Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance
Potential conflict of interest: Nothing to report.
Supported by the MUTHEC project (Institut National du Cancer, PRTK-14), Cancéropôle Emergence, and a fellowship from Fondation ARC (“Aides Individuelles,” to J.C.).
Abstract
We recently identified a histological subtype of hepatocellular carcinoma (HCC), designated as “macrotrabecular-massive” (MTM-HCC) and associated with specific molecular features. In order to assess the clinical relevance of this variant, we investigated its prognostic value in two large series of patients with HCC treated by either surgical resection or radiofrequency ablation (RFA). We retrospectively included 237 HCC surgical samples and 284 HCC liver biopsies from patients treated by surgical resection and RFA, respectively. Histological slides were reviewed by pathologists specialized in liver disease, and the MTM-HCC subtype was defined by the presence of a predominant (>50%) macrotrabecular architecture (more than six cells thick). The main clinical and biological features were recorded at baseline. Clinical endpoints were early and overall recurrence. The MTM-HCC subtype was identified in 12% of the whole cohort (16% of surgically resected samples, 8.5% of liver biopsy samples). It was associated at baseline with known poor prognostic factors (tumor size, alpha-fetoprotein level, satellite nodules, and vascular invasion). Multivariate analysis showed that MTM-HCC subtype was an independent predictor of early and overall recurrence (surgical series: hazard ratio, 3.03; 95% confidence interval, 1.38-6.65; P = 0.006; and 2.76; 1.63-4.67; P < 0.001; RFA series: 2.37; 1.36-4.13; P = 0.002; and 2.19; 1.35-3.54; P = 0.001, respectively). Its prognostic value was retained even after patient stratification according to common clinical, biological, and pathological features of aggressiveness. No other baseline parameter was independently associated with recurrence in the RFA series. Conclusion: The MTM-HCC subtype, reliably observed in 12% of patients eligible for curative treatment, represents an aggressive form of HCC that may require more specific therapeutic strategies. (Hepatology 2018;68:103-112).
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- Ang2
-
- angiopoietin 2
-
- BCLC
-
- Barcelona Clinic Liver Cancer
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- MTM-HCC
-
- macrotrabecular massive HCC
-
- RFA
-
- radiofrequency ablation
-
- VEGFA
-
- vascular endothelial growth factor A
Liver cancer ranks as the fifth most frequent cancer worldwide and the second most common cause of cancer-related death.1, 2 Hepatocellular carcinoma (HCC) is the most frequent primary liver tumor, and its main risk factors are hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcohol consumption, and metabolic syndrome.2, 3 Clinical outcome of HCC remains poor: the majority of patients present with advanced disease, and curative therapies, such as surgical resection and radiofrequency ablation (RFA), are hampered by high rates of tumor recurrence (50%-70%).3
Over the last decade, the emergence of high-throughput molecular technologies has allowed us to define the main molecular mechanisms involved in HCC development and progression. HCC appears as a highly heterogeneous entity, composed of distinct transcriptomic subgroups with various genetic alterations.4-9
A high degree of heterogeneity can also be observed at the histological level. Indeed, although neoplastic cells most often mimic the trabeculae of normal liver, numerous cytological variants and architectural patterns are recognized by the World Health Organization.10 Tumor differentiation is also assessed according to a three-grade system combining architectural and nuclear features.10, 11 Several histological subtypes, which feature distinctive and recognizable morphological features, have also been reported, such as the scirrhous subtype, characterized by a fibrous, hyaline stroma, and the lymphoepithelioma-like subtype, defined by a dense intratumor lymphocytic infiltration.12-14 Although some histological features have been shown to influence recurrence, their independent prognostic value, compared to microvascular invasion and satellite nodules, is not powerful enough to be included in therapeutic algorithms.15
We have recently shown that HCC morphology is tightly associated to specific underlying molecular alterations16 and noticeably identified a histological subtype, designated as “macrotrabecular-massive” (MTM-HCC), associated with an aggressive phenotype and particular biological (high alpha-fetoprotein [AFP] serum levels) and molecular (G3 transcriptomic subgroup, TP53 mutations, and FGF19 amplifications) features.16
Consistent with its microscopic appearance, which consists of thick trabeculae coated by endothelial cells and surrounded by vascular spaces, MTM-HCC was also characterized by high expression of angiopoietin 2 (Ang2) and vascular endothelial growth factor A (VEGFA), two key regulators of neoangiogenesis and vascular remodeling.16-18
In order to assess the clinical relevance of this particular histological HCC subtype, we aimed in the present work to validate its independent relationship with patient outcome in an independent series of patients treated by surgical resection and to determine its prognostic value when identified in biopsy samples of patients treated by RFA.
