Serum Angiopoietin-2 Predicts Mortality and Kidney Outcomes in Decompensated Cirrhosis
Abstract
Acute kidney injury in decompensated cirrhosis has limited therapeutic options, and novel mechanistic targets are urgently needed. Angiopoietin-2 is a context-specific antagonist of Tie2, a receptor that signals vascular quiescence. Considering the prominence of vascular destabilization in decompensated cirrhosis, we evaluated Angiopoietin-2 to predict clinical outcomes. Serum Angiopoietin-2 was measured serially in a prospective cohort of hospitalized patients with decompensated cirrhosis and acute kidney injury. Clinical characteristics and outcomes were examined over a 90-day period and analyzed according to Angiopoietin-2 levels. Primary outcome was 90-day mortality. Our study included 191 inpatients (median Angiopoietin-2 level 18.2 [interquartile range 11.8, 26.5] ng/mL). Median Model for End-Stage Liver Disease (MELD) score was 23 [17, 30] and 90-day mortality was 41%. Increased Angiopoietin-2 levels were associated with increased mortality (died 21.9 [13.9, 30.3] ng/mL vs. alive 15.2 [9.8, 23.0] ng/mL; P < 0.001), higher Acute Kidney Injury Network stage (stage I 13.4 [9.8, 20.1] ng/mL vs. stage II 20.0 [14.1, 26.2] ng/mL vs. stage III 21.9 [13.0, 29.5] ng/mL; P = 0.002), and need for renal replacement therapy (16.5 [11.3, 23.6] ng/mL vs. 25.1 [13.3, 30.3] ng/mL; P = 0.005). The association between Angiopoietin-2 and mortality was significant in unadjusted and adjusted Cox regression models (P ≤ 0.001 for all models), and improved discrimination for mortality when added to MELD score (integrated discrimination increment 0.067; P = 0.001). Conclusion: Angiopoietin-2 was associated with mortality and other clinically relevant outcomes in a cohort of patients with decompensated cirrhosis with acute kidney injury. Further experimental study of Angiopoietin/Tie2 signaling is warranted to explore its potential mechanistic and therapeutic role in this population.
Abbreviations
-
- ACLF
-
- acute-on-chronic liver failure
-
- AKI
-
- acute kidney injury
-
- AKIN
-
- Acute Kidney Injury Network
-
- Angpt-2
-
- Angiopoietin-2
-
- APRI
-
- aspartate aminotransferase to platelet ratio index
-
- ATN
-
- acute tubular necrosis
-
- CLIF-C ACLF
-
- Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure
-
- HR
-
- hazard ratio
-
- HRS
-
- hepatorenal syndrome
-
- IDI
-
- integrated discrimination index
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NRI
-
- net reclassification index
Hepatorenal syndrome (HRS) and acute kidney injury (AKI) are common and devastating complications of portal hypertension and end-stage liver disease.1, 2 Medical therapies for HRS are largely supportive, focused on mitigating the clinical manifestations of pathophysiology. HRS is caused by splanchnic vasodilation, which subsequently leads to decreased effective circulating volume, systemic vasoconstriction, and, ultimately, renal hypoperfusion.3 However, volume expansion (intravenous albumin) and splanchnic vasoconstrictors (terlipressin) effectively treat HRS in less than half of patients,4, 5 suggesting mechanistic pathways remain under-addressed by contemporary therapy.
Serum creatinine, as a marker of kidney function, is a key component of the Model for End-Stage Liver Disease (MELD)-Na mortality risk score.6, 7 However, MELD score underperforms in patients with HRS,8 further suggesting that there is a need to better phenotype this population. Emerging evidence implicates systemic inflammation and increased vascular permeability in acute-on-chronic liver failure (ACLF) and decompensated cirrhosis as potential contributors to the hemodynamic and circulatory dysfunction of HRS.9-11 Therefore, there is a pressing need to explore mechanisms related to inflammation and vascular function that may contribute to HRS and AKI in cirrhosis.
The Angiopoietin/Tie2 signaling axis is an important regulator of vascular integrity. Tie2 receptors are diffusely expressed on endothelial cells.12 When activated, Tie2 signaling fortifies interendothelial junctions and reduces the expression of leukocyte adhesion molecules.13, 14 Angiopoietin-2 (Angpt-2) is a context-specific antagonist of the Tie2 receptor that potentiates permeability and vascular inflammation by weakening adherens junctions, recruiting inflammatory cells, and promoting dysregulated thrombosis in the microvasculature.15-17 Targeted manipulations of Angiopoietin/Tie2 signaling—whether by genetic approaches, antibodies, or RNA interference, reported by independent groups—consistently implicate excess Angpt-2 in the end-organ injury and hemodynamic alterations that arise in experimental sepsis and liver disease.18-23 Notably, Angpt-2 is upregulated in human patients with hepatocellular carcinoma,24 advanced liver fibrosis,25 and kidney disease.26, 27 Therefore, our aim was to evaluate serum Angpt-2 levels in a population of hospitalized patients with decompensated cirrhosis and AKI for its ability to predict mortality. We also investigated the association of Angpt-2 levels with the causes and severity of AKI.
