The NACSTOP Trial: A Multicenter, Cluster-Controlled Trial of Early Cessation of Acetylcysteine in Acetaminophen Overdose
Abstract
Historically, intravenous acetylcysteine has been delivered at a fixed dose and duration of 300 mg/kg over 20 to 21 hours to nearly every patient deemed to be at any risk for hepatotoxicity following acetaminophen overdose. We investigated a 12-hour treatment regimen for selected low-risk patients. This was a multicenter, open-label, cluster-controlled trial at six metropolitan emergency departments. We enrolled subjects following single or staggered acetaminophen overdose with normal serum alanine transaminase (ALT) and creatinine on presentation and at 12 hours, and less than 20 mg/L acetaminophen at 12 hours. Patients were allocated to intervention (250 mg/kg over 12-hour) or control (300 mg/kg over 20-hour) regimens by site. The primary outcome was incidence of “hepatic injury” 20 hours following initiation of acetylcysteine treatment, defined as ALT doubling and peak ALT greater than 100 IU/L, indicating the need for further antidotal treatment. Secondary outcomes included incidence of hepatotoxicity (ALT > 1,000 IU/L), peak international normalized ratio (INR), and adverse drug reactions. Of the 449 acetaminophen overdoses receiving acetylcysteine, 100 were recruited to the study. Time to acetylcysteine (median 7 hours [interquartile ratio 6,12] versus 7 hours [6,10]) and initial acetaminophen (124 mg/L [58,171] versus 146 mg/L [66,204]) were similar between intervention and control groups. There was no difference in ALT (18 IU/L [13,22] versus 16 IU/L [13,21]) or INR (1.2 versus 1.2) 20 hours after starting acetylcysteine between groups. No patients developed hepatic injury or hepatotoxicity in either group (odds ratio 1.0 [95% confidence interval 0.02, 50]). No patients represented with liver injury, none died, and 96 of 96 were well at 14-day telephone follow-up. Conclusion: Discontinuing acetylcysteine based on laboratory testing after 12 hours of treatment is feasible and likely safe in selected patients at very low risk of liver injury from acetaminophen overdose.
Abbreviations
-
- ALT
-
- alanine transaminase
-
- APAP
-
- acetaminophen concentration at presentation
-
- AST
-
- aspartate transaminase
-
- CI
-
- confidence interval
-
- ED
-
- emergency department
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- NAAR
-
- nonallergic anaphylactic reaction
-
- NAC
-
- N-acetylcysteine
-
- OR
-
- odds ratio
-
- VAMPIRE
-
- Victorian Austin Monash Poisons Information, Research and Education
Acetaminophen is one of the most common medications taken in overdose around the world.(1) Acetylcysteine (N-acetylcysteine or NAC) is the antidote used to treat patients at risk of developing hepatotoxicity following acetaminophen overdose.(2) The decision to treat cases of single acute acetaminophen overdose with acetylcysteine is based on a timed acetaminophen concentration plotted on a treatment nomogram.(3) The standard acetylcysteine treatment regimen, used for several decades, calls for 300 mg/kg infused over a fixed interval of 20 to 21 hours (i.e., 150 mg/kg of acetylcysteine intravenously [IV] over 15-60 minutes, followed by 50 mg/kg of acetylcysteine over 4 hours and 100 mg/kg of acetylcysteine over 16 hours).(4) To simplify acetylcysteine dosing and reduce adverse reactions during the rapid initial load,(5-7) we recently instituted a two-bag acetylcysteine regimen consisting of 200 mg/kg over 4 hours followed by a further 100 mg/kg over 16 hours.(8, 9) Although the total dose and duration is similar to the standard acetylcysteine regimen,(4) the incidence of adverse reactions is substantially lower.(8-10) However, the fixed duration of 20 hours, which typically necessitates hospital admission, likely represents overtreatment for patients at very low risk, and ignores an opportunity for further risk stratification based on retesting midway through treatment.(11)
The administration of acetylcysteine within 8 hours of an acute single acetaminophen overdose prevents hepatotoxicity in nearly all patients.(4, 12-14) More recent work has shown that patients with normal liver function tests when treatment is started are unlikely to develop hepatotoxicity.(15-17) Moreover, very few patients in whom acetylcysteine is discontinued prematurely develop hepatotoxicity, provided treatment is started within 8 hours of overdose and at least 200 mg/kg are administered.(17) The initial 3-bag, 20-hour intravenous acetylcysteine regimen was first proposed in the 1970s. The 20-hour duration was arbitrarily selected and based on a crude estimate of acetaminophen elimination (i.e., 5 half-lives). This treatment recommendation was made at a time when the serum acetaminophen concentration was not readily measurable in most hospitals.(18) Serial acetaminophen and liver function assays are now easily performed and can provide reassurance that hepatic function is preserved. Indeed, an acetaminophen concentration that falls by at least 90% over 12 hours (i.e., greater than 3 half-lives) is the first readily available test of liver function following overdose, and provides a mathematically simple benchmark for early risk stratification.(17) Acetylcysteine elimination is slower than acetaminophen in low-risk patients, and antidote remains in the circulation for a few hours after cessation of the infusion.(19) A previous randomized trial of 300 mg/kg acetylcysteine administered over 12 hours demonstrated a decrease in adverse reactions to acetylcysteine when compared with the original 20-hour 3-bag regimen, with no sign of diminished efficacy.(6)