Materials and Methods
PATIENTS TREATED BY SURGICAL RESECTION
We retrospectively included 237 patients who underwent surgical resection for HCC at Henri Mondor (n = 176) or Beaujon (n = 61) university hospitals between 1995 and 2017.
Exclusion criteria were R1 or R2 resection, extrahepatic metastasis at the time of surgery, preoperative antitumoral treatment, unavailable histological slides or follow-up data, equivocal histological features suggestive of combined hepatocellular-cholangiocellular carcinoma, and inadequate material for histological analysis (necrosis >90%). This series is independent from our previous study.16 The following clinical and biological features were recorded: gender, age at surgery, fibrosis stage (assessed with the METAVIR scoring system19), risk factors of liver disease, and preoperative AFP serum level.
For each case, all available histological slides were reviewed, and tumors with a predominant (>50%) macrotrabecular (trabeculae of more than six cells thick) architectural pattern were classified as MTM-HCC, according to published criteria.16 A first set of 100 tumors was reviewed independently by three pathologists (N.P., V.P., J.C.) to assess the interobserver agreement. The following cases were reviewed by J.C. Poor tumor differentiation was defined according to the World Health Organization.10
Given that other HCC subtypes (steatohepatitic, scirrhous, and lymphoepithelioma-like) did not demonstrate any prognostic value in univariate analysis in our previous work, these parameters were not included in the present study.16 This noninterventional study was approved by an institutional review board.
PATIENTS TREATED BY RFA
Two hundred and eighty-four consecutive patients with HCC who underwent RFA between 2002 and 2014 at five French university liver units (Jean Verdier, Bondy; Henri Mondor, Créteil; Beaujon, Clichy; Centre Hospitalier Universitaire d'Angers, Angers; Centre Hospitalier Universitaire de Montpellier, Montpellier) were retrospectively included according to the following criteria: single Barcelona Clinic Liver Cancer (BCLC) 0/A HCC ≤50 mm, available tumor biopsy sample obtained during the RFA procedure, complete ablation defined by the absence of enhancing tissue at the tumor site 1 month after the RFA procedure, and follow-up of at least 3 months after RFA. All patients had established cirrhosis.
The following clinical features were available: gender, age, risk factors of liver disease, AFP serum level, and tumor size at imaging.
All biopsy samples were independently reviewed by two pathologists (M.Z. and J.C.), and tumors were classified as MTM-HCC if foci of macrotrabecular architectural pattern were observed. Cases with disagreement were analyzed using a multipipe microscope, and a consensus was reached.
FOLLOW-UP
Patients treated by RFA or resection were followed by liver imaging (magnetic resonance imaging or computed tomographic scan). Clinical endpoints were early and overall tumor recurrence. Early recurrence was defined as the emergence of one or several enhancing foci detected by imaging within 2 years after resection or RFA.20, 21 It is usually considered as a metastasis from the treated tumor rather than a result of de novo carcinogenesis.20, 21
STATISTICAL ANALYSIS
Statistical tests were performed using R software (R Foundation for Statistical Computing, Vienna, Austria, 3.2.2-R Studio). Comparisons between qualitative and quantitative data were performed using chi-squared and Fisher exact tests. The Monte Carlo simulation method was used for multiple testing corrections. Interobserver agreement was assessed with Cohen kappa and Fleiss kappa tests (“irr” package).
