Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial
See Editorial on page 1668
Potential conflict of interest: Nothing to report.
Catherine Properzi received an Australian Government Research Training Program Scholarship. Cobram Estate Olive Oil donated a portion of the olive oil supplied on the Mediterranean intervention.
Abstract
Although diet-induced weight loss is first-line treatment for patients with nonalcoholic fatty liver disease (NAFLD), long-term maintenance is difficult. The optimal diet for improvement in either NAFLD or associated cardiometabolic risk factors, regardless of weight loss, is unknown. We examined the effect of two ad libitum isocaloric diets (Mediterranean [MD] or low fat [LF]) on hepatic steatosis (HS) and cardiometabolic risk factors. Subjects with NAFLD were randomized to a 12-week blinded dietary intervention (MD vs. LF). HS was determined by magnetic resonance spectroscopy (MRS). From a total of 56 subjects enrolled, 49 completed the intervention and 48 were included for analysis. During the intervention, subjects on the MD had significantly higher total and monounsaturated fat, but lower carbohydrate and sodium, intakes compared to LF subjects (P < 0.01). At week 12, HS had reduced significantly in both groups (P < 0.01), and there was no difference in liver fat reduction between groups (P = 0.32), with mean (SD) relative reductions of 25.0% (±25.3%) in LF and 32.4% (±25.5%) in MD. Liver enzymes also improved significantly in both groups. Weight loss was minimal and not different between groups (–1.6 [±2.1] kg in LF vs –2.1 [±2.5] kg in MD; P = 0.52). Within-group improvements in Framingham Risk Score (FRS), total cholesterol, serum triglyceride (TG), and glycated hemoglobin (HbA1c) were observed in the MD (all P < 0.05), but not with the LF diet. Adherence was higher for the MD compared to LF (88% vs. 64%; P = 0.048). Conclusion: Ad libitum low-fat and Mediterranean diets both improve HS to a similar degree.
Nonalcoholic fatty liver disease (NAFLD) represents abnormal hepatic lipid deposition, related to increasing peripheral adipose accumulation and insulin resistance (IR). With the world-wide epidemics of obesity and diabetes, NAFLD is now the most prevalent liver disease worldwide, affecting up to 25% of the adult population.1 Subjects with NAFLD may develop liver injury, termed nonalcoholic steatohepatitis (NASH), with a subset developing progressive fibrosis, cirrhosis, and complications, including end-stage liver failure and hepatocellular carcinoma.2 Patients with NAFLD also have an increased risk of cardiovascular (CV) disease (CVD), which is the leading cause of mortality in this population.3 Thus, the morbidity and mortality associated with this condition represents an increasing health burden in an aging population.
Treatment for NAFLD is through lifestyle modification consisting of caloric restriction and exercise, with an emphasis on weight loss.4, 5 The applicability, tolerability, cost, and benefits of lifestyle modification mean that it is a powerful intervention that could greatly reduce the societal morbidity and mortality related to NAFLD. Unfortunately, the success and longevity of lifestyle changes that focus on weight loss are poor.6, 7 In the PREDIMED (Prevención con Dieta Mediterránea) study (median follow-up duration of 4.8 years), an ad libitum Mediterranean dietary approach emphasizing change in nutrient composition rather than weight loss, was used successfully to reduce cardiovascular events in older adults with type 2 diabetes (T2D) or major CV risk (CVR) factors.8 This approach therefore seems suitably efficacious and may provide appropriate levels of long-term adherence to intervention diets.
Within the setting of dietary intervention for NAFLD, the role of diet type and nutrient profile has received limited attention. Whereas the evidence around weight loss in NAFLD shows a strong correlation between reduction in body weight and improvement in NAFLD,9 the confounding influence of weight loss means that effects or benefits of nutrient composition remain relatively unexplored. Practice guidelines now reflect a small body of evidence highlighting the advantages of a Mediterranean style of eating,5, 10 and although this has been suggested as the optimal therapeutic approach in situations where weight loss is not achieved,11 this requires confirmation.
Dietary patterns, such as Mediterranean and low-fat diets, are associated with low rates of CVD and a reduction in general CVR.8 Consequently, a Mediterranean dietary pattern with predominantly unsaturated fat has been suggested by the American Heart Association12-14 and the National Heart Foundation of Australia15 to reduce CV events (CVEs). Given that CVD is the leading cause of death among patients with NAFLD, these diets are therefore logical recommendations for this population. Nevertheless, evidence from high-quality trials relating to Mediterranean diets and NAFLD is limited. One pilot study of 12 subjects has provided evidence of reductions in hepatic steatosis (HS) with a Mediterranean diet (MD)16; however, the small cohort makes it difficult to generalize these findings. Clearly, more evidence is required to strengthen recommendations about optimal evidence-based care.
In order to guide optimal nutritional treatment for NAFLD and examine the efficacy of focusing upon altering nutrient profiles without body weight loss, we performed a randomized controlled trial comparing Mediterranean and low-fat diets using an ad libitum approach to energy intake. Our primary outcome was HS, with secondary outcomes of CVR, arterial stiffness, glycated hemoglobin (HbA1c) and IR measures, liver enzymes, compliance, and health-related quality of life (HRQL).
Patients and Methods
SUBJECTS
From April 2013 to June 2016, adult patients were recruited from NAFLD clinics at a Perth tertiary hospital and from private clinics, by participating gastroenterologists. Subjects provided written informed consent for the study before completing any assessments. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was approved the Sir Charles Gairdner and Osborne Park Hospital Group Human Research Ethics Committee (No. 2012-113), and registered on Australia New Zealand Clinical Trials Registry (ACTRN12612000841875).