Our aim was to test the feasibility and safety of early discontinuation of the 2-bag regimen compared with the full 20-hour course in selected low-risk adult patients based on serial laboratory testing. These low-risk features included normal serum alanine transaminase (ALT) and creatinine both at presentation and after 12 hours of treatment, as well as an acetaminophen concentration that fell into the therapeutic range or lower by 12 hours.
Methods and Materials
Study Setting
This was a multicenter, cluster-controlled, open-label trial of patients with acute single or staggered acetaminophen overdose who presented to the emergency departments (EDs) of six Australian metropolitan hospitals from February 2016 to February 2018. There were three toxicology units, part of the Victorian Austin Monash Poisons Information, Research, and Education network and Western Sydney Toxicology Service, that were involved in managing patients at each hospital.
Study Eligibility and Recruitment
Study investigators and treating clinicians aware of the study approached eligible patients during the first 12 hours of acetylcysteine treatment, as the initial 12-hour infusion was identical for all subjects and standardized among sites. All subjects provided written informed consent to participate in the study.
- Age 16 years or older;
- Acute single or staggered (i.e., over 1 hour) acetaminophen ingestion, requiring acetylcysteine as per the Australian and New Zealand acetaminophen guidelines (i.e., above 150 mg/L at 4-hour treatment nomogram line for single ingestions)(3);
- Serum ALT less than the local laboratory upper limit of normal (40 IU/L) on presentation and after 12 hours of acetylcysteine infusion;
- Serum acetaminophen concentration less than 20 mg/L (132 μmol/L, the upper therapeutic concentration) after 12 hours of acetylcysteine infusion; and
- Serum creatinine within normal laboratory limits on presentation and after 12 hours of acetylcysteine infusion.
The exclusion criteria were known pregnancy, acetaminophen modified-release ingestion, and other supratherapeutic ingestions (i.e., unintentional ingestions of more than 10 g or more than 200 mg/kg over 24 hours, more than 6 g/day over 48 hours). Modified-release ingestions were excluded because of their erratic absorption, less predictable pharmacokinetics, and peak acetaminophen concentrations being delayed in overdose.(20) Supratherapeutic ingestions were excluded because the current Australian and New Zealand acetaminophen management guideline allows for acetylcysteine discontinuation based on laboratory testing 8 hours after initiation of acetylcysteine. Those requiring treatment with acetylcysteine and excluded from the study received management as per the guideline.(3)
Study Procedures
Six hospitals were assigned to the two study arms in a 1:1 fashion (i.e., three intervention and three control sites). The toxicology inpatient units were physically based at the intervention sites, hence available to oversee more closely the changed acetylcysteine regimen and to prevent dosing errors including protocol violations, but investigators also consented and consulted in person on subjects at the control sites. All eligible patients were started on the two-bag infusion acetylcysteine regimen (200 mg/kg over 4 hours followed by 6.25 mg/kg/hour prepared in 1,000 mL of 5% glucose to provide 100 mg/kg over the next 16 hours). After 12 hours of treatment (i.e., after 8 hours of the second bag and 250 mg/kg of acetylcysteine), the serum acetaminophen concentration, liver, and renal function tests were repeated. If the serum ALT was less than 40 IU/L, the serum creatinine was normal, and the acetaminophen was less than 20 mg/L (132 μmol/L), acetylcysteine was discontinued at intervention sites and 1 L Ringer's lactate was infused instead over the remaining 8 hours. At control sites, the remaining 50 mg/kg acetylcysteine was continued in all cases to provide 300 mg/kg total over 20 hours (Fig. 1). If the 12-hour laboratory tests did not meet eligibility criteria (i.e., were abnormal), the patient was excluded from the study and the remaining dose of acetylcysteine was continued for the full 300 mg/kg regardless of site.