The association between variables and tumor recurrence was investigated using univariate and multivariate Cox proportional hazards regression models using the R survival package. Variables with P < 0.10 were included in the multivariate analysis. All tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
PATIENTS TREATED BY SURGICAL RESECTION
Among the 237 patients, 81% were male. The main risk factors were HCV infection (32%), HBV infection (25%), and alcohol intake (23%) (Table 1). Mixed etiologies were observed in 13% of the patients. Disease stage was very early/early (BCLC 0/A) and intermediate/advanced (BCLC B/C) in 85% and 15% of the cases, respectively.
| Variables | Available Data (n) | n (%) | MTM-HCC n (%) | Non-MTM-HCC n (%) | P |
|---|---|---|---|---|---|
| Clinical and biological features | |||||
| Age >60 years | 237 | 133 (56) | 17/38 (45) | 116/199 (59) | 0.17 |
| Male gender | 237 | 193 (81) | 27/38 (71) | 165/199 (83) | 0.12 |
| Alcohol | 237 | 52 (23) | 3/35 (8) | 49/193 (25) | 0.05 |
| HCV | 228 | 73 (32) | 12/35 (34) | 61/193 (32) | 0.91 |
| HBV | 228 | 58 (25) | 15/35 (43) | 43/193 (22) | 0.02 |
| NASH | 228 | 41 (18) | 2/35 (6) | 39/193 (20) | 0.05 |
| Other/undetermined | 228 | 33 (15) | 6/35 (17) | 27/193 (14) | 0.62 |
| BCLC B-C | 237 | 35 (15) | 15/38 (39) | 20/199 (10) | <0.001 |
| AFP serum level >100 ng/mL | 191 | 55 (29) | 19/26 (73) | 36/165 (22) | <0.001 |
| Pathological features of tumors and nontumoral liver | |||||
| Tumor size >50 mm | 237 | 108 (45) | 25/38 (66) | 83/199 (42) | 0.01 |
| Satellite nodules | 237 | 61 (26) | 18/38 (47) | 43/199 (22) | 0.001 |
| Macrovascular invasion | 237 | 31 (13) | 15/38 (39) | 16/199 (8) | <0.001 |
| Microvascular invasion | 237 | 111 (47) | 34/38 (89) | 77/199 (39) | <0.001 |
| Poor differentiation | 237 | 36 (15) | 13/38 (34) | 23/199 (11) | <0.001 |
| Cirrhosis | 231 | 94 (39) | 18/36 (50) | 76/195 (39) | 0.29 |
- Abbreviation: NASH, nonalcoholic steatohepatitis.
Elevated preoperative AFP serum levels (>100 ng/mL) were detected in 29% of the cases (Table 1). As expected in a series of patients with HCC treated by surgical resection, a high rate of noncirrhotic livers was observed (F0-F3 61%) (Table 1).
Tumors were large (>50 mm, 45%), with satellite nodules, macrovascular invasion, and microvascular invasion in 26%, 13%, and 47% of the cases, respectively.
Thirty-eight cases of MTM-HCC were identified, representing 16% of the whole series (Fig. 1). Interobserver agreement between the three pathologists for the diagnosis of MTM-HCC was good (Fleiss kappa 0.74).