Patients and Methods
Population and Setting
From 2013 to 2017, adults (age 18 years or older) admitted to the Massachusetts General Hospital who met eligibility criteria were enrolled and followed prospectively for 90 days from the time of hospital admission. Patients were included if they had decompensated cirrhosis and AKI. Patients were excluded if they were pregnant, nursing, or had a prior kidney transplant. Three contemporaneous reference groups of patients were included: (1) hospitalized patients with decompensated cirrhosis without AKI, (2) outpatients with cirrhosis and refractory ascites referred for transjugular intrahepatic portosystemic shunt (TIPS) placement, and (3) outpatients with compensated cirrhosis.
Definitions
Diagnosis and cause of cirrhosis were determined by the treating hepatologist. Cause of cirrhosis was deemed multifactorial if there were ≥2 causes felt to be contributing to liver disease. A member of the research team (A.S.A.) performed an in-depth review of the medical records to ensure accuracy. The Acute Kidney Injury Network (AKIN) criteria were used to diagnose AKI.28 Acute kidney injury was subdivided by cause: (1) prerenal AKI (resolution of AKI within 48 hours of volume administration), (2) acute tubular necrosis (ATN; clinical history consistent with ischemic or nephrotoxic AKI and failure to respond to volume administration), (3) HRS (exclusion of other potential causes of AKI based on International Club of Ascites Criteria),1 or (4) other AKI (e.g., serologic or histologic evidence of glomerulonephritis, obstruction). Two members of the study team (A.S.A. and X.V.P.) adjudicated the cause of AKI, with a third member (S.K.) providing a tie-breaking diagnosis, if needed. Patients were classified as having an infection on a clinical basis, using culture and cell count data, or if they received antibiotics.
Data Collection
All data were abstracted from the medical records. MELD and Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure (CLIF-C ACLF) scores were calculated at the time of admission.6, 7, 29, 30 Patients provided serum and urine samples on enrollment and day 5 and 30 after enrollment (as available). Laboratory data were recorded from values closest to enrollment sample collection. Members of the study team did not participate or intervene in the clinical care of patients. Outcomes for this study, including the primary outcome of all-cause mortality, were determined at 90 days following the day of index hospitalization.
Measurement of Serum Angiopoietin-2
Serum Angpt-2 levels were measured using a commercially available quantitative enzyme-linked immunosorbent assay (ELISA; Catalog number DANG20, Human Angiopoietin-2 Quantikine ELISA kit, R and D systems, MN) and reported in ng/mL. The coefficient of variation for this assay was <10 %. All study samples were processed within 4 hours of collection and stored at −80°C, with Angpt-2 assays performed in batch at the end of the study. Results of Angpt-2 testing were not reported to the clinical team or to patients.
Analyses
Patients were divided into tertiles by serum Angpt-2 level at enrollment. The primary outcome of 90-day all-cause mortality among inpatients with decompensated cirrhosis was visualized using a Kaplan-Meier curve and compared across tertiles using a log-rank test. Multiple prespecified Cox regression models were used to evaluate the association between Angpt-2 level as a continuous variable and mortality. These included unadjusted models and models adjusted for MELD score, CLIF-C ACLF score, AKIN stage, cause of AKI, and presence of infection. Independent unadjusted models evaluating MELD and CLIF-C ACLF scores prediction of 90-day mortality were reported as reference. Three sensitivity analyses were performed: (1) excluding prior liver transplant recipients (n = 5), (2) excluding patients with hepatocellular carcinoma (n = 20), and (3) using Fine and Gray regression for a composite outcome of liver transplant or death by 90 days to account for these competing risks.31 Results of Cox models were summarized with hazard ratios (HR) and Wald asymptotic 95% confidence intervals (CI). Continuous variables were presented as median [interquartile range] given nonparametric distribution and categorical variables as percentages. The data were compared using a chi-squared test or a Fisher's exact test (for categorical variables), a Wilcoxon rank sum test (for comparison of differences in continuous variables between two independent groups), or a Pearson correlation test (for two continuous variables, with log transformation for nonparametric data). The incremental improvement of adding Angpt-2 to the existing predictive models of cirrhosis (MELD score, MELD-Na score, and CLIF-C ACLF score) was measured by change in C statistic, category-free net reclassification index (NRI), and integrated discrimination index (IDI).32 SAS version 9.4 (Cary, NC) was used for all analyses. Two-tailed P value < 0.05 was considered statistically significant.
Ethics Statement
The Partners Healthcare Institutional Review Board approved this study. All procedures and practices abide by guidelines set forth by the Declarations of Helsinki and Istanbul. Patients (or their health care designee) provided a written informed consent.