INCLUSION AND EXCLUSION CRITERIA
Subjects required a diagnosis of NAFLD, with HS quantified as >5.5% as determined by magnetic resonance spectroscopy (MRS) and an average alcohol consumption of <20 g/day or 140 g/week for females or <30 g/day or 210 g/week for males.
Exclusion criteria were: secondary causes of NAFLD (e.g., medication induced); unstable body weight (variation >5% within the preceding 3-month period); current use of weight loss medications (e.g., Orlistat); current use of pioglitazone; other liver disease (viral hepatitis, autoimmune or cholestatic liver disease, Wilson's disease, hemochromatosis, or alpha-1 anti-trypsin deficiency); unstable diabetes (HbA1c >8.5%); decompensated cirrhosis (international normalized ratio >1.3, platelets <100 × 109/mm, bilirubin >20 mmol/L, albumin <35 g/L, ascites, or hepatic encephalopathy); renal failure; malignancy (aside from skin cancer); inability to provide informed consent; claustrophobia preventing MRS examination; current smoking; atrial fibrillation preventing SphygmoCor assessment; and pregnancy or lactation.
STUDY DESIGN AND OUTCOMES
A 12-week, prospective, parallel-group, single-blinded, randomized controlled trial of subjects with NAFLD was conducted. The primary outcome was percentage of HS at the end of week 12, determined by MRS. Secondary outcomes were CVR measures consisting of: (1) Framingham Risk Score (FRS); (2) arterial stiffness assessed by pulse wave velocity (PWV) and aortic augmentation index (AIx) using SphygmoCor; (3) HbA1c and IR measures (homeostatic model assessment of insulin resistance; HOMA-IR); (4) quality of life (QoL) and (5) liver function tests; and (6) compliance.
RANDOMIZATION
At baseline, subjects were randomized in a single-blinded fashion into one of two dietary intervention groups: low fat (LF) or MD diet. Subjects were randomized in a 1:1 fashion using randomly selected envelope-concealed allocations in blocks of 4, which were prepared before trial commencement. Stratification by diabetes status was used, because of the prognostic effect on NAFLD severity and vascular risk. Following individual subject enrollment and consent by the trial nurse, the next sequential envelope was drawn.
DIETARY INTERVENTION
The LF diet was based on National Health and Medical Research Council17 and American Heart Association Dietary recommendations.13 Target macronutrient energy contributions for the LF diet were 50% from carbohydrate, 30% from fat (with <10% of energy as saturated fat), and 20% from protein. The MD was based on analysis of actual foods consumed in traditional Cretan diets,18 with alterations to allow for standardization of protein intake with the LF diet. Target macronutrient energy contributions were 40% from carbohydrate, 35%-40% from fat (with <10% of energy as saturated fat), and 20% of energy as protein.
Dietary interventions were standardized in terms of education, counseling and dietary care. Education materials included diet-specific summaries of the patterns of food intake and a food-group list specifying preferred choices and approximate numbers and size of servings to consume per day based on dietary modeling and individual requirements. Recipe books, designed specifically for each diet in this study, were provided.
To minimize financial disadvantage to subjects consuming core foods in the MD, all subjects were provided with two food supplements appropriate to their diet. At each 4-weekly visit, the foods provided were 750 g of nuts (almonds or walnuts) and 750 mL of olive oil for the MD and 1 kg of natural muesli and 200 g of low-fat snack bars for the LF diet.
Education and dietary prescription was individualized by the study dietitian within the diet-specific recommendations, to allow for personal food preferences. All subjects received equivalent intensity of care in terms of opportunities for contact, availability of individual dietary counseling, type and amount of written resources, and the number of food items provided. Subjects were aware of the number (1 or 2) of their individual dietary allocation; however, the diet types were not disclosed at any point during the screening, informed consent, or during the trial.
ASSESSMENT AND MONITORING
Subjects underwent assessment at baseline and end of study for dietary intake and composition, NAFLD, CVR, anthropometry, fasting biochemistry, physical activity, and QoL. The study dietitian contacted participants by phone on a weekly basis for the first 4 weeks, to assess compliance and provide support in making dietary change. Contact was then every 4 weeks at scheduled review visits, or through additional patient-initiated contact. At each scheduled visit, subjects completed a standardized compliance questionnaire19 and underwent fasting blood draw and anthropometric measures. Subjects received further food supplies, support, and could ask questions. Noncompliance was defined as less than 70% compliance with scored items. Subjects also completed daily self-assessed checklists of compliance with food-group targets.
DIETARY ASSESSMENT
Dietary intake and composition data were collected using a modified Burke diet history interview carried out by a single accredited practicing dietitian (C.P.) experienced in dietary intervention trials and analyzed using nutritional analysis software (Foodworks Professional version 8; Xyris Software, Spring Hill, QLD, Australia).
CLINICAL ASSESSMENT
Subjects completed a standardized interviewer-administered questionnaire that recorded smoking, hypertension, diabetes, family history, dyslipidemia, alcohol intake, medications, and history of vascular disease (cerebral, coronary, and peripheral).
ANTHROPOMETRY AND BODY COMPOSITION
Height and body mass (nearest 0.05 kg in light clothing, no shoes) were measured using a single set of calibrated electronic medical scales with a stadiometer. Waist circumference was measured using flexible steel girth tape (W606PM, Lufkin; Apex Tool Group, Sparks, MD) at the narrowest point between the 10th rib border and iliac crest. If narrowing was not obvious, then the midpoint was used.20 Resting blood pressure (BP) was assessed after a 5-minute, seated rest. The mean of three valid measurements was used for each assessment.
