Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis
Potential conflict of interest: Nothing to report.
Supported by grants from the National Basic Research Program (2012CB524900) and the National Natural Science Foundation (81230018, 81471006, 81430020, and 31640020) and National Key R&D Program (SQ2017YFSF090203) of China.
Abstract
Physiological opening of the mitochondrial permeability transition pore (mPTP) is indispensable for maintaining mitochondrial function and cell homeostasis, but the role of the mPTP and its initial factor, cyclophilin D (CypD), in hepatic steatosis is unclear. Here, we demonstrate that excess mPTP opening is mediated by an increase of CypD expression induced hepatic mitochondrial dysfunction. Notably, such mitochondrial perturbation occurred before detectable triglyceride accumulation in the liver of high-fat diet–fed mice. Moreover, either genetic knockout or pharmacological inhibition of CypD could ameliorate mitochondrial dysfunction, including excess mPTP opening and stress, and down-regulate the transcription of sterol regulatory element–binding protein-1c, a key factor of lipogenesis. In contrast, the hepatic steatosis in adenoviral overexpression of CypD–infected mice was aggravated relative to the control group. Blocking p38 mitogen-activated protein kinase or liver-specific Ire1α knockout could resist CypD-induced sterol regulatory element–binding protein-1c expression and steatosis. Importantly, CypD inhibitor applied prior to or after the onset of triglyceride deposition substantially prevented or ameliorated fatty liver. Conclusion: CypD stimulates mPTP excessive opening, subsequently causing endoplasmic reticulum stress through p38 mitogen-activated protein kinase activation, and results in enhanced sterol regulatory element–binding protein-1c transcription and hepatic steatosis. (Hepatology 2018;68:62-77).
Abbreviations
-
- Ad
-
- adenoviral
-
- CsA
-
- cyclosporine A
-
- CypD
-
- cyclophilin D
-
- EGFP
-
- enhanced green fluorescent protein
-
- ER
-
- endoplasmic reticulum
-
- HFD
-
- high-fat diet
-
- IRE1α
-
- inositol-requiring enzyme 1α
-
- LKO
-
- liver-specific knockout
-
- MAPK
-
- mitogen-activated protein kinase
-
- mPTP
-
- mitochondrial permeability transition pore
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- PBS
-
- phosphate-buffered saline
-
- pLV-PPIF
-
- CypD overexpression plasmid
-
- PPIF
-
- peptidylprolyl isomerase F
-
- ROS
-
- reactive oxygen species
-
- siRNA
-
- small interfering RNA
-
- SREBP
-
- sterol regulatory element-binding protein
-
- TC
-
- total cholesterol
-
- TG
-
- triglyceride
-
- Tm
-
- tunicamycin
-
- VO2
-
- oxygen consumption
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the world, with a global prevalence of 20%-40%.1 NAFLD patients can progress to nonalcoholic steatohepatitis, which ultimately leads to the development of severe and irreversible liver damage, including cirrhosis and hepatocellular carcinoma.2 However, specific therapeutic strategies are lacking as the exact pathological process remains unclear despite a large amount of research.3
The underlying mechanism for the development and progression of NAFLD is complex and multifactorial. Different theories have been formulated, from an initial “two-hit” hypothesis to the current “multiple-hit” hypothesis.4 In all of the hypotheses, fat accumulation in the liver (steatosis) is thought to be an early stage of NAFLD5 and may progress to end-stage liver disease.6 Evidence has shown that triglyceride (TG) de novo lipogenesis is a prominent abnormality in NAFLD and the key event that leads to massive steatosis.5, 7
Mitochondrial dysfunction, characterized by impaired hepatic lipid homeostasis and decreased energy-transducing capacity, is considered the initiating event in the development and progression of NAFLD and nonalcoholic steatohepatitis.8, 9 Futhermore, studies have raised the possibility that NAFLD might be a mitochondrial disease.10 However, abnormality of mitochondrial function is traditionally thought to be a consequence of steatosis and plays an important role in the “second-hit” process.10, 11 It is poorly understood how exactly mitochondria function in the initial pathogenesis of steatosis. Identifying the underlying mechanisms will be crucial for the early prevention and therapy of NAFLD.
The opening of the mitochondrial permeability transition pore (mPTP) in the physiological state has a central role in cell metabolism, but excessive opening will lead to mitochondrial stress, which is characterized by impaired mitochondrial respiratory chain function, mitochondrial swelling, reactive oxygen species (ROS) generation, and more.12, 13 Cyclophilin D (CypD), a peptidylprolyl isomerase F (PPIF) residing in the mitochondrial matrix, can modulate the permeability of the mPTP in response to various stress stimuli.14 Recently, CypD has been regarded as an initial factor in mPTP opening.15 CypD deficiency improves mitochondrial function in the mouse cerebral cortex14 and skeletal muscle16; consequently, it ameliorates learning and memory in Alzheimer's disease and insulin resistance. However, the role of CypD in hepatic steatosis requires further investigation. Sterol regulatory element-binding protein-1c (SREBP-1c) is the key lipogenic transcription factor,17 and our previous study identified that SREBP-1c is indispensable for TG accumulation in the liver.18 However, the relationship between mitochondrial stress and SREBP-1c is unclear.