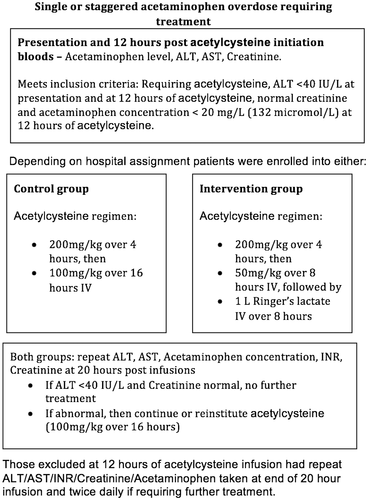
By study protocol, all patients had serum acetaminophen concentration, electrolytes, creatinine, aspartate transaminase (AST), and ALT measured on presentation and again after 12 hours and 20 hours of acetylcysteine infusion.
Study Follow-Up
A structured telephone interview performed by either of two study investigators (A.W./R.M.) after at least 14 days was performed to identify any unscheduled hospital visits for symptoms suggestive of liver injury including abdominal pain and jaundice, for another overdose, or for admission to hospital for any reason, including surgical procedures, laboratory testing, or referral to the regional liver transplant unit. Electronic medical records from each of the six study hospitals were also reviewed for this information at 14 days after enrollment. We queried the Australian and New Zealand liver transplant registries to identify whether any subject had been listed as a potential recipient within 14 days of enrollment. We also searched the study hospital records before study completion to establish vital status for the few patients who could not be contacted by phone.
Outcome Measures
The primary outcome was the incidence of hepatic injury at 20 hours after initiation of acetylcysteine treatment, defined as ALT doubling and peak ALT greater than 100 IU/L.(17) Secondary outcomes included the incidence of hepatotoxicity (peak ALT > 1,000 IU/L), change in ALT, peak international normalized ratio (INR both absolute and at a cutpoint of 1.3), and adverse reactions to acetylcysteine (grouped as gastrointestinal symptoms and nonallergic anaphylactic (NAARs or “anaphylactoid” reactions). NAARs were subclassified into cutaneous (flushing, rash, urticaria, wheals, itch) and more severe reactions, namely, respiratory (bronchospasm, wheeze, dyspnea, shortness of breath), angioedema, or cardiovascular instability (hypotension).
Data Collection/Management/Monitoring
Data were entered on study-specific case report forms by study investigators. Data included demographic details, reported dose, formulation and time of overdose, results of blood tests as per protocol (acetaminophen, ALT, AST, INR, creatinine), amount of acetylcysteine received, duration of acetylcysteine treatment, adverse reactions to acetylcysteine, length of stay, and discharge disposition.
Clinical data were retrieved from hospital electronic medical records (Cerner Millennium; Cerner, Kansas City, MO) and Symphony Version 2.29 (Ascribe, Bolton, United Kingdom) and transcribed manually into a secure password-protected research data set residing on secure hospital computer servers. All blood specimens were collected and analyzed at the participating hospital laboratories as per standard laboratory practice.
The study was approved by each institution's review board, with a requirement to immediately review and report any serious adverse events, including subject death deemed to be secondary to the altered acetylcysteine regimen or morbidity from dosing errors.
This study was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12615000938505).
Statistical Analysis
A sample size of 100 patients was selected based on expected recruiting efficiency and on the feasibility of the trial, given the number of sites participating. The first 50 patients to meet the inclusion criteria in each study arm were included. A low primary outcome event rate in such a sample would also provide some confidence with which to pursue a larger clinical trial, whereas the secondary outcomes could still have sufficient power to identify an important but subclinical safety signal.