Results
Demographics and Clinical Characteristics
Our study included 191 inpatients. The median length of stay was 15 [8, 23] days. The median time from admission to initial sample collection was 4 [2,7] days. The median age was 57 [49, 64] years, 27% were female, 91% were of white race, and 88% were of non-Hispanic ethnicity (see Table 1). The median admission MELD score was 23 [17, 30] and CLIF-C ACLF score was 44 [39, 50]. Serum creatinine was 1.9 [1.3, 3.0] mg/dL at the time of initial Angpt-2 measurement. Twenty patients (10%) had hepatocellular carcinoma. Sixty-six patients (35%) had evidence of infection. Twenty-eight patients (15%) received a liver transplant during follow-up. Among those with AKI, 48 patients (28%) had stage I AKI, 40 patients (23%) had stage II AKI, 86 patients (49%) had stage III AKI, and 49 patients (26%) required renal replacement therapy during follow-up.
| Characteristics | Low Tertile <13.5 ng/mL (n = 64) | Mid Tertile (n = 64) | High Tertile >23.0 ng/mL (n = 63) | P Value |
|---|---|---|---|---|
| Age (years) | 55 [48, 61] | 57 [49, 66] | 59 [49, 64] | 0.23 |
| Female sex (%) | 15 (25) | 17 (26) | 18 (29) | 0.90 |
| White race (%) | 54 (85) | 59 (91) | 59 (94) | 0.32 |
| Non-Hispanic ethnicity (%) | 52 (83) | 57 (88) | 59 (94) | 0.16 |
| Body mass index (kg/m2) | 28 [24, 33] | 29 [23, 33] | 28 [25, 32] | 0.73 |
| Comorbidities (%) | ||||
| Diabetes mellitus | 18 (30) | 20 (32) | 12 (20) | 0.28 |
| Chronic kidney disease | 25 (40) | 16 (25) | 12 (20) | 0.05 |
| Cardiovascular disease | 13 (21) | 10 (15) | 14 (22) | 0.59 |
| Hypertension | 25 (40) | 22 (35) | 24 (38) | 0.88 |
| Primary reason for admission (%) | 0.47 | |||
| Complications of liver disease | 30 (51) | 38 (59) | 38 (61) | |
| Acute kidney injury | 10 (17) | 11 (17) | 9 (15) | |
| Infectiona | 7 (12) | 10 (16) | 9 (15) | |
| Other | 12 (20) | 5 (8) | 6 (10) | |
| Etiology of cirrhosis (%) | 0.26 | |||
| Hepatitis C | 7 (11) | 14 (22) | 12 (19) | |
| Alcohol | 25 (40) | 19 (29) | 14 (23) | |
| Nonalcoholic steatohepatitis | 3 (5) | 5 (8) | 10 (16) | |
| Multifactorial | 17 (27) | 16 (25) | 15 (24) | |
| Other | 11 (17) | 11 (17) | 11 (18) | |
| Prior complications of liver disease (%) | ||||
| Ascites requiring prior paracentesis | 33 (52) | 40 (62) | 41 (65) | 0.32 |
| Encephalopathy | 15 (24) | 21 (32) | 26 (41) | 0.11 |
| Gastrointestinal bleeding | 14 (22) | 9 (14) | 11 (17) | 0.46 |
| Spontaneous bacterial peritonitis | 4 (6) | 8 (12) | 10 (16) | 0.24 |
| Hepatocellular carcinoma | 4 (6) | 7 (11) | 9 (14) | 0.35 |
| Prior liver transplantation (%) | 5 (8) | 0 (0) | 0 (0) | 0.001 |
| MELD score | 19 [16, 27] | 23 [17, 30] | 26 [22, 33] | <0.001 |
| CLIF-C ACLF score | 41 [33, 47] | 44 [39, 50] | 48 [43, 55] | <0.001 |
| APRI | 1.5 [0.8, 3.0] | 2.1 [1.5, 3.5] | 3.6 [1.8, 6.4] | <0.001 |
| Laboratory values | ||||
| Sodium (mEq/L) | 134 [130, 138] | 133 [130, 138] | 134 [130, 138] | 0.81 |
| Creatinine (mg/dL) | 1.8 [1.3, 2.7] | 1.9 [1.3, 2.5] | 2.4 [1.6, 3.6] | 0.04 |
| White blood count (K/uL) | 6.5 [4.4, 10.3] | 7.6 [5.2, 10.6] | 8.5 [6.2, 17.7] | 0.006 |
| Hemoglobin (g/dL) | 9.0 [8.0, 10.8] | 8.9 [7.8, 10.0] | 8.9 [8.0, 10.2] | 0.53 |
| Platelets (K/uL) | 100 [69, 145] | 83 [53, 108] | 71 [54, 113] | 0.05 |
| Albumin (g/dL) | 3.0 [2.5, 3.5] | 3.2 [2.6, 3.5] | 3.1 [2.6, 3.5] | 0.67 |
| International normalized ratio (INR) | 1.4 [1.3, 1.8] | 1.7 [1.5, 1.9] | 2.0 [1.6, 2.4] | <0.001 |
| Total bilirubin (mg/dL) | 2.1 [1.2, 5.1] | 4.8 [2.4, 10.4] | 10.4 [3.8, 24.8] | <0.001 |
| Aspartate aminotransferase (U/L) | 45 [32, 70] | 59 [41, 79] | 84 [54, 188] | 0.005 |
| Alanine aminotransferase (U/L) | 21 [14, 42] | 26 [19, 43] | 32 [21, 100] | 0.02 |
| Alkaline phosphatase (U/L) | 117 [79, 168] | 108 [85, 146] | 126 [88, 166] | 0.52 |
- Continuous variables given as median [interquartile range].