Here, we found that CypD-induced mitochondrial stress triggered hepatic TG accumulation. The mechanism was that CypD stimulated excessive mPTP opening and Ca2+ balance disruption, subsequently causing endoplasmic reticulum (ER) stress through p38 mitogen-activated protein kinase (MAPK) activation and resulted in enhanced SREBP-1c transcription. Increased expression of hepatic CypD is an early event that leads to steatosis in the liver. Therefore, inhibition or deletion of CypD may be vital to the prevention and treatment of NAFLD.
Materials and Methods
ANIMALS
Eight-week-old C57BL/6j male mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and housed in colony cages with a 12-hour light/dark cycle in a temperature-controlled environment. For high-fat diet (HFD) feeding experiments, mice were fed a diet containing 60 kcal% fat (Research Diets; D12492). All mice were killed from 10:00 pm to midnight and the liver tissues acquired. The protocol was approved by the Research Ethics Committee of Shandong Provincial Hospital.
Ppif–/– mice and Srebp1c–/– mice were kindly provided by Dr. Heng Du and Prof. Youfei Guan (Dalian Medical University, China), respectively. These mice were backcrossed to the C57BL/6j strain for 10 generations.
Liver-specific Ire1α knockout (Ire1α-LKO) mice were produced by intercrossing Ire1αflox/flox mice, which were kindly provided by Prof. Yong Liu (Chinese Academy of Sciences, China), with Alb-Cre transgenic mice.
Mouse adenoviral (Ad) PPIF (CypD overexpression adenovirus) and adenoviral enhanced green fluorescent protein (AdEGFP; empty vector adenovirus) were purchased from Hanbio Biotechnology Co., Ltd.; dissolved in sterile phosphate-buffered saline (PBS); and injected through the caudal vein. The amount of viral particles used in these experiments was 2 × 108 pfu per mouse, given three times with a 6-day interval.
Clinical-grade cyclosporine A (CsA; Novartis) was diluted fresh in PBS. PBS or CsA (10 mg • kg–1 • day–1 or 20 mg • kg–1 • day–1) was injected intraperitoneally daily to C57BL/6j mice for 6 weeks. Tunicamycin (Tm; Sigma; no. T7765) was soluble in dimethyl sulfoxide at 10 mg • mL–1 and diluted fresh in PBS. PBS or Tm (1 mg • kg–1 • day–1) was injected intraperitoneally daily to Ppif–/– (CypD knockout) mice and Ppif+/+ mice for 2 days.
STATISTICAL ANALYSES
The analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL). The results were reported as the mean ± SD. Comparison of different groups was performed using one-way analysis of variance. Two-tailed P < 0.05 was considered significant.
Further materials and methods are described in the Supporting Information.
Results
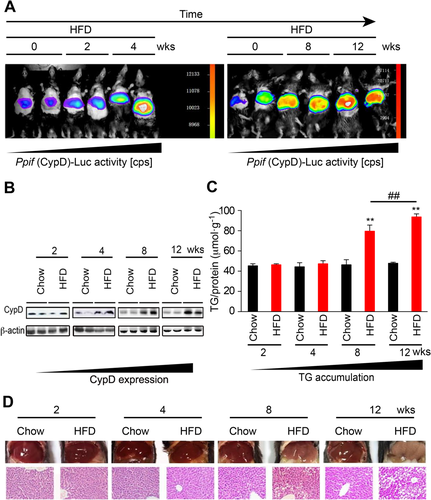
HEPATIC STEATOSIS IS SECONDARY TO THE INCREASE OF CypD EXPRESSION
To assess the role of CypD in the etiology of hepatic steatosis, we fed mice a chow diet or the HFD containing 60 kcal% fat for different time periods, and the mice were examined at the end of the second, fourth, eighth, and twelfth weeks. The in vivo imaging analysis (Fig. 1A) showed that the luciferase activity of CypD was increased as early as the end of week 4 and gradually enhanced as time passed. Elevated CypD protein expression also started from the end of week 4, as shown by western blot analysis (Fig. 1B). However, hepatic TG deposition and steatosis, corroborated by gross morphology and hematoxylin and eosin staining, were observed until the end of the eighth week of HFD feeding and continued to increase (Fig. 1C,D). The hepatic total cholesterol (TC) content was not significantly changed (data not shown). The results showed that increased CypD expression occurred earlier than the TG accumulation in the liver, suggesting a potential relationship between CypD and the etiology of steatosis.