Descriptive data are reported as medians and interquartile ranges (IQR), as appropriate. Odds ratios (95% confidence interval) were used for binary outcomes, the Mann Whitney U test to compare nonparametric data, and the Wilcoxon matched-pairs signed rank test to compare ALT pairs taken at presentation and after 20 hours following initiation of acetylcysteine. For subjects with an acute ingestion at a known time, we calculated the ratio between each subject's acetaminophen concentration at presentation (APAPs) and the threshold concentration for treatment based on the time interval following ingestion using the Rumack-Matthew nomogram (APAPt), as customary in risk stratification.(21) This is to allow comparison of acetaminophen concentrations in relation to time following ingestion. We also calculated the acetaminophen X ALT multiplication product(22) at presentation and after 12 hours of acetylcysteine, and the ψ parameter(23) at the start of acetylcysteine, for further risk stratification. GraphPad Prism Version 7 (GraphPad Software, San Diego, CA) was used to perform the statistical analysis.
Results
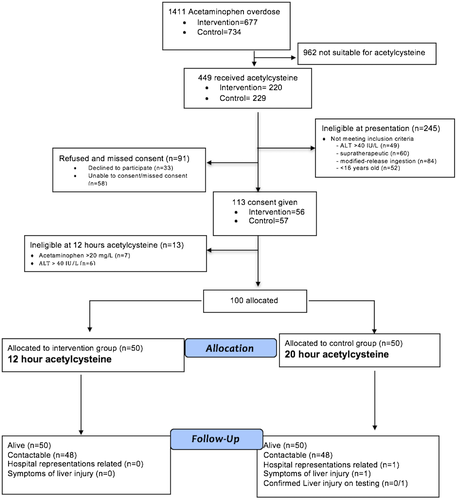
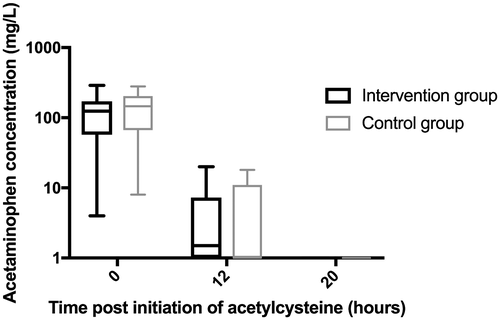
During the study period, 1,411 patients presented with acetaminophen overdose at the six study sites, of whom 449 received acetylcysteine (Fig. 2). One hundred subjects were enrolled into the trial (50 intervention and 50 control group) and all were intentional overdose. Most (77%) were female, aged 23 years (median, IQR 18-32). The two groups were well balanced with regard to demographics including age, sex, ethnicity, as well as ingested acetaminophen dose, initial acetaminophen concentration (Fig. 3), initial serum ALT, and time to acetylcysteine treatment (Table 1). The APAPs:APAPt ratio was also similar between groups (1.25 [1, 1.6] versus 1.3 [1.1,1.7], P = 0.38); almost all (94%) had a ratio between 1 and 2. All study subjects were admitted to a short-stay observation unit for acetylcysteine treatment, and adherence to protocol was excellent.
| Intervention Group (n = 50) | Control Group (n = 50) | P-Value | |
|---|---|---|---|
| Participants enrolled at each site, n | Austin (24) | Monash Medical (23) | |
| Dandenong (18) | Casey (19) | ||
| Westmead (8) | Blacktown (8) | ||
| Median age (IQR), years | 23 (19, 38) | 22 (18, 32) | NS |
| Female | 37 (74%) | 40 (80%) | NS |
| Time from ingestion to treatment, < 8 hours | 27 (54%) | 30 (60%) | NS |
| Alcohol co-ingested | 3 (6%) | 3 (6%) | NS |
| Other drugs co-ingested | 21 (42%) | 13 (26%) | |
| Opiates | 8 | 3 | |
| Antihistamines | 2 | 1 | |
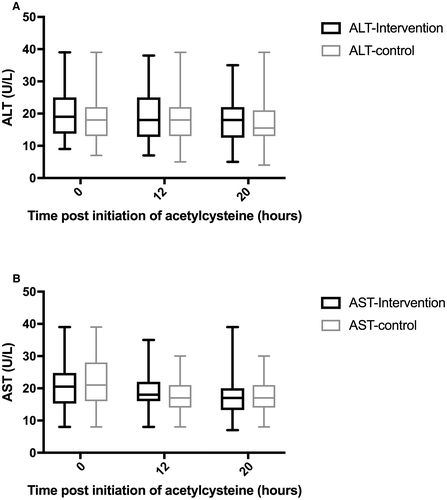
| Median presentation ALT (IQR), IU/L | 19 (14, 25) | 18 (13, 22) | NS |
| Median presentation AST (IQR), IU/L | 21 (16, 28) | 21 (16, 28) | NS |
| Median 20 hours following acetylcysteine ALT (IQR), IU/L | 18 (13, 22) | 16 (13, 21) | NS |
| Median 20 hours following acetylcysteine AST (IQR), IU/L | 18 (14, 20) | 17 (14, 21) | NS |