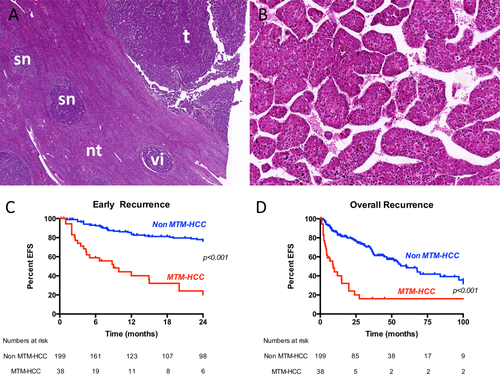
Regarding clinical and biological features, MTM-HCC was associated with HBV infection (P = 0.02), BCLC B-C disease stages (P < 0.001), and elevated AFP serum levels (P < 0.001). At the pathological level, MTM-HCCs were more frequently large (>50 mm, P = 0.01), with satellite nodules (P = 0.001), macrovascular invasion (P < 0.001), and microvascular invasion (P < 0.001) (Table 1).
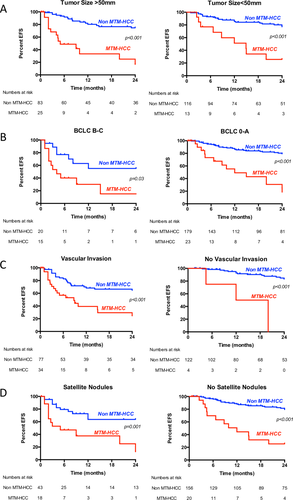
Ninety-one recurrences were observed, including 61 early recurrences (67%). Variables associated with early tumor recurrence in univariate analysis were HBV infection (hazard ratio [HR], 1.86; P = 0.02), BCLC B-C stages (HR, 3.93; P < 0.001), AFP serum levels >100 ng/mL (HR, 2.53; P = 0.001), satellite nodules (HR, 2.89; P < 0.001), microvascular invasion (HR, 3.60; P < 0.001), poor tumor differentiation (HR, 2.25; P = 0.008), and MTM-HCC subtype (HR, 5.81; P < 0.001) (Table 2). Multivariate analysis showed that MTM-HCC was an independent predictor of both early (P = 0.006) and overall (P < 0.001) recurrence. MTM-HCC subtype retained a prognostic value even after stratification according to common clinical, biological, and pathological features of aggressiveness (Table 2 and Fig. 2). Indeed, patients with MTM-HCC and small tumors (n = 13), without satellite nodules (n = 20), classified BCLC 0/A (n = 23), or without vascular invasion (n = 4) had significantly lower event-free survival compared to patients with other histological subtypes (Fig. 2). The other variable independently associated with overall recurrence was satellite nodules (P = 0.03) (Table 2).
| Early Tumor Recurrence | Overall Tumor Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Clinical and biological features | ||||||||
| Age >60 years | 0.59 (0.36-0.98) | 0.04 | 0.93 (0.44-1.96) | 0.86 | 0.89 (0.59-1.35) | 0.59 | ||
| Male gender | 1.23 (0.62-2.43) | 0.54 | 0.89 (0.53-1.50) | 0.67 | ||||
| BCLC stage B-C | 3.93 (2.26-6.84) | <0.001 | 2.27 (0.82-6.23) | 0.11 | 2.71 (1.65-4.47) | <0.001 | 1.70 (0.89-3.21) | 0.10 |
| AFP serum level >100 ng/mL | 2.53 (1.42-4.51) | 0.001 | 1.12 (0.57-2.17) | 0.74 | 1.51 (0.91-2.52) | 0.11 | ||
| Alcohol | 0.57 (0.28-1.17) | 0.12 | 0.61 (0.34-1.10) | 0.09 | 0.62 (0.34-1.14) | 0.13 | ||
| HCV | 0.63 (0.35-1.16) | 0.13 | 0.90 (0.57-1.42) | 0.65 | ||||
| HBV | 1.86 (1.09-3.15) | 0.02 | 1.50 (0.71-3.15) | 0.28 | 1.23 (0.77-1.95) | 0.38 | ||
| NASH | 0.37 (0.15-0.92) | 0.03 | 0.30 (0.09-1.00 | 0.05 | 0.58 (0.31-1.10) | 0.09 | 0.63 (0.33-1.20) | 0.16 |