PHYSICAL ACTIVITY ASSESSMENT
Physical activity was assessed at both baseline and end of study using the International Physical Activity Questionnaire long form.21 Subjects were advised to maintain their usual level of activity for the duration of the intervention.
NAFLD ASSESSMENT
Hepatic triglyceride content (HTGC) was quantified by MRS/proton density fat fraction (MRS-PDFF) using a two-voxel point-resolved spectroscopy sequence performed on a Philips 3T Ingenia scanner (Philips Medical Systems, Best, The Netherlands). PDFF methods are recommended to assess treatment efficacy for clinical trials in NAFLD.22 For each patient, an e-THRIVE sequence was run before the MRS sequence to obtain sagittal, coronal, and axial slices through the entire liver in order to position the volume of interest away from tissue boundaries, major blood vessels, or intrahepatic bile ducts. In order to minimize the influence of abdominal motion on field homogeneity, iterative shimming around the volume of interest was used. HTGC was measured in two different locations across the liver to account for heterogeneity of fat deposition within the liver using voxels of size 20 × 20 × 20 mm. Colocalization of voxels was performed using screen captures of voxel placement at baseline so as to replicate the positioning at study end. Acquisition of spectra was initiated on expiration (where chest movement is minimized) using the following parameters: echo time = 50 ms, repetition time = 1,800 ms, flip angle = 90 degrees, four averages, and eight individual dynamic scans. Quantitative analysis of HTGC was performed using the AMARES nonlinear least square quantitation algorithm in version 4.0 of the jMRUI software package (available at: http://www.jmrui.eu/). HTGC was calculated as described in Longo et al.23
Standard liver function tests (alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase [ALT], bilirubin, and gamma-glutamyl transferase [GGT]) were assessed at baseline and week 12 by the state reference laboratory (Pathwest, Nedlands WA, Australia). Normal ALT levels were defined according to this laboratory (<40 IU/L for males, <35 IU/L for females). Noninvasive assessment of hepatic fibrosis was undertaken using FibroScan and HepaScore. HepaScore is a serum-based model consisting of age, sex, bilirubin, GGT, alpha-2 macroglobulin, and hyaluronic acid, which has been validated as a predictive model for fibrosis in NAFLD.24 FibroScan was performed following an overnight fast by an experienced assessor with >200 acquisitions.
METABOLIC ASSESSMENT
Twelve-hour fasting blood samples were collected for analysis of: plasma glucose, insulin (with calculated HOMA1-IR and HOMA2-IR), HbA1c, sodium, potassium, urea, creatinine, liver function tests, cholesterol, triglycerides (TGs), high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol levels.
VASCULAR ASSESSMENT
Arterial stiffness using applanation tonometry (SphygmoCor) was used to measure AIx and PWV. Carotid-femoral PWV is considered to be the gold-standard noninvasive assessment of arterial stiffness, which predicts future CVEs, cardiovascular events and mortality.25 Patients had carotid-femoral PWV and AIx assessed by a single operator under standardized conditions as per consensus recommendations.26
QoL ASSESSMENT
HRQL was assessed using The Assessment of Quality Of Life (AQoL-8D) tool.27 The AQoL-8D was developed in an Australian population, is valid and reliable, and has a higher correlation than other instruments with subjective well-being.28
POWER CALCULATION AND STATISTICAL ANALYSIS
We aimed to recruit 55 subjects. Allowing for 10% dropout, this would provide 25 subjects per treatment group to detect diet-induced differences in HTGC of ≥13% (absolute value, 2.5%), based on a significance level of 5%, power of at least 80% and mean (SD) for intrahepatic TG content in NAFLD patients of 19.5% (3.1%).29
Endpoints were analyzed on an intention-to-treat basis at study completion (week 12). Repeated-measures analysis of covariance (ANCOVA) was used to examine differences in outcomes between diet groups at week 12 after adjusting for baseline values. Statistical differences within groups were analyzed by paired t tests or nonparametric Wilcoxon signed-rank tests. ANCOVA was also used to investigate the effect of measured adherence on HS. Significance was determined using a value of 0.05. Statistical analyses were carried out with SPSS statistical analysis software for Mac (version 24.0; SPSS, Inc., Chicago, IL).
Results
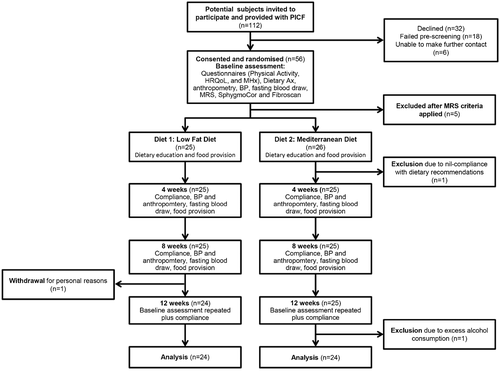
A total of 56 subjects were recruited with 5 subjects subsequently deemed ineligible after MRS examination showed insufficient steatosis (<5.5%). Of the 51 randomized subjects, 1 was excluded at week 2 for failing to implement any recommended dietary changes, and another subject left the study at week 8 for personal reasons. Forty-nine subjects completed the full 12 weeks of intervention. One subject was excluded from endpoint analysis because of excess alcohol consumption that was not declared at baseline, but found on analysis of the dietary interview data. A flow diagram of study participation is shown in Fig. 1, and baseline characteristics of subjects can be found in Table 1. The cohort was middle-aged with an even sex distribution. Approximately one third had diabetes or hypertension. No subjects were with cirrhosis. Subjects randomized to the MD group had a higher mean HS percentage and lower HDL-cholesterol. Groups did not differ on body mass index (BMI), BP, total cholesterol, TGs, or HbA1c.