ABLATION OF CypD PREVENTS DIET-INDUCED HEPATIC MITOCHONDRIAL STRESS AND STEATOSIS
In view of the increased expression of CypD associated with hepatic TG contents, we explored whether CypD serves as a mitochondrial target potentiating diet-induced steatosis. Hepatic lipid accumulation induced by the HFD was ameliorated in Ppif–/– (CypD deficiency) mice compared with Ppif+/+ (littermate counterparts) mice (Fig. 2A). And the hepatic TG levels in Ppif–/– mice were lower than those in Ppif+/+ mice, while this difference was enlarged by HFD treatment (Fig. 2B). However, the hepatic TC level showed no significant change (Fig. 2C). In addition, the serum TG and fasting blood glucose levels were decreased in Ppif–/– mice with the HFD, while the serum TC level was not significantly changed (Supporting Fig. S1A,B). This murine model also revealed increased phosphorylation of kinases associated with insulin signaling, including Akt and glycogen synthase kinase 3β, in the liver (Supporting Fig. S1C). On the other hand, no significant changes in serum TG and TC levels were observed between Ppif–/– and Ppif+/+ mice on the chow diet (Supporting Fig. S1D).
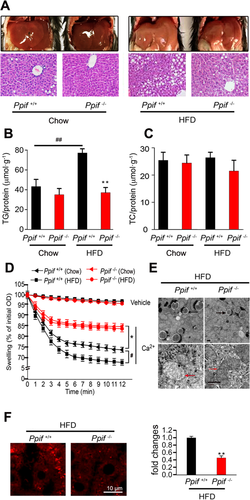
To confirm whether remission of steatosis in Ppif–/– mice was related to the promotion of mitochondrial function, we detected hepatic mitochondrial stress in HFD-induced mice. Mitochondrial swelling is an index that reflects mPTP opening and mitochondrial function.14 Hepatic mitochondria of Ppif–/– mice were more resistant to swelling and permeability transition in response to Ca2+ than those of Ppif+/+ mice. The mitochondria in HFD-treated Ppif+/+ mice showed greater swelling than those in the corresponding chow group, but this difference was eliminated between Ppif–/– mice fed the chow diet and those fed the HFD diet, implying the indispensable role of CypD in HFD-induced swelling (Fig. 2D). Further, transmission electron microscopic analysis showed that mitochondrial swelling, vacuolation, and destruction of cristae in HFD-fed mouse livers were attenuated in the liver of Ppif–/– mice (Fig. 2E). Mitochondrial swelling reflects excessive mPTP opening, which results in mitochondrial ROS generation, cytochrome c release and cell apoptosis.19, 20 To evaluate mitochondrial ROS generation, we used MitoSox red staining, a unique fluorogenic dye that allows for selective detection of superoxide production in the mitochondria. Ppif–/– mice showed much less MitoSox staining compared to Ppif+/+ mice (Fig. 2F), indicating that the absence of CypD reduced HFD-induced mitochondrial ROS generation.
It is well known that approximately 90% of mammalian oxygen consumption (VO2) is mitochondrial and that an increase in the VO2 level is a direct reflection of increased mitochondrial oxidative metabolism.15 We also checked the contribution of CypD to VO2 and energy expenditure (H) at the whole-body level using metabolic cage analysis. Ppif–/– mice fed the HFD have lower body weight and percentage of fat mass, which may be influenced by an increase in physical activity,15 while food intake was the same as that in Ppif+/+ mice (Supporting Table S1). Higher VO2 and energy expenditure (H) in Ppif–/– mice fed the HFD indicated accelerated fatty acid oxidation (Supporting Fig. S1E). The higher body temperature found in Ppif–/– mice on the HFD suggests that thermogenic mechanisms may be more active in these mice to maintain homeothermy (Supporting Table S1). Metabolic cage results showed no clear changes between Ppif–/– and Ppif+/+ mice on the chow diet (Supporting Fig. S1F and Table S1). Taken together, these results showed that CypD deficiency alleviated HFD-induced mitochondrial dysfunction and hepatic steatosis.
