC in the HIV/HCV group vs. 61.8 years for the 179 patients who developed HCC in the HCV group), and that the time frame elapsed since the last normal US examination (6.3 vs. 6.5 months) was quite similar. A dedicated prospective study performed in this type of population would be useful to confirm this point. However, several retrospective comparative studies, including one from our groups, have already shown that, at the time of diagnosis, HCCs were more aggressive as reflected by a more-frequent multinodular presentation and an increased frequency of portal thrombosis.18, 19 Brau et al.20 also compared 63 HIV/HCV co-infected patients with 226 HCV patients having developed an HCC and showed that HCC developed faster in HIV/HCV co-infected than in HCV mono-infected patients (mean, 26 vs. 34 years after HCV infection; P = 0.002). As in our study, they showed that HIV-positive patients were younger than controls (52 vs. 64 years; P < 0.001), were more frequently symptomatic (51% vs. 38%; P = 0.048), and had a higher median AFP level (227 vs. 51 ng/mL; P = 0.005), but, contrary to in our series, HCC staging score was similar in both groups.
However, even though in some points the HIV/HCV co-infected population becomes increasingly similar to the HCV mono-infected population, some specificity persists in the co-infected population.
An important difference between HIV/HCV co-infected and HVC mono-infected patients shown by our study was the persistent higher risk of mortality of HIV/HCV co-infected patients attributed to extrahepatic conditions, after adjusting for age. It is of particular interest to note that the first cause of non-liver-related death was bacterial infection, after excluding bacterial ascites, whose risk is synergistically increased by cirrhosis and HIV. The second cause of non-liver-related death was extrahepatic cancer.
This reinforces the message to treat all HIV/HCV co-infected patients for their hepatitis as for HCV mono-infected patients, especially given that there are no more differences in the response rate to new DAAs between both populations, with a rate of SVR reaching 93%-95% in clinical trials20-25 as in real-life studies whatever the combinations of DAAs used.26 Moreover, eradication of HCV infection in patients with cirrhosis has been shown to reduce the risk of HCC, decompensation, and nonliver complications such as cardiovascular events and bacterial infections.27 The only minimal differences that still persist between the two populations is the slight increased complexity to treat attributed to the risk of interactions between antiretrovirals and DAA and the increased risk of HCV reinfections, which seems to be higher in HIV-infected MSM.28
The main strength of the study relies on the quality and exhaustiveness of the data collection, which was performed every 6 months in two prospective ANRS French cohorts, both implemented at the same period in 2005. Very importantly, the two populations were defined on the same criteria: biopsy-proven cirrhosis, no previous complications, and Child Pugh A, with an exclusion of the cases with previous decompensating or HCC at baseline.
However, our study has some limits; the first one being that some events may have been missed in patients. The second one is that some causes of death, mainly those that occurred suddenly at home, could not be precisely identified, underestimating the rate of cardiovascular death or psychiatric death attributed to suicide or overdoses. Better knowledge of these causes could increase the rate of extrahepatic mortality and would reinforce our observation.
There is also a possible bias of exposition. Because of the fact that HIV/HCV co-infected patients died 10 years younger than HCV-infected patients, the median duration of HCV disease could be shorter in the HIV/HCV group, depending on the age at HIV/HCV exposure. Moreover, it is well known that age increases the risk of hepatic disease aggravation. The validity of these results will be verified once all patients are treated and cured from HCV with direct active drugs.
In conclusion, because of well-tolerated and very efficient cART and easy access to anti-HCV therapies, the risk of LD and HCC observed in HIV/HCV co-infected patients is no longer higher than that observed in HCV mono-infected patients. HIV/HCV co-infected patients remain, however, at higher risk of death from nonhepatic causes. Current guidelines can apply to both populations, and the only specificity of HIV/HCV co-infection is the higher risk of drug-drug interaction between DAAs and cART.
Acknowledgment
This study was sponsored by the ANRS (France Recherche Nord & sud Sida-hiv Hépatites: FRENSH). The funding sponsor had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Appendix
Patients of the HEPAVIH Cohort
Scientific Committee of the ANRS CO13 HEPAVIH Study Group: D. Salmon-Ceron (Principal Investigator), L. Wittkop (co-Principal Investigator), L Esterle (project manager), P. Sogni, P. Trimoulet, J. Izopet, L. Serfaty, V. Paradis, B. Spire, P. Carrieri, MA. Valantin, G. Pialoux, J. Chas, I. Poizot-Martin, K Barangue, A Naqvi, E. Rosenthal, A. Bicart-See, O. Bouchaud, A. Gervais, C. Lascoux-Combe, C. Goujard, K. Lacombe, C. Duvivier, D. Vittecoq, D. Neau, P. Morlat, F. Bani-Sadr, L. Meyer, F. Boufassa, S. Dominguez, B. Autran, A.M. Roque, C. Solas, H. Fontaine, D. Costagliola, L. Piroth, A. Simon, D. Zucman, F. Boué, P. Miailhes, E. Billaud, H. Aumaître, D. Rey, G. Peytavin, V. Petrov-Sanchez, and A. Pailhe.