| Other/undetermined | 1.39 (0.71-2.76) | 0.33 | 1.31 (0.73-2.38) | 0.36 | ||||
| Histological and gross features of the tumors | ||||||||
| Tumor size >50 mm | 1.51 (0.91-2.50) | 0.10 | 1.26 (0.83-1.90) | 0.27 | ||||
| Satellite nodules | 2.89 (1.72-4.85) | <0.001 | 1.30 (0.55-3.07) | 0.54 | 2.45 (1.56-3.83) | <0.001 | 1.89 (1.07-3.31) | 0.03 |
| Microvascular invasion | 3.60 (2.07-6.24) | <0.001 | 1.94 (0.90-4.17) | 0.08 | 2.00 (1.32-3.03) | <0.001 | 1.14 (0.70-1.87) | 0.60 |
| Poor differentiation | 2.25 (1.22-4.17) | 0.008 | 0.68 (0.28-1.65) | 0.39 | 1.45 (0.83-2.53) | 0.19 | ||
| Macrotrabecular-massive subtype | 5.81 (3.46-9.76) | <0.001 | 3.03 (1.38-6.65) | 0.006 | 3.83 (2.40-6.11) | <0.001 | 2.76 (1.63-4.67) | <0.001 |
| Cirrhosis | 1.20 (0.72-2.01) | 0.48 | 1.24 (0.81-1.88) | 0.32 | ||||
- Abbreviations: CI, confidence interval; NASH, nonalcoholic steatohepatitis.
PATIENTS TREATED BY RFA
As observed in the surgical series, a strong male predominance was observed among the 284 patients (75%), with HCV infection and alcohol intake being the most frequent risk factors of liver disease (Table 3). Preoperative AFP was elevated in 13% of the patients. Tumor size at imaging was >30 mm in 26% of the cases.
| Variables | Available Data (n) | n (%) | MTM-HCC n (%) | Non-MTM-HCC n (%) | P |
|---|---|---|---|---|---|
| Clinical and biological features | |||||
| Age >60 years | 284 | 203 (71) | 18/24 (75) | 185/260 (71) | 0.87 |
| Male gender | 284 | 213 (75) | 20/24 (83) | 193/260 (74) | 0.46 |
| Alcohol | 284 | 129 (45) | 12/24 (50) | 117/260 (45) | 0.80 |
| HCV | 284 | 135 (47) | 12/24 (50) | 123/260 (47) | 0.97 |
| HBV | 284 | 23 (8) | 1/24 (4) | 22/260 (8) | 0.70 |
| NASH | 284 | 52 (18) | 6/24 (25) | 46/260 (18) | 0.54 |
| Other/undetermined | 284 | 8 (3) | 1/24 (4) | 7/260 (3) | 0.67 |
| AFP serum level >100 ng/mL | 262 | 36 (13) | 8/23 (35) | 28/211 (13) | 0.005 |
| Pathological features | |||||
| Tumor size >30 mm | 284 | 74 (26) | 11/24 (46) | 63/260 (24) | 0.04 |
| Poor differentiation | 284 | 46 (16) | 7/24 (33) | 39/260 (15) | 0.13 |
- Abbreviation: NASH, nonalcoholic steatohepatitis.
Pathological review of biopsy samples identified 24 cases (8.5%) of MTM-HCC, with an excellent interobserver agreement (Cohen kappa, 0.82). MTM-HCC subtype samples were easily identified at low-power field examination of biopsies due to the characteristic dyscohesive aspect of trabeculae (Fig. 3).
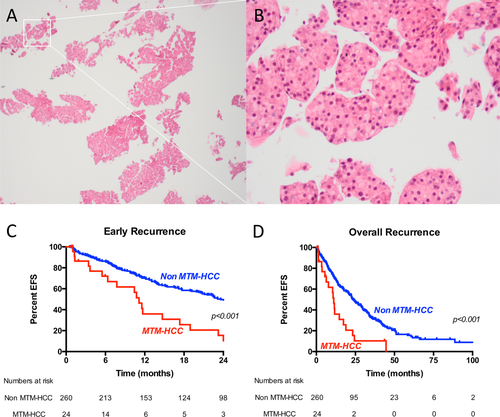
Cases classified as MTM-HCC were associated with high AFP serum levels (P = 0.005) and high tumor size (>30 mm, P = 0.04) (Table 3).