| LF Diet (n = 25) | MD (n = 26) | Signiificance (P Value) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 53 (9.06) | 51 (13.36) | 0.538 |
| Female (%) | 14 (56.0) | 11 (42.3) | 0.406b |
| Ethnicity (%) | 0.547c | ||
| White | 21 (84.0) | 21 (80.8) | |
| Asian | 3 (12.0) | 4 (15.4) | |
| Other | 1 (4.0) | 1 (3.8) | |
| Anthropometry | |||
| Height (cm) | 165 (10.6) | 167 (7.96) | 0.326 |
| Weight (kg) | 81.3 (13.0) | 88.1 (12.9) | 0.065 |
| Waist (cm) | 98.3 (11.8) | 104.5 (10.7) | 0.058 |
| BMI (kg/m2) | 30.2 (5.6) | 31.5 (4.1) | 0.333 |
| CV indicators | |||
| Systolic BP (mm Hg) | 130 (16) | 126 (14) | 0.361 |
| Diastolic BP (mm Hg) | 79 (9) | 81 (7) | 0.396 |
| AIx @75 bpm (%) | 23.5 (11.2) | 20.2 (10.5) | 0.298 |
| PWV (m/s) | 9.3 (2.8) | 8.8 (2.8) | 0.561 |
| Lipids | |||
| Total cholesterol (mg/dL) | 202.2 (34.0) | 183.7 (48.3) | 0.123 |
| TGs (mg/dL) | 143.5 (58.5) | 166.5 (76.2) | 0.297a |
| HDL-cholesterol (mg/dL) | 49.5 (10.1) | 41.8 (8.9) | 0.006 a |
| LDL-cholesterol (mg/dL) | 123.7 (29.8) | 108.3 (43.3) | 0.130 |
| Lifestyle | |||
| Activity (MET-h/wk) | 31.3 (56.2)d | 40.7 (60.0)d | 0.598a |
| Quality of Life Score (/100) | 80.0 (11.6) | 80.0 (9.5) | 0.916 |
| Glucose (mg/dL) | 99.0 (17.6) | 102.6 (23.0) | 0.456a |
| Insulin (mU/L) | 14.4 (11.9) | 14.7 (6.7) | 0.229a |
| HbA1c (%) | 5.9 (0.8) | 6.1 (1.1) | 0.350a |
| HOMA-IR | 3.0 (1.9) | 3.7 (1.9) | 0.103a |
| HOMA2-IR | 1.6 (0.8) | 1.9 (0.9) | 0.117a |
| Liver | |||
| Raw hepatic fat (%) | 21.4 (10.0) | 32.8 (16.5) | 0.010 a |
| HepaScore | 0.29 (0.30) | 0.37 (0.33) | 0.372a |
| LS (kPa) | 7.03 (3.75) | 12.05 (15.19) | 0.108a |
| ALT (IU/L) | 66.8 (64.9) | 76.5 (51.2) | 0.187a |
| GGT (IU/L) | 119.6 (122.8) | 95.2 (116.8) | 0.801a |
| Comorbidities (%) | |||
| Diabetes | 7 (28.0) | 8 (30.8) | 1.00bb |
| Hypertension | 10 (40.0) | 9 (34.6) | 0.773bb |
| Hypercholesterolaemia | 11 (44.0) | 15 (57.7) | 0.406bb |
| CVD | 3 (12.0) | 5 (19.2) | 0.703bb |
- Data presented as mean (SD) or number (percentage). Bold type indicates P < 0.05.
- a Non-normal distribution. Normality determined using the Shapiro-Wilk test (P < 0.05). Non-normal distributions were analyzed for differences between groups using the Mann-Whitney U test.
- b Significance determined using Fisher’s exact (two-sided).
- c Significance determined using Pearson’s chi-square (exact significance). To be interpreted with caution because of low counts (four cells with expected count less than 5).
- d Median and interquartile range.
- Abbreviations: bpm, beats per minute; MET, metabolic equivalent; kPa, kilopascals.
DIETARY INTERVENTION
Total daily energy and macronutrient intake was not different between groups at baseline (Table 2); however, fiber intake was higher (P = 0.015) and energy (%) from saturated fat was lower (P = 0.012) in the MD group. A significant alteration in diet was achieved between groups at week 12 and from baseline within each group (Table 2). Measured macronutrient intakes of both groups were comparable with predicted intakes for the diets. At completion, energy, fiber, saturated fat, and alcohol intakes across the two groups were not significantly different. Carbohydrate and sugar intakes were both significantly higher in the LF diet. Intakes of total fat and monounsaturated fat were significantly higher and sodium was significantly lower in the MD. Saturated fat intake was not different between diets (9.3 [2.9]% vs. 9.5 [1.9]% of energy for LF and MD, respectively; P = 0.54).