Clinical Centers (ward/participating physicians): APHP Cochin, Paris (Médecine Interne et Maladies Infectieuses: D. Salmon, L. Alagna; Hépato-gastro-entérologie: P. Sogni; Anatomo-pathologie: B. Terris; Virologie: A. Krivine); APHP Pitié-Salpétrière, Paris (Maladies Infectieuses et Tropicales: C. Katlama, M.A. Valantin, and H. Stitou; Hépato-gastroentérologie: Y. Benhamou; Anatomo-pathologie: F. Charlotte; Virologie: S. Fourati); APHP Pitié-Salpétrière, Paris (Médecine Interne: A. Simon, P. Cacoub, and S. Nafissa); APHM Sainte-Marguerite, Marseille (Service d'Immuno-Hématologie Clinique-CISIH: I. Poizot-Martin, O. Zaegel, and M. Porcher; Virologie: C. Tamalet); APHP Tenon, Paris (Maladies Infectieuses et Tropicales: G. Pialoux, J. Chas, and L. Slama; Anatomo-pathologie: P. Callard, F. Bendjaballah; Virologie: C. Le Pendeven); CHU Purpan, Toulouse (Maladies Infectieuses et Tropicales: B. Marchou; Hépato-gastro-entérologie: L. Alric, K. Barange, and S. Metivier; Anatomopathologie: J. Selves; Virologie: F. Larroquette); CHU Archet, Nice (Médecine Interne: E. Rosenthal; Infectiologie: Alissa Naqvi; Anatomo-pathologie: J. Haudebourg, M.C. Saint-Paul; Virologie: C. Partouche); APHP Avicenne, Bobigny (Médecine Interne–Unité VIH: O. Bouchaud; Anatomo-pathologie: M. Ziol; Virologie: Y. Baazia); Hôpital Joseph Ducuing, Toulouse (Médecine Interne: M. Uzan, A. Bicart-See, D. Garipuy, and M.J. Ferro-Collados; Anatomo-pathologie: J. Selves; Virologie: F. Nicot); APHP Bichat–Claude-Bernard, Paris (Maladies Infectieuses: A. Gervais, Y. Yazdanpanah; Anatomo-pathologie: H. Adle-Biassette; Virologie: G. Alexandre); APHP Saint-Louis, Paris (Maladies infectieuses: C. Lascoux-Combe, J.M. Molina; Anatomo-pathologie: P. Bertheau, J. Duclos; Virologie: P. Palmer); APHP Saint-Antoine (Maladies Infectieuses et Tropicales: K. Lacombe, J. Bottero; P.M. Girard, Anatomo-pathologie: D. Wendum, P. Cervera, J. Adam; Virologie: C. Viala); APHP Bicêtre, Paris (Médecine Interne: C. Goujard, E. Teicher; Virologie: C. Pallier; Maladies Infectieuses: D. Vittecoq); APHP Necker, Paris (Maladies Infectieuses et Tropicales: O. Lortholary, C. Duvivier, C. Rouzaud, J. Lourenco, F. Touam, and C. Louisin; Virologie: A. Mélard); CHU Pellegrin, Bordeaux (Maladies Infectieuses et Tropicales: D. Neau, A. Ochoa, E. Blanchard, S. Castet-Lafarie, C. Cazanave, D. Malvy, M. Dupon, H. Dutronc, F. Dauchy, and L. Lacaze-Buzy; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas); Hôpital Saint-André, Bordeaux (Médecine Interne et Maladies Infectieuses: P. Morlat, D. Lacoste, F. Bonnet, N. Bernard, M. Bonarek Hessamfar, J. Roger-Schmeltz, P. Gellie, P. Thibaut, F. Paccalin, C. Martell, M. Carmen Pertusa, M. Vandenhende, P. Mercier, D. Malvy, T. Pistone, M.C. Receveur, and S. Caldato; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas); Hôpital du Haut-Levêque, Bordeaux (Médecine Interne: J.L. Pellegrin, J.F. Viallard, E. Lazzaro, and C. Greib; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas); Hôpital FOCH, Suresnes (Médecine Interne: D. Zucman, C. Majerholc; Virologie: F. Guitard); APHP Antoine Béclère, Clamart (Médecine Interne: F. Boué, J. Polo Devoto, I. Kansau, V. Chambrin, C. Pignon, L. Berroukeche, R. Fior, and V. Martinez; Virologie: C. Deback); CHU Henri Mondor, Créteil (Immunologie Clinique: Y. Lévy, S. Dominguez, J.D. Lelièvre, A.S. Lascaux, and G. Melica); CHU Hôtel Dieu, Nantes (Maladies Infectieuses et Tropicales: E. Billaud, F. Raffi, and C. Alavena; Virologie: A. Rodallec); Hôpital de la Croix Rousse, Lyon (Maladies Infectieuses et Tropicales: P. Miailhes, D. Peyramond, C. Chidiac, F. Ader, F. Biron, A. Boibieux, L. Cotte, T. Ferry, T. Perpoint, J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, and M. Amiri; Virologie: Caroline Scholtes, T.T. Le-Thi); CHU Dijon, Dijon (Département d'infectiologie: L. Piroth, P. Chavanet M. Duong Van Huyen, M. Buisson, A. Waldner-Combernoux, S. Mahy, R. Binois, A.L. Simonet-Lann, and D. Croisier-Bertin); CH Perpignan, Perpignan (Maladies infectieuses et tropicales: H. Aumaître); CHU Robert Debré, Reims (Médecine interne, maladies infectieuses et immunologie clinique: F. Bani-Sadr, D. Lambert, Y Nguen, C. Rouger, and J.L. Berger); CHRU Strasbourg (Le Trait d'Union: D. Rey, M. Partisani, M.L. Batard, C. Cheneau, M. Priester, C. Bernard-Henry, and E. de Mautort; Virologie: P. Gantner, S. Fafi-Kremer), APHP Bichat-Claude Bernard (Pharmacologie: G. Peytavin).