HEPATIC CypD OVEREXPRESSION INDUCED MITOCHONDRIAL STRESS AND LIPID ACCUMULATION
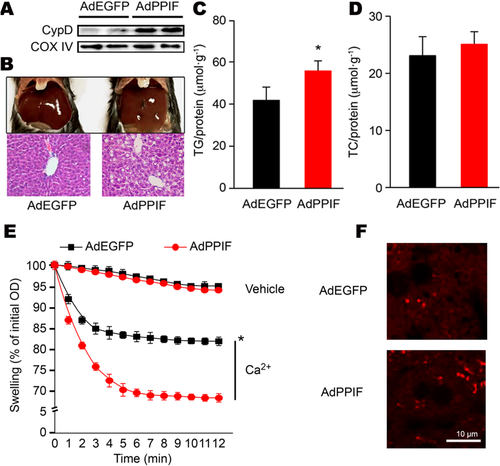
In order to directly determine the role of CypD in hepatic TG accumulation, we overexpressed CypD in mouse liver by infecting AdPPIF (adenoviral overexpression of CypD) through the caudal vein. Compared to the control group, the alanine aminotransferase and aspartate aminotransferase levels in the CypD overexpression group showed no significant changes, which means that CypD adenoviral infection did not result in obvious hepatic toxicity (Supporting Table S2). We verified the localization in the mitochondria and expression of CypD by double immunofluorescence staining (Supporting Fig. S2A) and western blot (Fig. 3A), respectively. Compared to AdEGFP (empty vector adenovirus), AdPPIF in the mice did not change the physical activity, body weight, percentage of fat mass, and VO2 significantly (Supporting Fig. S2B and Table S1) but increased lipid accumulation (Fig. 3B-D). Serum free fatty acid, TG, and TC levels did not show a clear change with CypD overexpression adenovirus injection (Supporting Table S2). Furthermore, in vivo overexpression of CypD aggravated mitochondrial swelling (Fig. 3E) and increased mitochondrial ROS generation (Fig. 3F), suggesting more serious mitochondrial dysfunction. All of the above results showed that hepatic TG accumulation depended on mitochondrial CypD.
CypD MEDIATES HEPATIC TG ACCUMULATION BY UP-REGULATING SREBP-1c
To explore the mechanism of CypD-induced TG accumulation, we performed RT-PCR array to analyze the expression of fatty liver disease–focused genes involved in TG biosynthesis, fatty acid uptake, and oxidation (Supporting Table S3). The representative genes were validated by real-time quantitative PCR. It was demonstrated that the mRNA expression of synthesis and oxidation-related genes was significantly changed in Ppif–/– (CypD deficiency) mice fed the HFD for 8 weeks (Fig. 4A). SREBP-1c is the key transcription factor of lipogenesis, and we further detected the protein expression of the precursor and mature forms. Mature SREBP-1c could translocate into the nucleus and bind to specific sterol regulatory element DNA sequences, leading to the up-regulation of genes involved in TG biosynthesis. CypD was increased as early as the end of the fourth week of HFD treatment (Fig. 1B). And mature SREBP-1c expression was increased at the end of the eighth week of HFD treatment (Fig. 4B), while CypD deficiency eliminated HFD-induced SREBP-1c up-regulation (Fig. 4C), indicating the potential regulation of SREBP-1c by CypD.
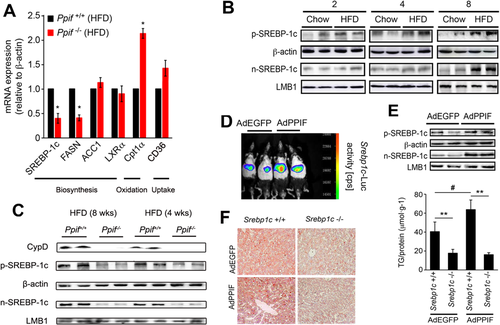
Further, the promoter activity and protein expression of SREBP-1c in AdPPIF (CypD overexpression adenovirus)–infected mice were increased compared with the AdEGFP (empty vector adenovirus) group (Fig. 4D,E). To determine whether SREBP-1c was indispensable for CypD-induced TG accumulation, Srebp1c–/– and Srebp1c+/+ (littermate counterparts) mice were injected with AdPPIF and AdEGFP, respectively. Unlike the littermate Srebp1c+/+ mice, the lipid contents in Srebp1c–/– mice were not increased even in those treated with AdPPIF (Fig. 4F), which supported the idea that CypD stimulates TG accumulation through SREBP-1c.