| Baseline | 12 Weeks | LF (Baseline vs. 12 Weeks) | MD (Baseline vs. 12 Weeks) | |||||
|---|---|---|---|---|---|---|---|---|
|
LF Diet (n = 25) M (SD) |
MD (n = 26) M (SD) |
Significance (P Value) |
LF Diet (n = 24) M (SD) |
MD (n = 24) M (SD) |
Significance (P Value) | Significance (P Value) | Significance (P Value) | |
| Energy (kJ) | 9,930 (2,240) | 10,500 (2,080) | 0.348 | 9,839 (2,108) | 10,915 (2,244) | 0.097 | 0.541 | 0.110 |
| Protein (g) | 113 (20.5) | 116 (22.8) | 0.637 | 122.4 (22.5) | 118.6 (22.8) | 0.570 | 0.010 | 0.428 |
| Total fat (g) | 99 (32.2) | 100 (29.8) | 0.936a | 82.8 (28.9) | 132.6 (34.5) | <0.001 | 0.003 | <0.001 |
| Saturated (g) | 37 (12.6) | 33 (11.1) | 0.269 | 25.7 (12.9) | 27.9 (7.23) | 0.187a | <0.001 a | 0.007 |
| Monounsaturated (g) | 40 (14.9) | 40 (13.5) | 1.000a | 32.3 (12.0) | 69.3 (22.9) | <0.001 a | 0.004 a | <0.001 |
| Carbohydrate (g) | 231 (54.1) | 254 (71.7) | 0.205 | 247.1 (59.7) | 203.6 (64.0) | 0.019 a | 0.073a | <0.001 |
| sugars (g) | 105 (45.4) | 111 (45.3) | 0.459a | 122.0 (40.0) | 91.2 (26.2) | 0.003 a | 0.078a | 0.012 a |
| Fiber (g) | 27.4 (6.7) | 31.8 (6.5) | 0.015 | 39.9 (8.9) | 40.4 (9.5) | 0.847 | <0.001 | <0.001 |
| Sodium (mg) | 2,775 (711) | 2,795 (617) | 0.919 | 2,560 (736) | 2,085 (627) | 0.021 | 0.084 | <0.001 |
| Alcohol (g) | 8.0 (14.3) | 9.6 (14.2) | 0.723a | 6.09 (9.23) | 5.80 (8.50) | 0.982a | 0.650a | 0.362a |
| % energy from protein | 19.8 (2.6) | 19.0 (2.9) | 0.360 | 21.4 (2.30) | 18.7 (2.37) | <0.001 | 0.006 | 0.549 |
| % energy from carbohydrateb | 43.7 (5.4) | 45.7 (6.5) | 0.214 | 48.0 (5.4) | 36.7 (5.9) | <0.001 | 0.010 | <0.001 |
| % energy from fat | 36.4 (5.3) | 35.0 (5.9) | 0.387 | 30.6 (6.37) | 44.7 (6.43) | <0.001 | 0.001 | <0.001 |
| % energy from saturated fat | 13.5 (3.0) | 11.4 (2.6) | 0.012 | 9.32 (2.93) | 9.50 (1.89) | 0.537a | 0.003 a | 0.001 |
| % of fat as monounsaturated | 42.7 (4.7) | 42.9 (4.0) | 0.870 | 42.7 (5.73) | 55.3 (6.09) | <0.001 | 0.003 | <0.001 |
- Bold type indicates P < 0.05.
- a Non-normal distribution. Normality determined using the Shapiro-Wilk test (P < 0.05). Non-normal distributions were also analyzed for differences between groups using the Mann-Whitney U test.
- b Includes energy from carbohydrates, fiber, alcohol, and polydextrose compounds.
HS AND LIVER MEASURES
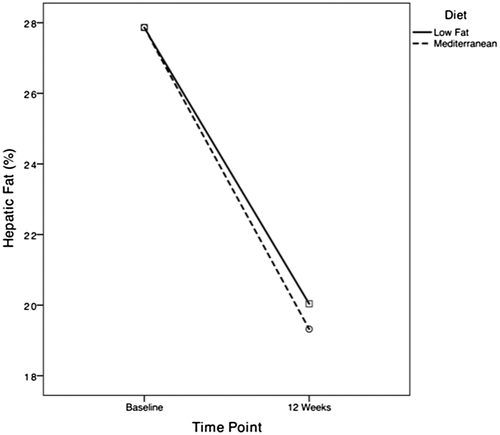
HTGC reduced significantly from baseline in both groups (P < 0.001), with relative change of –25.0% (25.3%) in the LF group and –32.4 (25.5)% in the MD group (Table 3; Fig. 2). After adjustment for baseline, hepatic TG was not significantly different between groups at completion (P = 0.28; Table 4). The proportion of subjects with NAFLD resolution was higher in the LF group (37.5% vs. 12.5%; P = 0.046). This was likely to be related to a lower baseline hepatic fat within the LF group, given that there was no significant difference in NAFLD resolution following adjustment for baseline hepatic fat content (odds ratio, 0.2; 95% confidence interval [CI], 0.05-1.30; P = 0.11). At end of treatment, there were no significant differences in liver function tests, HepaScore, or liver stiffness measurement (LSM) between the two groups. LSM and HepaScore did not change significantly during the period of the intervention. Within groups, however, ALT reduced significantly in both groups, with GGT falling significantly in the MD group (P < 0.001), and trended toward improvement in the LF group (P = 0.055). Five subjects with elevated ALT at baseline normalized their ALT, with no difference between LF and MD (8% vs. 21%; P = 0.6).