- * Includes only infections listed as primary reason for admission. For any infection during admission: n = 18 (low tertile), n = 20 (mid tertile), n = 28 (high tertile); P = 0.15.
- Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; CLIF-C ACLF, Chronic Liver Failure Consortium Organ Failure Acute-on-Chronic Liver Failure Score; MELD, Model for End-Stage Liver Disease.
Serum Angiopoietin-2 Levels
Serum Angpt-2 levels were measured in 191 hospitalized patients (176 with cirrhosis and AKI and 15 reference patients with cirrhosis and no AKI). The median enrollment Angpt-2 level was 18.2 [11.2, 26.5] ng/mL. Ninety-three patients also had Angpt-2 measured on day 5, and 40 patients had Angpt-2 measured on day 30. Among the 93 patients with interval samples, Angpt-2 levels were stable between enrollment (19.3 [11.8, 26.4] ng/mL) and day 5 (18.1 [12.5, 27.9]; P = 0.94). Patients were divided into tertiles by Angpt-2 level at enrollment: low tertile (Angpt-2 < 13.5 ng/mL; n = 64), mid tertile (Angpt-2 between 13.5 and 23.0 ng/mL; n = 64), and high tertile (Angpt-2 > 23.0 ng/mL; n = 63). Patients with higher Angpt-2 levels had higher MELD scores (low Angpt-2 tertile 19 [16, 27] vs. mid Angpt-2 tertile 23 [17, 30] vs. high Angpt-2 tertile 26 [22, 33]; P < 0.001) and higher CLIF-C ACLF scores (low tertile 41 [33, 47] vs. mid tertile 44 [39, 50] vs. high tertile 48 [43, 55]; P < 0.001). There was no difference across Angpt-2 tertiles by the etiology of cirrhosis (P = 0.26). Although patients with cirrhosis and AKI had higher MELD scores (24 [18, 31] vs. 18 [15, 22]; P = 0.004) and higher CLIF-C ACLF scores (45 [40, 51] vs. 38 [30, 41]; P < 0.001), Angpt-2 levels were comparable between those with cirrhosis and AKI (17.9 [11.7, 27.5] ng/mL) and those with cirrhosis and no AKI (21.4 [12.6, 22.5] ng/mL; P = 0.89). In outpatient reference groups, Angpt-2 was 7.1 [4.4, 11.1] ng/mL among 18 patients with decompensated cirrhosis referred for TIPS for refractory ascites and was 2.3 [1.6, 3.3] ng/mL among 10 patients with compensated cirrhosis (see Supporting Table S1).
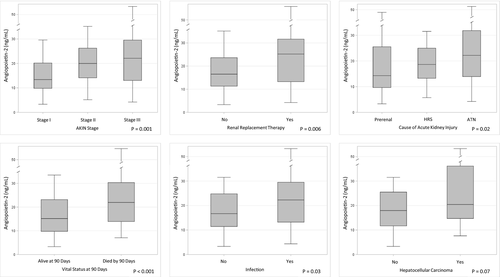
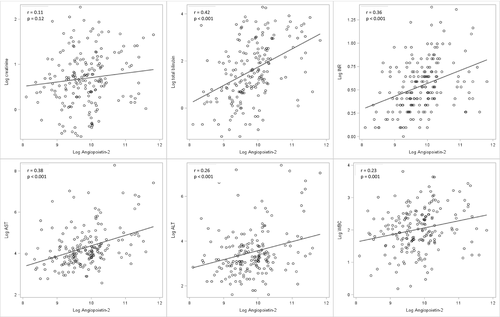
Evaluation of continuous Angpt-2 levels against clinical outcomes are presented in Fig. 1. Higher Angpt-2 was associated with higher AKI stage (stage I 13.4 [9.8, 20.1] ng/mL vs. stage II 20.0 [14.1, 26.2] ng/mL vs. stage III 21.9 [13.0, 29.5] ng/mL; P = 0.002) and the need for renal replacement therapy (no renal replacement 16.5 [11.3, 23.6] ng/mL vs. need for renal replacement 25.1 [13.3, 30.3] ng/mL; P = 0.005). Among those with AKI, 61 patients (35%) were diagnosed with prerenal AKI, 54 patients (31%) were diagnosed with HRS, 30 patients (29%) were diagnosed with ATN, and 10 patients (6%) were diagnosed with other AKI. Angpt-2 was lower in those diagnosed with prerenal AKI (14.3 [9.6, 25.5] ng/mL) compared with HRS (18.6 [13.2, 25.0] ng/mL) and ATN (22.0 [13.9, 31.8] ng/mL; P = 0.02). Angpt-2 was higher with presence of infection (no infection 16.7 [11.4, 24.8] ng/mL vs. infection 22.1 [13.2, 29.5] ng/mL; P = 0.03), and trended toward statistical association with presence of hepatocellular carcinoma (no hepatocellular carcinoma 17.9 [11.7, 25.5] ng/mL vs. hepatocellular carcinoma 20.4 [14.7, 51.1] ng/mL; P = 0.07). To account for heterogeneity in sample collection timing, these analyses were repeated after exclusion of samples in the top quartile of time from admission to sample collection (>7 days). Among the remaining 144 patients, all Fig. 1 analyses remained stable, except for Angpt-2's association with cause of AKI, which crossed the P value threshold to 0.055). Pearson correlation between continuous serum Angpt-2 and several clinical variables (with log transformation for skewed distributions) was reported in Fig. 2, including serum creatinine, total bilirubin, international normalized ratio (INR), aspartate aminotransferase, alanine aminotransferase, and white blood cell count.