INOSITOL-REQUIRING ENZYME 1α IS INVOLVED IN THE PROCESS OF CypD-STIMULATED SREBP-1c EXPRESSION
Based on the above results, we wondered how CypD affected SREBP-1c. CypD-induced ROS accumulation could activate the downstream p38 MAPK signaling pathway in synaptic damage.21 In our study, we found that the phosphorylation of p38 MAPK was suppressed in Ppif–/– (CypD deficiency) mice (Fig. 5A). However, phosphorylation of both extracellular signal–regulated kinase and c-Jun N-terminal kinase, the other two important members of the MAPK family, was not changed. These results supported the idea that CypD-induced mitochondrial stress might affect p38 MAPK. Previous studies showed that p38 MAPK might associate mitochondrial dysfunction with ER stress.22-24 We found that ER dilatation was attenuated in Ppif–/– mice by transmission electron microscopy (Fig. 5B). The phosphorylation of inositol-requiring enzyme 1α (IRE1α), a representative ER stress–related protein, was down-regulated in Ppif–/– mice, while phospho-PERK, phospho-eIF2α, and ATF6 were not changed (Fig. 5C). Tm, one of the most common ER stress inducers,25 was used as a positive control in our study to test the sensitivity of Ppif–/– mice to the ER-active chemical. Tm could induce the activation of all three unfolded protein response arms of ER stress in Ppif–/– mice; more importantly, SREBP-1c expression could also be activated by Tm in Ppif–/– mice (Supporting Fig. S3). Our results indicated that CypD might cause ER stress through p38 MAPK activation.
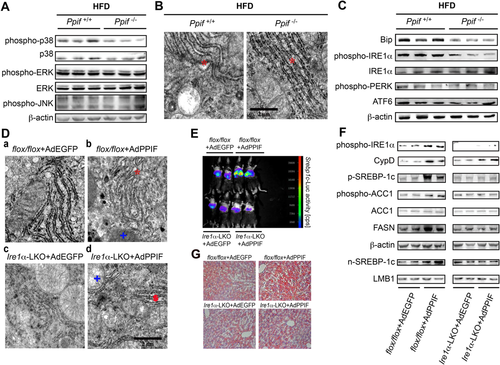
ER stress can trigger SREBP-1c expression and/or activation.26-28 Next, to determine the role of IRE1α in CypD-induced SREBP-1c expression, we established an Ire1α-LKO mouse model. Ire1α-LKO mice and littermate counterpart (flox/flox) mice were injected with AdPPIF (adenoviral overexpression of CypD) and AdEGFP (empty vector adenovirus), respectively. In flox/flox littermates, both the hepatic mitochondria and ER were destroyed by AdPPIF injection relative to AdEGFP (Fig. 5D, b versus a), while AdPPIF-infected Ire1α-LKO mice had abnormal mitochondria compared to the AdEGFP-infected Ire1α-LKO group, but no significant difference was observed in the ER (Fig. 5D, d versus c). Moreover, the expression of hepatic SREBP-1c (Fig. 5E,F) and lipid accumulation (Fig. 5G) in Ire1α-LKO mice were not increased even after treatment with AdPPIF compared to littermate flox/flox mice. These results suggested the indispensable role of IRE1α in CypD-induced SREBP-1c expression.
Activated IRE1 can cleave the mRNA encoding a specific transcription factor called XBP1, giving rise to an active spliced mRNA (XBP1s) that regulates the transcription of genes involved in protein folding.25 Our results showed that IRE1α activation and Xbp1 mRNA splicing were increased when hepatic steatosis was aggravated (Supporting Fig. S4), which is in accordance with the study of Lee et al.29
CypD INCREASES SREBP-1c EXPRESSION THROUGH Ca2+/p38 MAPK/IRE1α SIGNALING
Considering the complexity of the in vivo experiments, we set up palmitate-treated HepG2 cells and found that lipid accumulation was significant at the end of the 12 hours (Fig. 6A) but that corresponding expression of CypD was up-regulated as early as the end of the 6 hours before detectable lipid accumulation (Fig. 6B,C). Similarly, mitochondrial ROS generation was increased following the CypD change (Supporting Fig. S5A). As soon as CypD expression was increased, the phosphorylation level of p38 MAPK was elevated at the end of 6 hours, while IRE1α and SREBP-1c were changed at the end of 12 hours (Supporting Fig. S5B). When PPIF small interfering RNA (siRNA; the siRNA of CypD) was used, the phosphorylation levels of p38 MAPK and IRE1α and the expression level of SREBP-1c were suppressed with palmitate treatment (Fig. 6D). These results verified that CypD-induced mitochondrial stress occurred before detectable TG accumulation.