|
Baseline: LF Diet (n = 25) |
Week 12: LF Diet |
Significance (P Value) | Baseline: MD (n = 26) | Week 12: MD | Significance (P Value) | |
|---|---|---|---|---|---|---|
| Anthropometry | ||||||
| Weight (kg) | 81.3 (13.3) | 79.6 (13.5) | 0.001 | 89.3 (12.7) | 87.3 (12.5) | <0.001 |
| Waist (cm) | 98.0 (12.0) | 93.9 (10.6) | <0.001 | 105.6 (10.3) | 102.9 (10.4) | 0.001 |
| BMI (kg/m2) | 30.1 (5.69) | 29.5 (5.8) | 0.001 | 31.8 (4.0) | 31.1 (4.0) | <0.001 |
| CV indicators | ||||||
| Systolic BP | 130 (16) | 126 (14) | 0.08 | 126 (14) | 122 (13) | 0.09 |
| Diastolic BP | 79 (9) | 76 (9) | 0.07 | 81 (7) | 78 (8) | 0.07 |
| AIx @75 bpm (%) | 23.3 (11.4) | 24.0 (12.5) | 0.98 | 19.6 (10.2) | 19.0 (10.3) | 0.66 |
| PWV (m/s) | 9.3 (2.8) | 8.9 (3.8) | 0.92 | 8.6 (2.9) | 8.9 (2.1) | 0.70 |
| FRS (%) | 4.1 (4.3) | 4.0 (4.3) | 0.34b | 4.3 (5.8) | 3.8 (5.2) | 0.007 b |
| Lipids | ||||||
| Total cholesterol (mg/dL) | 202.2 (34.8) | 199.2 (41.2) | 0.27 | 184.8 (49.9) | 175.2 (49.5) | 0.010 |
| TGs (mg/dL) | 144.4 (59.3) | 139.9 (0.64) | 0.38 | 165.6 (76.2) | 144.2 (76.2) | 0.008 b |
| HDL-cholesterol (mg/dL) | 49.5 (10.4) | 48.3 (9.7) | 0.31b | 41.8 (8.5) | 41.4 (8.5) | 0.52 |
| LDL-cholesterol (mg/dL) | 123.7 (30.2) | 122.6 (33.3) | 0.34 | 109.4 (44.9) | 105.6 (43.3) | 0.24 |
| Lifestyle | ||||||
| Activity (MET-h/wk) | 31.3 (56.2)a | 45.5 (75.6)a | 0.881b | 40.7 (60.0)a | 46.1 (72.9)a | 0.568b |
| Quality of Life Score (/100) | 81.2 (10.1) | 86.3 (6.4) | 0.003 b | 79.6 (9.49) | 83.4 (8.7) | 0.035 |
| Glucose (mg/dL) | 99.2 (17.3) | 97.0 (27.4) | 0.07b | 100.3 (29.7) | 105.8 (31.7) | 0.92b |
| Insulin (mU/L) | 14.5 (12.2) | 11.64 (9.16) | 0.05b | 12.2 (6.69) | 15.33 (8.8) | 0.33b |
| HbA1c (%) | 6.0 (0.8) | 5.8 (0.8) | 0.39b | 6.1 (1.1) | 5.9 (1.0) | 0.045 b |
| HOMA-IR | 2.76 (1.52) | 2.95 (4.32) | 0.040b | 3.91 (1.92) | 3.63 (1.93) | 0.263b |
| HOMA2-IR | 1.49 (0.62) | 1.40 (1.14) | 0.073b | 2.02 (0.86) | 1.89 (0.89) | 0.44b |
| Liver | ||||||
| Raw hepatic fat (%) | 21.5 (10.0) | 15.3 (7.7) | <0.001 | 34.2 (16.3) | 24.0 (14.7) | <0.001 |
| HepaScore | 0.26 (0.28) | 0.30 (0.29) | 0.09 | 0.39 (0.33) | 0.41 (0.32) | 0.78 |
| LS (kPa) | 7.0 (3.8) | 7.0 (6.0) | 0.20b | 12.4 (15.4) | 11.7 (15.3) | 0.11b |
| ALT (IU/L) | 68 (66) | 56 (45) | 0.004 b | 77 (51) | 69 (47) | 0.049 b |
| GGT (IU/L) | 121 (125) | 102 (110) | 0.055b | 102 (120) | 83 (99) | <0.001 b |
- Data presented as mean (SD). Bold type indicates P < 0.05.
- a Median and interquartile range.
- b Non-normal distribution(s). Significance levels derived from the Wilcoxon signed-rank test.
- Abbreviations: bpm, beats per minute; MET, metabolic equivalent; kPa, kilopascals.
| Grouping | Variable |
LF Diet M (CI) |
MD M (CI) |
Significance (P Value) |
|---|---|---|---|---|
| Anthropometry | Weight (kg) | 83.6 (82.6-84.5) | 83.3 (82.3-84.3) | 0.658 |
| Waist (cm) | 97 (96-99) | 100 (98-101) | 0.041 | |
| BMI (kg/m2) | 30.4 (30.0-30.7) | 30.2 (29.9-30.6) | 0.608 | |
| CV | Systolic BP | 124 (120-129) | 123 (119-128) | 0.714 |
| Diastolic BP | 76 (73-79) | 78 (75-81) | 0.396 | |
| AIx @75 bpm (%) | 22.3 (18.8-25.7) | 20.7 (17.3-24.0) | 0.513 | |
| PWV (m/s) | 8.87 (7.46-10.3) | 8.94 (7.65-10.23) | 0.937 | |
| FRS | 4.06 (3.42-4.70) | 3.57 (2.90-4.24) | 0.294 | |
| Lipids | Total cholesterol (mg/dL) | 187.9 (178.3-197.6) | 182.5 (172.9-192.6) | 0.457 |
| TGs (mg/dL) | 145.3 (132.0-159.4) | 135.5 (121.3-148.8) | 0.284 | |
| HDL-cholesterol (mg/dL) | 45.2 (43.3-47.2) | 44.5 (42.2-46.4) | 0.605 | |
| LDL-cholesterol (mg/dL) | 113.3 (105.2-121.8) | 111.8 (103.6-119.9) | 0.755 | |
| Lifestyle | Activity (MET-h/wk) | 54.8 (43.4-70.0) | 58.2 (43.4-73.1) | 0.745 |
| Quality of Life Score (/100) | 85.4 (83.3-87.4) | 83.3 (81.2-85.3) | 0.153 | |
| Glucose (mg/dL) | 99.0 (90.0-108.0) | 102.6 (95.4-111.6) | 0.485 | |
| Insulin (mU/L) | 12.1 (9.7-14.5) | 14.0 (11.6-16.3) | 0.281 | |
| HbA1c (%) | 5.9 (5.8-6.1) | 5.8 (5.7-6.0) | 0.278 | |
| HOMA-IR | 3.73 (2.7-4.8) | 2.9 (1.8-3.9) | 0.268 | |
| HOMA2-IR | 1.65 (1.31-1.98) | 1.65 (1.33-1.98) | 0.978 | |
| Liver | Raw hepatic fat (%) | 20.0 (17.3-22.8) | 19.3 (16.6-22.0) | 0.722 |
| HepaScore | 0.35 (0.29-0.42) | 0.36 (0.29-0.42) | 0.885 | |
| LS (kPa) | 9.8 (8.1-11.6) | 9.4 (7.6-11.1) | 0.697 | |
| ALT (IU/L) | 60 (51-69) | 66 (56-75) | 0.363 | |
| GGT (IU/L) | 95 (80-109) | 91 (76-106) | 0.716 |
- Bold type indicates P < 0.05.