During the study period, 66/176 (38%) patients had resolution of AKI (creatinine improved within 0.3 mg/dL of baseline). Patients with AKI resolution had lower initial Angpt-2 levels (14.5 [9.7, 24.8] ng/mL vs. 19.4 [13.0, 28.0] ng/mL; P = 0.001). Of 176 patients, 57 (32%) had progression of AKI (increase in AKI stage or presenting with AKI stage III and later requiring dialysis). Patients with AKI progression had higher initial Angpt-2 levels (21.6 [13.0, 30.0] ng/mL vs. 16.1 [11.3, 25.0] ng/mL; P = 0.03).
Angiopoietin-2 and Mortality
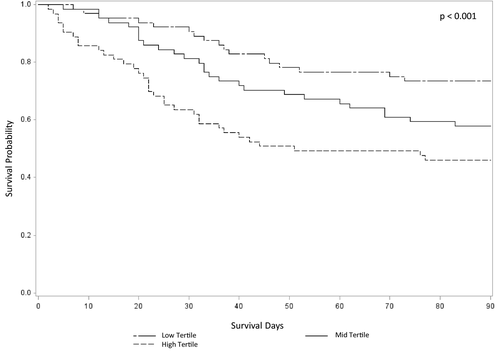
Of 191 patients, 78 (41%) died within 90 days of follow-up. Initial Angpt-2 levels were higher among those who died compared with those who survived (died 21.9 [13.9, 30.3] ng/mL vs. alive 15.2 [9.8, 23.0] ng/mL; P < 0.001). Kaplan-Meier analysis of survival over 90 days by tertiles of Angpt-2 suggested a graded association (Fig. 3; P < 0.001). Clinical characteristics by vital status at 90 days are presented in Table 2. In addition to serum Angpt-2, there were several significant predictors of 90-day mortality in univariate analyses. Comparing alive versus died by 90 days, these included age, presence of hepatocellular carcinoma, MELD score, CLIF-C ACLF score, AKIN stage, need for renal replacement therapy, and cause of AKI. Among 93 patients with interval samples collected, there was no significant change in Angpt-2 between enrollment and day 5 when stratifying by vital status at 90 days: for 51 patients alive at day 90 (enrollment 15.6 [11.4, 29.4] ng/mL vs. day 5 15.0 [8.4, 23.6] ng/mL; P = 0.22) or for 42 patients who died by day 90 (enrollment 20.7 [14.5, 26.1] ng/mL vs. day 5 19.7 [14.4, 37.6] ng/mL; P = 0.15).
| Characteristics | Alive at 90 days (n = 113) | Died by 90 days (n = 78) | P Value |
|---|---|---|---|
| Age (years) | 56 [47, 61] | 60 [52, 66] | 0.002 |
| Female sex (%) | 31 (27) | 20 (26) | 0.78 |
| White race (%) | 100 (89) | 73 (94) | 0.24 |
| Non-Hispanic ethnicity (%) | 96 (85) | 73 (94) | 0.07 |
| Etiology of cirrhosis (%) | 0.65 | ||
| Hepatitis C | 23 (21) | 10 (13) | |
| Alcohol | 35 (31) | 23 (30) | |
| Nonalcoholic steatohepatitis | 10 (9) | 8 (10) | |
| Multifactorial | 28 (25) | 20 (26) | |
| Other | 17 (15) | 16 (21) | |
| Hepatocellular carcinoma (%) | 6 (5) | 14 (18) | 0.005 |
| Presence of infection (%) | 38 (34) | 28 (36) | 0.75 |
| MELD score | 22 [17, 29] | 26 [18, 32] | 0.02 |
| CLIF-C ACLF score | 43 [36, 48] | 46 [42, 53] | 0.001 |
| APRI | 1.9 [1.0, 4.0] | 2.3 [1.5, 5.4] | 0.11 |
| Laboratory values | |||
| Sodium (mEq/L) | 133 [130, 137] | 134 [130, 139] | 0.40 |
| Creatinine (mg/dL) | 1.7 [1.2, 3.4] | 2.4 [1.8, 3.6] | <0.001 |
| White blood cell count (K/uL) | 6.8 [4.9, 10.4] | 8.9 [5.5, 13.6] | 0.02 |
| Hemoglobin (g/dL) | 8.9 [7.9, 10.2] | 9.1 [8.1, 10.4] | 0.38 |