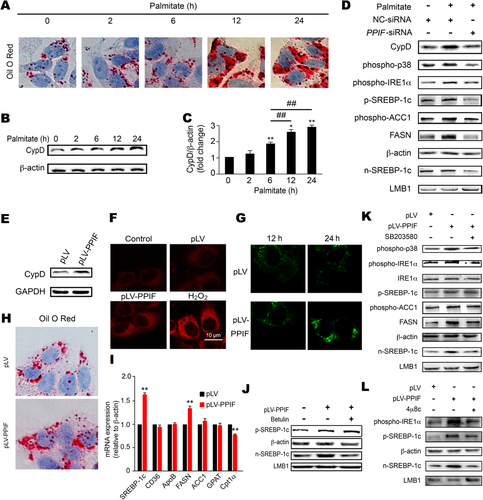
Next, pLV-PPIF (CypD overexpression plasmid) was used to confirm the effects of CypD on TG accumulation in HepG2 cells and mouse primary hepatocytes, respectively (Fig. 6E; Supporting Fig. S6A). In HepG2 cells, the pLV-PPIF plasmid increased mitochondrial ROS generation (Fig. 6F) and the intracellular concentration of Ca2+ (Fig. 6G), which was consistent with the result that CypD-induced ROS accumulation may cause Ca2+ balance disruption.21 CypD overexpression also aggravated mitochondrial dysfunction, including decreasing the oxygen consumption ratios of spare respiratory capacity and adenosine triphosphate production (Supporting Fig. S5C,D), which identified its role in mitochondrial respiratory function. Overexpression of CypD caused TG deposition (Fig. 6H) and increased mRNA and protein expression of SREBP-1c (Fig. 6I,J). Betulin, an inhibitor of SREBP maturation,30 impeded the effect of CypD on SREBP-1c maturation (Fig. 6J). At the same time, we found that CypD could specifically activate the IRE1α branch without changing the other two pathways in mouse primary hepatocytes from Ire1α-LKO and flox/flox mice (Supporting Fig. S7), so an IRE1α inhibitor (4μ8C) was used in our study. SB203580 (p38 MAPK inhibitor) or 4μ8C significantly decreased the expression of SREBP-1c promoted by CypD overexpression in HepG2 cells (Fig. 6K,L).
Similar to the results for HepG2 cells, in primary hepatocytes SB203580 or Ire1α-LKO significantly decreased the expression of SREBP-1c and TG accumulation promoted by CypD overexpression plasmid (Supporting Fig. S6B-G). These results indicated that Ca2+/p38 MAPK/IRE1α signaling might be the connection point between CypD and SREBP-1c expression. Given that activation of the ER stress response might parallel that of p38 MAPK, we tested the effect of p38 MAPK inhibitor on ER stress in primary hepatocytes (Supporting Fig. S6D). The results indicated that p38 MAPK inhibitor (SB203580) can suppress the IRE1α branch and decrease SREBP-1c expression promoted by CypD overexpression. On the other hand, in hepatocytes treated with SB203580, the effect of CypD on IRE1α phosphorylation also disappeared, while in Ire1α-deficient hepatocytes, CypD still could increase the expression of phospho-p38, which confirmed that Ire1α was the downstream target of the p38 signaling pathway (Supporting Fig. S6G).
PREVENTION AND THERAPEUTIC EFFECT OF CypD INHIBITOR IN HEPATIC STEATOSIS
Considering the effect of hepatic CypD on hepatic TG accumulation, we applied clinical-grade CsA (Sandimmune), an inhibitor of CypD, to determine the preventive and therapeutic effects of CypD as a potential target in NAFLD.
Mice fed the HFD for 4 weeks were given successive intraperitoneal injections of CsA for another 6 weeks (Fig. 7A). CsA could suppress HFD-induced mitochondrial ROS generation in a dose-dependent manner (Fig. 7B), and malondialdehyde levels were also decreased (Fig. 7C). Furthermore, CsA down-regulated SREBP-1c promoter activity (Fig. 7D) and its protein expression (Fig. 7E), which was followed by decreased lipid accumulation (Fig. 7F,G), especially TG levels (Fig. 7H).
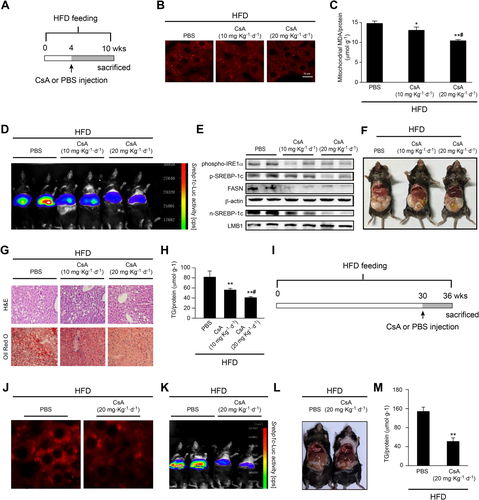
We also checked the effect of CsA in a long-term HFD-fed mouse model. Mice were fed the HFD for 30 weeks, followed by intraperitoneal injections of CsA for another 6 weeks (Fig. 7I). Similar to the 4-week model, CsA also suppressed HFD-induced mitochondrial ROS generation (Fig. 7J), down-regulated SREBP-1c activity (Fig. 7K), and inhibited hepatic steatosis (Fig. 7L,M). Overall, these results suggested that CypD might be a preventive and therapeutic target for hepatic TG accumulation and that CsA would be a new pharmacological approach for improving NAFLD.