- Abbreviations: bpm, beats per minute; MET, metabolic equivalent; kPa, kilopascals.
CVR
FRS did not differ between diet groups at week 12; however, there was a significant improvement in FRS within the MD group, which was not observed in the LF group (Tables 3 and 4). Total cholesterol, plasma TGs, and HbA1c all improved significantly from baseline in the MD group, but not the LF group (all P < 0.05). End-of-treatment values of fasting lipids and measures of IR were not significantly different between the two groups. No differences were noted between or within groups in arterial stiffness measures of PWV or AIx.
ANTHROPOMETRY AND ACTIVITY
Despite the ad libitum nature of the diet and instruction to subjects that weight maintenance was preferred, both groups lost small amounts of body weight with resultant reductions in BMI and waist measurements. Weight loss was similar between groups (1.6 [2.1] vs. 2.1 [2.5] kg; P = 0.52, in LF and MD, respectively) and represents a relative reduction of 2.1% and 2.3% from baseline, respectively. Physical activity remained equivalent between the two groups and did not change significantly over the course of the study.
QoL AND COMPLIANCE
Both LF and MD subjects experienced significant improvements in overall QoL scores (Table 3). The LF group experienced significant improvements in five of the eight domains (independent living, mental health, self-worth, coping, and relationships) and the MD group reported significantly improved scores in three (mental health, coping, and relationships).
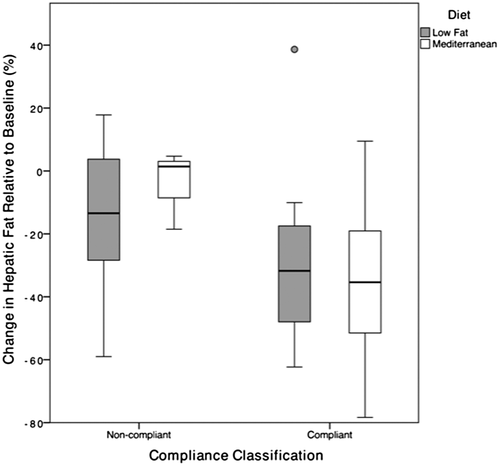
Nine subjects (36%) were categorized as noncompliant with the intervention diet in the LF group, compared to 3 (16%) from the MD group (P = 0.048). Improvement in HS was equivalent among subjects who adhered to either diet (Fig. 3).
Discussion
Our study addresses a gap in evidence about the effect of diets on HS and CVR when weight loss is not achieved or appropriate, by demonstrating that both Mediterranean and low-fat diets lead to a significant improvement in HS and measures of liver function. This finding complements the clear evidence of the effectiveness of diet-induced weight loss as the primary treatment for NAFLD.5, 9, 10 The disease-related burden of NAFLD advanced fibrosis and NASH-related cirrhosis has been increasing at rates beyond that of diabetes and obesity in the last decade,30 and diet represents a low-cost, low-risk strategy to reduce this.
CVR measures were not different between diet groups at the end of the study; however, within-group improvements in CVR measures were only observed within the MD group. Within this group, a mean 0.5% reduction in FRS from baseline was observed, representing a 0.5% reduction in estimated 10-year risk of a major CVE or death.31 This is consistent with previous findings that the MD lowers the risk of future CVEs and death in patients with established CVR factors.32 Given that CVD is the leading cause of death in patients with NAFLD, this study supports practice guidelines that recommend the adjustment of medical nutrition therapy in line with the MD.5, 10
We demonstrated in a relatively short time frame, that both an MD rich in olive oil and an LF diet could induce significant changes in hepatic fat with only a 2% body weight loss. This is less than the 3%-5% body weight loss required to improve hepatic fat.9, 33 In addition, the slight increase in energy intake we observed over the intervention, with no change to physical activity, suggests that weight loss depends on factors other than just caloric deficit.34 Both groups had significant reductions in saturated fat intake over the duration of the trial; saturated fat ingestion has been demonstrated to increase HTGC and hepatic IR.35, 36 Both groups also significantly increased dietary fiber consumption, which has been associated with reduced hepatic fat.37 The increase in total fiber and the concomitant increase in prebiotic factors within the diet may also influence the microbiota and therefore gut-liver axis, which is implicated in NAFLD development and progression.37, 38 In addition, the MD has high levels of polyphenols found in fruit and vegetables and high levels of monounsaturated fats found in olive oil. These compounds have been implicated in having wide-ranging benefits, including inhibiting de novo hepatic lipogenesis, improving peripheral insulin sensitivity, and reducing CVR.37, 39 Thus, there is potential for significant improvement in HS in the absence of significant weight loss in patients with NAFLD and for preferential improvements in cardiometabolic risk with the MD.