| Platelets (K/uL) | 87 [57, 131] | 74 [55, 109] | 0.35 |
| Albumin (g/dL) | 2.9 [2.5, 3.4] | 3.3 [2.7, 3.6] | 0.02 |
| International normalized ratio (INR) | 1.6 [1.4, 2.0] | 1.8 [1.5, 2.1] | 0.06 |
| Total bilirubin (mg/dL) | 3.7 [1.6, 9.1] | 7.0 [2.4, 20.2] | 0.006 |
| Aspartate aminotransferase (U/L) | 55 [41, 78] | 70 [41, 127] | 0.08 |
| Alanine aminotransferase (U/L) | 24 [17, 44] | 29 [20, 63] | 0.17 |
| Alkaline phosphatase (U/L) | 112 [86, 158] | 113 [80, 166] | 0.65 |
| AKIN stage (%)a | 0.001 | ||
| Stage I | 36 (36) | 12 (16) | |
| Stage II | 26 (26) | 14 (19) | |
| Stage III | 38 (38) | 48 (65) | |
| Required renal replacement therapy (%)a | 21 (19) | 28 (36) | 0.007 |
| Cause of Acute Kidney Injury (%)a | 0.02 | ||
| Prerenal acute kidney injury | 42 (46) | 19 (26) | |
| Hepatorenal syndrome | 26 (29) | 28 (38) | |
| Acute tubular necrosis | 23 (25) | 27 (36) | |
| Serum Angiopoietin-2 (ng/mL) | 15.2 [9.8, 23.0] | 21.9 [13.9, 30.3] | <0.001 |
- Continuous variables given as median [interquartile range].
- * Among 176 patients with acute kidney injury.
- † Excluding 10 patients with other AKI.
- Abbreviations: AKIN, Acute Kidney Injury Network; APRI, aspartate aminotransferase to platelet ratio index; CLIF-C ACLF, Chronic Liver Failure Consortium Organ Failure Acute-on-Chronic Liver Failure Score; MELD, Model for End-Stage Liver Disease.
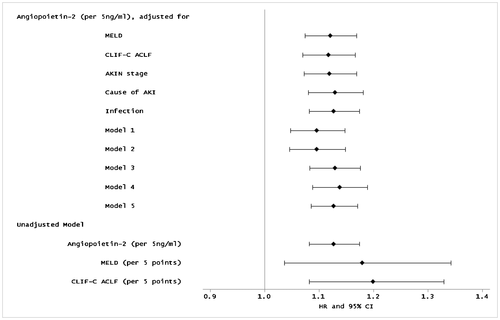
Multivariable Cox regression models to evaluate Angpt-2's ability to predict 90-day mortality are presented in Fig. 4, with hazard ratios reflecting risk per 5 ng/mL change in Angpt-2. In unadjusted models, higher serum Angpt-2 was significantly associated with mortality (HR 1.13 [95% CI 1.08, 1.17]; P < 0.001). Similarly, all adjusted models demonstrated a stable association between Angpt-2 level and increased mortality, including adjustment for (1) MELD score (HR 1.12 [1.07, 1.17]; P < 0.001), (2) CLIF-C ACLF score (HR 1.12 [1.07, 1.17]; P < 0.001), (3) AKIN stage (HR 1.12 [1.07, 1.17]; P < 0.001), (4) cause of AKI (HR 1.13 [1.08, 1.18]; P < 0.001), (5) presence of infection (HR 1.13 [1.08, 1.17]; P < 0.001), (6) MELD score and age (HR 1.09 [1.05, 1.15]; P < 0.001), and (7) MELD score, age, and presence of infection (HR 1.09 [1.04, 1.15]; P = 0.001). In sensitivity analyses, Angpt-2 was associated with increased mortality after (1) excluding 5 patients with a history of prior liver transplant (HR 1.13 [1.08, 1.18]; P < 0.001), (2) excluding 20 patients with a history of hepatocellular carcinoma (HR 1.14 [1.09, 1.19]; P < 0.001), and (3) in a competing risk analysis for liver transplant or death (HR 1.13 [1.09, 1.17]; P < 0.001). Exclusion of samples collected >7 days from admission did not change results (HR 1.12 [1.08, 1.17]; P < 0.001), nor did adjusting for time from admission to sample collection (HR 1.13 [1.08, 1.17]; P < 0.001).