Discussion
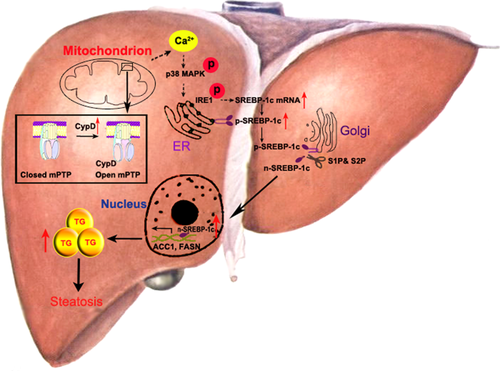
Our main finding here was that increased mitochondrial CypD expression could induce hepatic steatosis and, conversely, that deletion of CypD ameliorated TG accumulation in HFD-fed mice. Specifically, it was concerning that the mitochondrial stress induced by CypD was earlier than TG accumulation in the liver. The mechanism of CypD-mediated TG accumulation was that CypD stimulated excessive mPTP opening and mitochondrial stress, subsequently causing ER stress through Ca2+/p38 MAPK activation and finally resulted in the increase of SREBP-1c-mediated TG biosynthesis (Fig. 8). This is confirmation of the function of mitochondrial stress as the mechanism that CypD triggers steatosis in NAFLD.
We noted that the change of CypD was earlier than TG accumulation in the liver. Chen et al. detected mitochondrial respiratory dysfunction and TG accumulation in mouse liver at 12 weeks of HFD feeding.31 To check the exact relationship between CypD and TG contents, we fed mice the HFD for different time periods and confirmed that CypD expression was up-regulated at the end of the fourth week of HFD feeding and gradually enhanced as time passed. However, we failed to detect increased TG levels in the liver until the end of 8 weeks, which suggested that HFD-induced hepatic steatosis might be related to CypD expression. We detected a similar time course of CypD expression, IRE1α phosphorylation, and lipid accumulation in response to palmitate treatment in vitro. Tanaka et al. reported that apoptosis was observed until the end of 12 hours in palmitate (0.4 mM)–treated HepG2 cells relative to a control group (4.6% and 3.8% apoptotic cells, respectively).32 So we think palmitate-induced mitochondrial stress, ER stress, and TG accumulation were considered in the context of the extent of apoptosis in our study.
CypD is thought to be associated with metabolic disorders, including hyperglycemia and insulin resistance.16, 33 Similarly, in our study, Ppif–/– (CypD deficiency) mice fed the HFD also showed reduced serum glucose levels and alleviation of insulin resistance in the liver. The most striking finding was that the serum lipid levels and hepatic steatosis were also decreased in HFD-fed Ppif–/– mice, which indicated the indispensable role of CypD in HFD-induced steatosis. The indirect calorimetric data showed increased dark cycle activity and accelerated fatty acid oxidation in Ppif–/– mice on the HFD, which indicated that increased systemic metabolism may also be involved in the relief of hepatic steatosis in Ppif–/– mice on the HFD. To check if CypD could regulate systemic metabolism, we constructed a CypD overexpression mouse model. The results showed that VO2 and energy expenditure were not changed significantly, while the hepatic steatosis in CypD overexpression mice was aggravated relative to the control group. Our study also found that there were no clear changes in VO2 and energy expenditure between Ppif–/– and Ppif+/+ mice on the chow diet, which demonstrated that the change of systemic metabolism may not be a direct effect of CypD knockout. Similar to our results, the VO2 of cardiac mitochondria was not changed between Ppif–/– and Ppif+/+ mice on the chow diet.34 Further work is needed to elucidate the role of systemic metabolism change in the relief of hepatic steatosis in Ppif–/– mice fed the HFD.
CypD overexpression may cause apoptosis.35, 36 Our results showed that CypD overexpression can increase hepatic steatosis, especially TG contents when TG contents have been normalized by the corresponding tissue weight or protein.37 So we think we eliminate the possibility that CypD-induced steatosis is secondary to elevated apoptosis, at least to a certain degree.
CypD deficiency also showed improved mitochondrial function, such as relieved mitochondrial vacuolation, decreased mitochondrial swelling and ROS generation, as well as increased oxygen consumption, similar to the findings of other studies.38 Given the compensatory adjustment of other factors, HFD mitochondria are still able to swell without CypD. But, at least, we can confirm that HFD induces mitochondrial swelling through CypD because HFD cannot aggravate swelling relative to a chow diet when CypD is knocked out. By contrast, hepatic CypD overexpression aggravated TG accumulation and mitochondrial dysfunction. In conclusion, our findings demonstrated that TG accumulation depended on mitochondrial CypD expression in mouse liver. Although researchers have proved that human liver mitochondria possess a CypD-sensitive permeability transition,39 no evidence supports the close relationship between CypD and NAFLD in humans now.