Our findings of the preferential impact of the MD on cardiometabolic risk are supported by two smaller studies,16, 40 where an isocaloric MD led to improved hepatic insulin sensitivity in 12 patients with NAFLD over 6 weeks16 and reduced HbA1c levels in 36 patients with T2D.40 A greater reduction in HS levels in subjects consuming MDs, in comparison to low-fat, high-carbohydrate control diets, was observed in these studies, but not in our study. This may be attributed to the shorter duration of these interventions (6 and 8 weeks), which was perhaps insufficient time to see a reduction in HS with the low-fat arm.41 The greater proportion of energy from carbohydrates in the low-fat diet administered to subjects with T2D40 (53% ± 2.1 compared to the 48.0% ± 5.4% in our trial) may be implicated in increasing hepatic de novo lipogenesis.42 Another longer trial found that subjects following a low-carbohydrate MD had a greater reduction in HS than those following a low-fat diet. However, the absolute magnitude of this difference was small (1.5%).43 The significant caloric restriction, very low carbohydrate intake in the Mediterranean arm, greater weight loss, and inclusion criteria (only one half of subjects had NAFLD) are all meaningful differences from our trial.
Importantly, our trial established the ability of subjects to achieve nutrient intakes comparable to documented traditional Mediterranean diets. The ad libitum approach and the achievement of the Mediterranean nutrient profile without the limitations of consuming a local-style cuisine mean that the results of this randomized controlled trial are readily translatable to a clinical setting and prescription within medical nutrition therapy. Moreover, the high level of compliance and improved QoL suggest that it may be associated with greater long-term efficacy.
The strengths of our study lie in the randomized design and the ability to achieve nutrient targets within personal food preferences. Dietary change has been noted as having poor long-term adherence,44 an essential factor in achieving meaningful outcomes and reducing health care costs.45 Our examination of adherence, especially in the context of usual food preferences and availability, provides real insight into the relevance of these diets as clinical treatment tools.
The comprehensive assessment of HS and CVR and the monitoring of potential confounders, such as activity, further strengthen our results. Although this study is similar in duration to previous dietary studies,36, 40, 46 more long-term trials are required to determine whether these diets are efficacious treatments for NAFLD. The short duration of the intervention may also explain why there were no changes to HepaScore, liver stiffness (LS), and markers of vascular disease.
The use of multiple methods for assessment of dietary compliance (self- and investigator administered), the ad libitum approach, and the use of a single study dietitian for collection and analysis of all data are all means by which bias was addressed. We expect that bias would be concentrated in reporting of discretionary foods. Given that these were limited to an equal extent in both diets, we assume that the potential bias would be similar across groups. The small and nonsignificant increases in both energy intakes and activity levels within our populations are within expected variability considering the small cohort.
In summary, we have demonstrated that there is no difference in the reduction in HS produced by a Mediterranean or a low-fat diet over 12 weeks, when energy intake is not significantly altered from baseline. Both diets led to a similar degree of reduction (25%-32%) in HS and resolution of NAFLD. The dietary patterns are easily transferrable to practice, and treatment is relatively inexpensive. Our results show that a Mediterranean diet can be adhered to by subjects consuming from a local Western food supply. We suggest that from the evidence presented, Mediterranean and low-fat diets prescribed within the framework of individualized dietary care are viable and efficacious even without clinically significant body weight loss. The improvement in CVR observed in the Mediterranean diet builds on evidence suggesting that it may be the preferred dietary pattern for people with NAFLD.
Acknowledgment
We thank Dr. Briohny Smith and Dr. Wendy Cheng for assistance with recruitment; and Jeanette Atkinson, Thomas Greig, Department of Medical Technology & Physics, Stephanie Hopa, and the MRI Department, Sir Charles Gairdner Hospital, all for assistance with MRS.
REFERENCES
Abbreviations
-
- AIx
-
- aortic augmentation index
-
- ALT
-
- alanine aminotransferase
-
- ANCOVA
-
- analysis of covariance
-
- BMI
-
- body mass index
-
- BP
-
- blood pressure
-
- CV
-
- cardiovascular
-
- CVD
-
- CV disease
-
- CVE
-
- CV event
-
- CVR
-
- CV risk
-
- FRS
-
- Framingham Risk Score
-
- GGT
-
- gamma-glutamyl transferase
-
- HbA1c
-
- glycated hemoglobin
-
- HDL
-
- high-density lipoprotein
-
- HOMA-IR
-
- homeostatic model assessment of insulin resistance
-
- HS
-
- hepatic steatosis
-
- HTGC
-
- hepatic triglyceride content
-
- IR
-
- insulin resistance
-
- LDL
-
- low-density lipoprotein
-
- LF
-
- low-fat diet
-
- LS
-
- liver stiffness
-
- LSM
-
- liver stiffness measurement
-
- MD
-
- Mediterranean diet
-
- MRS
-
- magnetic resonance spectroscopy
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- PWV
-
- pulse wave velocity
-
- QoL
-
- quality of life
-
- T2D
-
- type 2 diabetes
-
- TG
-
- triglyceride