To compare the added usefulness of serum Angpt-2 to existing prediction models for 90-day mortality in this population, models containing Angpt-2, MELD, MELD-Na, and CLIF-C ACLF scores were compared using C statistics, category-free NRI, and IDI for their ability to predict 90-day mortality (see Table 3). As independent measures, C statistic for prediction of 90-day mortality was 0.65 for serum Angpt-2, 0.59 for MELD score, 0.57 for MELD-Na score, and 0.64 for CLIF-C ACLF score. Addition of serum Angpt-2 to MELD score significantly improved its discrimination for 90-day mortality by change in C statistic (0.59 to 0.66; P = 0.03), IDI (0.067; P = 0.001), but not category-free NRI (0.22; P = 0.15). Addition of serum Angpt-2 to MELD-Na score significantly improved its discrimination for 90-day mortality by change in C statistic (0.57 to 0.66; P = 0.04) and IDI (0.071; P < 0.001), but not category-free NRI (0.21; P = 0.17).
| Angpt-2 | MELD | MELD + Angpt-2 | P Value | MELD-Na | MELD-Na + Angpt-2 | P Value | CLIF-C ACLF | CLIF-C ACLF + Angpt-2 | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| C statistic | 0.65 | 0.59 | 0.66 | 0.03 | 0.57 | 0.66 | 0.04 | 0.64 | 0.68 | 0.14 |
| Category-free NRI | 0.22 | 0.15 | 0.21 | 0.17 | 0.10 | 0.50 | ||||
| IDI | 0.067 | 0.001 | 0.071 | <0.001 | 0.061 | 0.002 |
- Abbreviations: Angpt-2, Angiopoietin-2; CLIF-C ACLF, Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure Score; IDI, integrated discrimination increment; MELD, Model for End-Stage Liver Disease; NRI, net reclassification index.
Angiopoietin-2 Before and After Liver Transplant
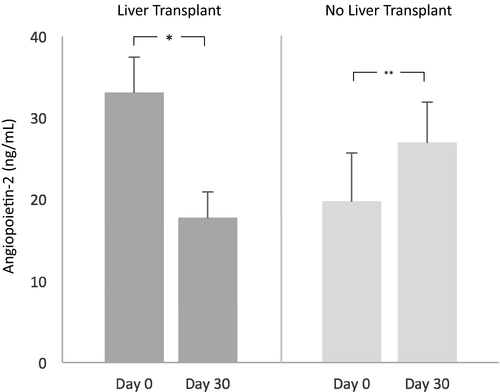
Forty patients had serum Angpt-2 levels measured 30 days after their initial sample. Twelve of those patients underwent liver transplantation between measurements. Angpt-2 decreased significantly from 28.2 [16.6, 33.1] ng/mL to 13.5 [6.1, 17.2] ng/mL after liver transplantation (P = 0.002). Among the remaining 28 patients who did not undergo liver transplantation, 9/28 (32%) died by 90 days, and Angpt-2 increased significantly from 13.5 [9.6, 19.7] ng/mL to 19.7 [11.1, 26.9] ng/mL (P = 0.001) over 30 days in this group (see Fig. 5).
Discussion
In this prospective cohort study, we found that higher serum Angpt-2, a known antagonist of vascular quiescence, was associated with increased mortality and AKI severity in patients with cirrhosis and AKI. As a single measure, Angpt-2 had a similar or better predictive ability for 90-day mortality compared with commonly used prognostic scores such as MELD, MELD-Na, and CLIF-C ACLF scores, and addition of Angpt-2 to these scores improved their discrimination for mortality.
The importance of vascular inflammation in acute-on-chronic liver failure, HRS, and AKI in cirrhosis has increasingly been recognized, although relatively few studies have examined underlying mechanisms. General markers of systemic inflammation such as pro-inflammatory cytokines have been reported with increasing ACLF grades and associated increased mortality.9, 10 Studies of systemic and nonabsorbable antibiotics in cirrhosis have demonstrated that such treatments can decrease inflammatory cytokines and improve hemodynamic circulation in the context of intestinal decontamination and decreased gut bacterial translocation.33-38 However, HRS is not completely preventable by antibiotics, nor can we reverse HRS associated with infection by treatment of infection alone.39, 40 Even with regimens including supportive care and HRS-directed therapies such as terlipressin and intravenous albumin, there is an increased HRS treatment-failure rate at higher ACLF grades.41 Therapies focusing on new pathways around an elevated inflammatory state may improve outcomes of these critically ill patients.
Tie2 signaling has been shown to be a pivotal molecular switch that toggles the vasculature between quiescence and inflammation. As a cell-surface receptor modulated by secreted ligands, Tie2 and Angiopoietins constitute a highly promising set of candidate drug targets for human disease. In experimental models of sepsis, systemic inflammatory syndrome, and disseminated intravascular coagulation, Angpt-2 upregulation and Tie2 inhibition have been linked to thrombocytopenia, increased INR, elevated D-dimer, overexpression of pro-inflammatory cytokines, multisystem organ dysfunction (e.g., AKI), decreased systemic circulating blood pressure, and ultimately poorer survival; conversely, interventions to restore or enhance Tie2 signaling not only ameliorate these abnormalities but also improve survival.16, 18, 23, 42 Strikingly, this same constellation of features is present among patients with decompensated cirrhosis and ACLF.10, 14, 29, 43, 44 Although there are no studies, to our knowledge, specifically examining Angpt-2/Tie2 interventions in combined liver-kidney injury animal models, our prospective human findings suggest that this pathway may be similarly important in HRS/AKI and cirrhosis.