| Median 20 hours following acetylcysteine INR, (IQR) | 1.2 (1.1, 1.3) | 1.2 (1.1, 1.3) | NS |
| Ingestion type | |||
| Single | 49 (98%) | 46 (92%) | |
| Staggered | 1 (2%) | 4 (8%) | |
| Median dose ingested acetaminophen (IQR), mg/kg | 250 (180, 385) | 269 (198, 346) | NS |
| Median presentation acetaminophen concentration (IQR), mg/L | 124 (58, 171) | 146 (66, 204) | NS |
| Presentation acetaminophen concentration > 200 mg/L (%) | 9 (18%) | 11 (22%) | NS |
| Acetaminophen concentration fall > 90% from presentation to 12 hours following acetylcysteine initiation tests, N (%) | 48 (96%) | 47 (94%) | NS |
| Median acetaminophen concentration 12 hours following initiation of acetylcysteine (IQR), mg/L | 2 (<1, 7) | < 1 (<1, 11) | NS |
| Median acetaminophen concentration 20 hours following initiation of acetylcysteine (IQR), mg/L | < Lower limit of detection | < Lower limit of detection | NS |
| Median acetylcysteine duration (IQR), hours | 13 (13, 13.5) | 20 (20, 20) | < 0.001 |
| Median time to acetylcysteine from overdose (IQR), hours | 7 (6, 12) | 7 (6, 10) | NS |
| Median total acetylcysteine dose during admission (IQR), mg/kg | 250 (250, 250) | 300 (300, 300) | < 0.001 |
| Median acetaminophen × ALT (IQR),IU × mg/L | NS | ||
| Presentation | 2,595 (1,323, 3,680) | 2,614 (4,120, 10,164) | |
| After 12 hours of acetylcysteine | 109 (46, 160) | 145 (98, 230) | |
| Median ψ parameter | NS | ||
| (IQR), mmol/L × hour | 0.499 (0.001, 1.328) | 0.465 (0.001, 1.124) | |
| Median length of stay for medical treatment (i.e., excluding psychiatric admission) (IQR), days | 1 (1, 1) | 1 (1, 1) | NS |
| Discharge disposition post acetylcysteine | |||
| Home, n | 45 (90%) | 37 (74%) | |
| Admitted psychiatric ward, n | 5 (10%) | 13 (26%) | |
- Abbreviation: NS, not significant (P > 0.05).
No subjects met the primary outcome of hepatic injury (OR 1.0 [95% CI 0.02, 50]). There was no significant difference in 20-hour ALT between intervention and control groups (median 18 IU/L [13, 22] versus 16 [13, 21], P = 0.51) (Fig. 4), nor in change from baseline (3 IU/L [0, 5.5] versus 2 IU/L [0, 4.25], P = 0.48). No subjects demonstrated a doubling of ALT regardless of peak. The 20-hour creatinine was also similar between groups (61 μmol/L [52, 70] versus 61 μmol/L [51, 68], P = 0.44). In addition, no subjects developed hepatotoxicity, died, or received a liver transplant.
There was no difference in 20-hour INR between groups (1.2 [1.1-1.3] in both groups, P = 0.98). However, there were more subjects with an INR greater than 1.3 in the control group (n = 8, 16%) versus the intervention group (n = 3, 6%; OR 3.0 [95% CI 0.74, 12]) at 20 hours. Median acetylcysteine duration was 13 hours (IQR 13-13.5) in the intervention group and 20 hours (20-20) in those receiving the full course. No subjects required an extension in acetylcysteine treatment. Those who did not require an inpatient psychiatric admission were discharged with community follow-up.