One-hundred and ninety-four recurrences were observed, including 135 (69%) early recurrences. MTM-HCC subtype was an independent predictive factor of both early and overall recurrence in multivariate analysis (HR, 2.37; P = 0.002; and HR, 2.19; P = 0.001, respectively) (Table 4). No other clinical, biological, or histological factor was related to recurrence.
| Early Tumor Recurrence | Overall Tumor Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Clinical and biological features | ||||||||
| Age >60 years | 1.01 (0.69-1.49) | 0.94 | 1.26 (0.90-1.77) | 0.17 | ||||
| Male gender | 1.20 (0.79-1.82) | 0.39 | 1.21 (0.86-1.71) | 0.21 | ||||
| AFP serum level >100 ng/mL | 1.55 (0.98-2.49) | 0.06 | 1.32 (0.80-2.17) | 0.27 | 1.24 (0.82-1.88) | 0.30 | ||
| Alcohol | 0.82 (0.59-1.16) | 0.27 | 0.86 (0.65-1.15) | 0.32 | ||||
| HCV | 1.04 (0.74-1.45) | 0.83 | 1.08 (0.81-1.43) | 0.61 | ||||
| HBV | 0.98 (0.53-1.81) | 0.94 | 0.97 (0.58-1.62) | 0.91 | ||||
| NASH | 1.25 (0.82-1.89) | 0.29 | 1.05 (0.72-1.51) | 0.81 | ||||
| Other/undetermined | 0.61 (0.19-1.91) | 0.40 | 0.51 (0.21-1.25) | 0.13 | ||||
| Histological and gross features of tumors | ||||||||
| Tumor size >30 mm | 1.26 (0.87-1.81) | 0.22 | 1.21 (0.89-1.65) | 0.22 | ||||
| Poor differentiation | 1.45 (0.94-2.22) | 0.09 | 1.33 (0.84-2.11) | 0.22 | 1.43 (0.99-2.07) | 0.06 | 1.38 (0.95-1.99) | 0.09 |
| Macrotrabecular-massive subtype | 2.40 (1.44-4.00) | <0.001 | 2.37 (1.36-4.13) | 0.002 | 2.23 (1.38-3.60) | <0.001 | 2.19 (1.35-3.54) | 0.001 |
- Abbreviations: CI, confidence interval; NASH, nonalcoholic steatohepatitis.
Discussion
The present work demonstrates that the macrotrabecular-massive subtype of HCC is robustly recognized by pathologists on resected and biopsied samples. We further show, in two large series of HCC patients treated by resection or RFA, that the identification of this histological entity has a major impact on clinical outcome, exceeding known predictors such as size, vascular invasion, satellite nodules, BCLC stage, and AFP levels. This subtype, observed in 16% of surgical samples and 8.5% of biopsy samples, independently predicted early and overall tumor recurrence in both investigated series.
The definition of this particular subtype of HCC, which represents 12% of our overall series of tumors, relies on morphological features; and overall good and excellent interobserver agreements were observed among the different pathologists involved in the study. Therefore, this tumor variant may be reliably diagnosed in both surgical and small biopsy samples without specific training.
We were able to confirm the association of MTM-HCC with various adverse biological and pathological parameters (AFP serum level, tumor size, vascular invasion, and satellite nodules) and to show that this subtype was a key determinant of patient prognosis, with a strong and independent predictive value for early and overall tumor recurrence in both investigated series, even after stratification according to main classical HCC prognostic indicators. Interestingly, the relative risk of relapse associated with MTM-HCC appeared much higher than that associated with classical HCC outcome predictors, such as AFP serum levels, vascular invasion, or satellite nodules. Of note, although this variant is more prevalent in intermediate or advanced HCC, it still represents a significant fraction (10%) of tumors classified as very early or early (BCLC 0/A).