The TG levels in the liver are determined by biosynthesis, fatty acid uptake and oxidation, and secretion18; and we were concerned about how CypD triggered TG accumulation. When we scanned fatty liver disease–related genes using a PCR array and validated the representative genes by real-time quantitative PCR, SREBP-1c, a key transcription factor of TG synthesis,18 was chosen. The transcription of SREBP-1c was identified to be dependent on CypD. TG accumulation was not changed in Srebp1c–/– mice, even in those treated with CypD overexpression adenovirus, supporting the idea that SREBP-1c was indispensable for CypD-induced steatosis. In addition, the mRNA expression of Cpt1α was found to be increased in CypD-deficient mice, implying that fatty acid oxidation might be also involved in CypD-induced hepatic steatosis, which needs further work to confirm.
Research has shown that intracellular Ca2+ is crucial for cell homeostasis23, 24 and that p38 MAPK activation can cause ER stress.40 In our model, CypD overexpression triggered cytosolic Ca2+ elevation and showed an imbalance of cell metabolism such as p38 MAPK activation and ER stress. ER stress can induce expression of SREBP-1c and activation of lipogenesis.26-28, 41, 42 Either blocking p38 MAPK or Ire1α-LKO could resist CypD-induced SREBP-1c expression and steatosis in our study. We also noted that Shao et al.37 reported increased hepatic TG accumulation in their Ire1α-LKO mice, most likely due to the feed and the long period of fasting.
Similar to reported results,25 Tm could up-regulate the expression of phospho-IRE1α, phospho-eIF2α, and cleaved ATF6 in our Ppif+/+ mice; more importantly, ER stress and SREBP-1c expression could also be activated by Tm in Ppif–/– mice, implying the sensitivity of Ppif–/– mice to the ER-active chemical. In Ppif+/+ mice, Tm could induce the activation of three unfolded protein response arms of ER stress, whereas CypD knockout suppressed Tm-induced phospho-IRE1α expression, without affecting the other two unfolded protein response arms. We also used CypD overexpression plasmid and Tm in hepatocytes of Ire1α-LKO mice and their littermate counterparts (flox/flox) mice, respectively. ATF6 and eIF2α could be activated by Tm even when IRE1α was knocked out, while they could not be regulated by CypD with or without IRE1α. All of the above results suggested a significant effect of CypD on the induction of IRE1α and that the mice or hepatocytes we used were sensitive to the ER-active chemical.
Our results showed that Xbp1 mRNA splicing, a marker of IRE1α activation, was increased when hepatic steatosis was aggravated, which is in accordance with the study of Lee et al.29 XBP1 is reported to be a regulator of lipogenesis and has important implications for human dyslipidemias.29 Studies in animal models have implicated XBP1s in lipid metabolism,43 and the hepatic IRE1α–XBP1 pathway was shown to play a crucial role in hepatic lipid homoeostasis.44 We also noted that Herrema et al. reported that XBP1s is an antilipogenic protein, most likely due to the feed.45
Given the high prevalence of NAFLD worldwide, we attempted to focus on its early prevention and therapy. CsA is known as an inhibitor of CypD,46 which blocks mPTP opening and gives partial protection in myocardial infarction and atherosclerotic plaque formation.47, 48 To evaluate these topics, HFD-fed mice were given successive intraperitoneal injections of CsA at two specific time points. Following CsA usage, mitochondrial dysfunction/stress was decreased and, astonishingly, steatosis was blocked for early treatment, while fatty liver was ameliorated for relatively later application. That is to say, the former presented a preventive effect and the latter presented a therapeutic effect. Kockx et al. have confirmed that CsA-induced hyperlipidemia is associated with abnormal serum cholesterol clearance.49 So CsA may mediate lipid metabolism by down-regulating the contents of TG in liver, inhibiting the clearance of cholesterol in blood, or both. Considering the wide pharmacological effects of CsA,50 it is necessary to eliminate the possibility that decreased hepatic TG accumulation was derived from secondary effects. We used the siRNA of CypD in vitro and gained consistent results, suggesting that CypD is a specific target for prevention and therapy of NAFLD.
Taken together, as the key factor of the mitochondria, CypD plays a crucial role in TG accumulation through the Ca2+/p38 MAPK/IRE1α/SREBP-1c signaling pathway, which could provide new approaches to NAFLD prevention and treatment.
Acknowledgment
We acknowledge Prof. Lin Li (Chinese Academy of Sciences, China) for providing professional suggestions. We thank Prof. Yong Liu for providing Ire1αflox/flox mice. We thank Prof. Youfei Guan for providing Srebp1c–/– mice.
REFERENCES
Author names in bold designate shared co-first authorship.