With regard to adverse reactions to the acetylcysteine infusion, only 1 subject in the control group developed a NAAR, namely, dyspnea 2 hours after commencing the infusion. This resolved spontaneously within 15 minutes and acetylcysteine was ceased temporarily. No subjects in either group developed systemic or cutaneous reactions. The most common adverse reactions were gastrointestinal (Table 2). There was no significant difference in the incidence of gastrointestinal symptoms between groups (28% versus 24%, OR 1.23 [95% CI 0.50, 3.01], P = 0.64). Five (10%) subjects in the intervention group and 4 (8%) in the control group had gastrointestinal symptoms before starting acetylcysteine. There were no serious adverse events noted during the trial.
| Gastrointestinal Symptoms | Intervention Group (n = 14 or 28%) | Control Group (n = 12 or 24%) |
|---|---|---|
| Nausea | 7 (14%) | 3 (6%) |
| Vomiting | 5 (10%) | 7 (14%) |
| Both nausea and vomiting | 2 (4%) | 2 (4%) |
At the 14-day follow-up, 96 (96%) subjects were contacted by telephone, of whom only 1 reported returning to hospital with abdominal pain on day 2 following the overdose. Repeated liver function tests and abdominal ultrasound were both normal at that time, and the patient was discharged. No other subjects reported any persisting gastrointestinal symptoms on phone follow-up. Of the 4 who could not be contacted, all were known to be alive based on subsequent and unrelated presentations to hospital.
Discussion
For many years, treatment of acetaminophen poisoning with acetylcysteine has taken a “one size fits all” approach.(11) Improvements in our understanding of risk stratification following acetaminophen overdose(14-16, 24) and wide availability of rapid and accurate laboratory testing present an opportunity for more individualized dosing of antidotal therapy. It has long been recognized that the treatment threshold for acetylcysteine is very conservative.(11, 13, 18) Moreover, many patients present and are treated within hours of an acute overdose, and demonstrate persistently normal liver function including acetaminophen elimination.(14, 16) We believe such patients to be an ideal target for abbreviated therapy, and set out to systematically describe their outcomes in a carefully controlled prospective and comparative trial.
In this trial, subjects who had persistently normal serum ALT at the start and after 12 hours of treatment, and in whom the serum acetaminophen fell at least into the therapeutic range within 12 hours of treatment, had identical and entirely benign clinical and biochemical outcomes, whether the abbreviated or the complete two-bag acetylcysteine regimen was administered. Moreover, there was no indication of any subclinical biochemical differences in any of the secondary outcomes, including any doubling of the ALT regardless of peak, or slowed elimination of acetaminophen. The only exception was a marginally higher proportion of subjects with an INR greater than 1.3 at 20 hours in the control group, almost certainly due to the well-known effect of acetylcysteine on prothrombin time.(25)
In addition, with a median time of 7 hours to initiation of acetylcysteine, all patients had ALTs performed greater than 24 hours following ingestion that were normal. A normal ALT on presentation has a high negative predictive value (100%) of individuals developing any serious liver injury (ALT > 1,000 IU/L) in those receiving acetylcysteine.(26)
Of note, we identified eligible patients for abbreviated treatment after 12 hours of antidotal therapy. We required that they have, by definition, a 12-hour APAP × ALT product below 800 mg/L × IU/L,(15, 24) a value substantially lower than is seen in patients who go on to develop hepatotoxicity or hepatic failure.(20, 22, 24, 27) Other risk stratification measures were also reassuring in these patients: The ψ parameter at the start of treatment was generally less than or equal to 2 mmol/L × hour,(23) and the serum acetaminophen concentration almost always had fallen by at least 90% in 12 hours.
Other lines of reasoning provide further reassurance of the safety of abbreviated antidotal therapy. Population-pharmacokinetic modeling of the two-bag acetylcysteine regimen suggests that when acetylcysteine is discontinued at 12 hours, it remains detectable in the bloodstream at appreciable concentrations for up to 8 hours.(28) This may provide some degree of ongoing liver protection once acetylcysteine is ceased, although neither the minimum nor optimum therapeutic serum concentration of acetylcysteine is known.