Data collection: F. Roustant, I. Kmiec, L. Traore, S. Lepuil, S. Parlier, V. Sicart-Payssan, E. Bedel, F. Touam, C. Louisin, M. Mole, C. Bolliot, M. Mebarki, A. Adda-Lievin, F.-Z. Makhoukhi, O. Braik, R. Bayoud, R. Usubillaga, M.-P. Pietri, V. Le Baut, D. Bornarel, C. Chesnel, D. Beniken, M. Pauchard, S. Akel, S. Caldato, C. Lions, L. Chalal, Z. Julia, H. Hue, A. Soria, M. Cavellec, S. Delarue, S. Breau, A. Joulie, P. Fisher, C. Ondoeyene, S. Ogoudjobi, C. Brochier, and V. Thoirain-Galvan.
Management, statistical analyses: E. Boerg, P. Carrieri, V. Conte, L. Dequae-Merchadou, M. Desvallees, N. Douiri, L. Esterle, C. Gilbert, S. Gillet, R. Knight, F. Marcellin, L. Michel, M. Mora, S. Nordmann, C. Protopopescu, P. Roux, B. Spire, S. Tezkratt, A. Vilotitch, and I. Yaya.
Scientific Committee of the ANRS CO12 CIRVIR Study Group:
Funding: ANRS (France Recherche Nord & Sud Sida-HIV Hépatites)
Patients of the CIRVIR Cohort
Scientific committee: P. Nahon (Principal Investigator), R. Layese and F. Roudot-Thoraval (data management), P. Bedossa, M. Bonjour, V. Bourcier, S. Dharancy, I. Durand-Zaleski, H. Fontaine, D. Guyader, A. Laurent, V. Leroy, P. Marche, D. Salmon, V. Thibault, V. Vilgrain, J. Zucman-Rossi, C. Cagnot (ANRS), and V. Petrov-Sanchez (ANRS).
Clinical centers (ward/participating physicians): CHU Jean Verdier, Bondy (P. Nahon, V. Bourcier); CHU Cochin, Paris (S. Pol, H. Fontaine); CHU Pitié-Salpétrière, Paris (Y. Benhamou); CHU Saint-Antoine, Paris (L. Serfaty); CHU Avicenne, Bobigny (D. Roulot); CHU Beaujon, Clichy (P. Marcellin); CHU Henri Mondor (A. Mallat); CHU Paul Brousse (P. Attali); CHU Tenon, Paris (J.D. Grangé); CHRU Hôpital Nord, Amiens (D. Capron); CHU Angers (P. Calès); Hôpital Saint-Joseph, Marseille (M. Bourlière); CHU Brabois, Nancy (J.P. Bronowicki); Hôpital Archet, Nice (A. Tran); Institut Mutualiste Montsouris, Paris (F. Mal, C. Christidis); CHU Poitiers (C. Silvain); CHU Pontchaillou, Rennes (D. Guyader); CH Pays d'Aix, Aix-en-Provence (C. Wartelle); CHU Jean Minjoz, Besancon (V. Di Martino); CHU Bordeaux–Hôpital Haut-Leveque, Pessac (V. de Ledinghen); CHU Bordeaux–Hôpital Saint-André, Bordeaux (J.F. Blanc); CHU Hôtel Dieu, Lyon (C. Trepo, F. Zoulim); CHU Clermont-Ferrand (A. Abergel); Hôpital Foch, Suresnes (S. Hillaire); CHU Caen (T. Dao); CHU Lille (P. Mathurin); CH Le Mans (C. Pilette); CHU Michallon, Grenoble (J.P. Zarski); CHU St Eloi, Montpellier (D. Larrey); CHU Reims (B. Bernard-Chabert); CHU Rouen (O. Goria, G. Riachi); Institut Arnaud Tzanck, St Laurent-du-Var (D. Ouzan); CHU Purpan, Toulouse (J.M. Péron, L.Alric); CHU Tours (Y. Bacq).