The aggressiveness of this variant may be related to molecular determinants. We indeed previously showed that this subtype was characterized by an activation of neoangiogenesis, with both Ang2 and VEGFA overexpression.16 Initially identified as an antagonist of Ang1, which promotes vascular stabilization by recruiting pericytes and smooth muscle cells to growing blood vessels, Ang2 is known to promote neoangiogenesis in cooperation with VEGFA and is an indicator of adverse outcome in various solid tumors.22 Ang2 promotes vascular sprouting and destabilizes blood vessels by disrupting interactions between endothelial cells and periendothelial cells, which results in increased sensitivity to VEGFA and thus favors neoangiogenesis activation.18, 23
It has also been shown that secretion of Ang2 by various primary solid tumors is capable of inducing metastasis.24 Indeed, by loosening endothelial cell junctions and increasing the vascular permeability of distant organs, Ang2 allows subsequent extravasation of circulating tumor cells and is considered a key regulator of the premetastatic niche.24-26 Satellite nodules are neoplastic nodules of HCC located <2 cm away from the main tumor and are usually considered early intrahepatic metastases,11 and we may thus hypothesize that Ang2 produced by neoplastic cells may disseminate in the directly adjacent liver parenchyma and contribute to the increased frequency of satellite nodules observed in MTM-HCC.
The underlying oncogenic pathways associated with this particular HCC subtype may also open therapeutic perspectives, and further studies will have to determine if MTM-HCC may be sensitive to potent inhibitors of neoangiogenesis. Noticeably, bispecific anti-Ang2 and anti-VEGFA antibodies have been reported to induce strong angiogenesis inhibition and tumor necrosis in various preclinical models of human malignancies.23, 27-29 Interestingly, Schmittnaegel et al. also demonstrated that blocking angiogenesis using these types of antibodies enhanced antitumor immunity. Indeed, the normalization and maturation of blood vessels induced by the treatment increased extravasation and perivascular accumulation of antitumor cluster of differentiation 8+, interferon-γ-producing cytotoxic T lymphocytes.28
The role of tumor biopsy for the management of patients with HCC is one of the most active debates in the liver cancer community.30-33 European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines currently do not require histological confirmation due to the reported excellent diagnostic performance of imaging procedures.34, 35 Moreover, although numerous histological tumor variants have been reported over the last decades, they did not demonstrate robust clinical impact and have thus aroused no or very little interest for physicians involved in the clinical care of patients with HCC. We provide in the present study evidence that MTM-HCC can be accurately diagnosed in biopsy samples and believe that the identification of this very aggressive subtype may lead to a reconsideration of the role of biopsy for HCC. Indeed, the identification of this variant during pretherapeutic workup has strong prognostic implications, and patients with this particular HCC subtype may benefit from adjuvant therapies and/or upfront registration on liver transplant waiting lists after resection or RFA. However, further studies are needed to assess the prognostic value of this subtype on recurrence after transplantation. In the same line, the activation of angiogenesis in this subset of tumors may also result in distinctive imaging features, and radiologic–pathologic correlations will have to determine if MTM-HCC may be diagnosed or at least suspected by computed tomography or magnetic resonance imaging.
In conclusion, we have demonstrated the value of the MTM-HCC subtype in predicting tumor recurrence in two large series of patients treated by distinct curative therapeutic modalities. We additionally show that this histological variant can reliably be identified on biopsy samples. Representing 12% of our overall series of patients treated by resection or RFA, we believe that MTM-HCC should be recognized as a distinct group of tumors that may require more personalized and aggressive treatments than conventional HCC.
Acknowledgment
We warmly thank Jacqueline Fontugne for English-language editing.
REFERENCES
Author names in bold designate shared co-first authorship.