In addition, preliminary work in a subset of NACSTOP trial subjects has shown similar peak microRNA-122 expression among groups, a measure of hepatic injury following acetaminophen overdose.(29) MicroRNA-122 can detect subclinical liver injury prior to rises in ALT.(30)
The current acetaminophen treatment nomogram in Australia uses a 25% lower acetaminophen concentration threshold for instituting acetylcysteine treatment (150 mg/L or 1,000 μmol/L at 4 hours) when compared with the original acetaminophen treatment nomogram used in the United Kingdom until recently (200 mg/L or 1,300 μmol/L at 4 hours)(18) and in Australia prior to 2008.(31) Patients in this so-called “possible hepatotoxicity” zone (i.e., in the absence of treatment) represent most of the acetaminophen overdoses receiving treatment, and are clearly ideal candidates for abbreviated therapy. However, one-fifth of subjects in our trial had an initial acetaminophen concentration greater than 200 mg/L yet still met the inclusion criteria after 12 hours of acetylcysteine by virtue of rapid acetaminophen metabolism and complete absorption, with a fall in acetaminophen of at least 90%.
In addition, almost half of the subjects in our study who received the abbreviated acetylcysteine regimen had treatment commence more than 8 hours following overdose. Therefore, using this low-risk feature as an inclusion criterion would substantially reduce the number eligible for abbreviated treatment, and demonstrates the advantage of using serial testing instead. We did not observe any biochemical evidence of liver injury even in these subjects in whom the ALT was normal at presentation and after 12 hours of treatment. Another important benefit of serial testing is reduced reliance on knowing with accuracy the time of ingestion, or insisting that the ingestion be taken at a single point in time.
Efforts to abbreviate acetylcysteine protocols have made slow gains in the past, but may now be gathering momentum. Previous modestly sized studies were part of the evidence base used to abbreviate oral acetylcysteine in the United States when the 72-hour oral course was routine.(32, 33) There have now been at least three studies of abbreviated intravenous acetylcysteine regimens.(6, 17, 34) A randomized clinical trial(6) of a 300-mg/kg over 12-hour Scottish Newcastle acetylcysteine protocol resulted in a decreased incidence of adverse reactions to antidotal treatment, but was not powered for clinical efficacy. Lucyk et al. reported that hepatic injury or hepatotoxicity were uncommon when the 20-hour intravenous acetylcysteine protocol was discontinued early, perhaps because of adverse effects, especially if the aminotransferases were normal and acetaminophen falling.(17) A retrospective review of supratherapeutic acetaminophen ingestions receiving an 8-hour acetylcysteine regimen reported liver injury in 0 of 46 patients if ALT was less than 50 IU/L on presentation.(34)
In practice, just under half of the patients requiring acetylcysteine treatment would be eligible for the NACSTOP protocol (Fig. 2) and more in countries with lower treatment thresholds. Benefits of a decreased duration of medical treatment might include an earlier opportunity for mental health assessment and interventions, fewer hospitalizations, and perhaps lower incidence of acetylcysteine dosing errors. These advantages may be especially important in jurisdictions that apply an even lower treatment threshold of 100 mg/L at 4 hours,(35) or which treat empirically(36) or suffer increasingly crowded emergency departments due to hospital access block.(37, 38)
This study also confirmed the very low rate of non-IgE mediated “anaphylactoid” reactions by modifying the load to 200 mg/kg acetylcysteine infusion over 4 hours. Other studies now support this administration regimen and have demonstrated lower adverse event rates to acetylcysteine.(8-10) The incidence of gastrointestinal adverse events remains high and was similar in both study groups, and included a substantial number with symptoms before receiving acetylcysteine. Gastrointestinal adverse rates have been up to 40% of subjects in previous studies, and can often be attributed to the ingestion itself.
Limitations
There are several limitations of this study. We cannot discount the potential for selection bias in this open-label study, yet refusals and missed enrollments were similar in frequency between arms. The study was not randomized at the individual subject level, and a cluster design for the study was chosen for both simplicity and minimization of protocol violations and crossover. Although the study was open-label, the primary endpoint was based on biochemical test results and hence unlikely to be unduly biased. The sample size in this study was small, but appropriate for an initial safety and feasibility study. We believe that future studies can now test our abbreviated acetylcysteine regimen using comparable inclusion criteria.
In conclusion, an abbreviated 12-hour acetylcysteine regimen is feasible and likely safe in selected patients at low risk of liver injury from acetaminophen overdose based on laboratory testing after 12 hours of treatment.
Potential conflict of interest
Nothing to report.
Acknowledgments
We thank our colleagues in the treating hospitals who assisted with recruitment.