Our results show robust multivariable adjusted relationships between Angpt-2 and outcomes in cirrhosis and AKI, with consistent effect sizes after adjustment for multiple potential confounders. We controlled for characteristics with known associations with Angpt-2 (infection, hepatocellular carcinoma) as well as mortality in cirrhosis (MELD and CLIF-C ACLF scores) and observed durable statistical significance between Angpt-2 levels and survival. In our cohort, severity of AKI was associated with higher risk of death, seen in both serum creatinine and AKI stage in univariate analysis, as well as in the increased MELD points given to those with worsening kidney function. Consistent with another important metric in current guidelines, those who experienced AKI progression had the highest initial Angpt-2 levels (21.6 ng/mL), and those who had AKI resolution had the lowest initial levels (14.5 ng/mL).1 Even then, addition of Angpt-2 to the MELD score still added incremental value in predicting mortality, and as a single lab test, Angpt-2 had a slightly better C statistic than the composite of the MELD score. The limitations of the MELD score have been debated since its adoption,45 and other studies of markers of inflammation or kidney injury have shown the potential to augment its prognostic ability.46, 47 The specific interest around Angpt-2 extends beyond its association with mortality, given its potential as a mechanistic regulator in other similar disease models. Although this study is not designed to examine the causal relationship between Angpt-2 and HRS/AKI in cirrhosis, this strong prospective association warrants further investigation. Angpt-2 may not only help predict outcomes, but in future trials that evaluate vascular stabilizing strategies, Angpt-2 levels could be used to enrich for at-risk participants, monitor pharmacodynamics drug responses, and even serve as a surrogate of efficacy in early-stage investigations.
One important observation was that our inpatient reference group (decompensated cirrhosis without AKI) had similar Angpt-2 levels to cirrhosis with AKI. Although small sample size (n = 15) may contribute to this lack of statistical significance, it should be noted that elevated Angpt-2 may be a reflection of overall acute decompensation of liver disease, rather than from the AKI itself. Our reference group of outpatients with cirrhosis had lower Angpt-2 levels than those hospitalized with ACLF, and were in the same range as in previously published outpatient cirrhotic levels.24 Angpt-2's molecular weight (66 kDa) is similar to that of albumin, a molecule only found in urine if there is damage to the glomerular basement membrane.48 To our knowledge, detectable urine levels of Angpt-2 have only been reported in the context of albuminuria in diabetic nephropathy.49 This makes an increase in Angpt-2 due to poor renal clearance itself unlikely. We generally view HRS/AKI in cirrhosis as a sequela of overall liver decompensation, rather than an inciting incident. If the Angpt-2 pathway proves to be a causative mechanism of morbidity/end organ damage in ACLF, its potential as a druggable target may be less dependent on the presence of AKI and more so on the degree of overall decompensation or inflammation. These conclusions around the broader implications of Angpt-2 across a wide spectrum of liver disease are tempered by small sample size and require validation in larger cohorts.
Finally, we noted that Angpt-2 decreased dramatically following liver transplantation, suggesting that serum levels may be influenced directly or indirectly by liver fibrosis or hepatic synthetic function. Angpt-2 is highly expressed in the liver endothelium and has been shown to be important for hepatic regeneration.21, 48 In this context, one could speculate that hepatically derived Angpt-2 may represent the molecular equivalent of regenerating nodules. There may be some additional effect from transplant immunosuppressive medications on serum Angpt-2 levels as well, although this is hard to quantify. Because our sample size of eventual transplant recipients with pre- and posttransplant samples in this cohort was small (n = 12), additional study of this subgroup will be needed to draw firm conclusions. The stability of Angpt-2 levels over 5-day intervals among the 93 patients with repeat testing available and the similar results seen when adjusting for time from admission to sample collection suggest that the timing of testing was not greatly confounded by inpatient medical therapy or short-term evolution of clinical status.
This study should be interpreted in the context of its limitations. This is a single-center study, limiting generalizability. This is highlighted by the relatively low predictive value of validated prognostic scores such as MELD, MELD-Na, and CLIF-C ACLF, compared with other populations of patients with liver disease.50 Results likely best apply to hospitalized patients with decompensated cirrhosis and AKI, and further study should be performed across a broader spectrum of patients with advanced liver disease. Our study was not powered to adjust for all potential clinical confounders. Despite these limitations, we believe our results provide an important extension of the potential role of Angpt-2 in patients with cirrhosis.
In conclusion, serum Angiopoietin-2 levels were strongly associated with mortality and other relevant clinical outcomes in a cohort of patients with decompensated cirrhosis hospitalized with acute kidney injury. Future study to examine the link between vascular inflammation and altered vascular activation and clinical outcomes in advanced kidney and liver disease are required. Angiopoietin/Tie2 signaling represents a potential therapeutic target in this population and requires further experimental study to validate its mechanism.
Potential conflict of interest
Dr. Allegretti consults for Ferring. Dr. Chung received grants from Gilead, AbbVie, Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Janssen, and Roche. Dr. Fuchs consults for Gilead. He received grants from Enanta, Blade, and Collagen Medical.




