Wnt/β-catenin signaling controls intrahepatic biliary network formation in zebrafish by regulating notch activity
Potential conflict of interest: Nothing to report.
Supported in part by grants from the National Institutes of Health (DK101426, DK109365, to D.S.; DK105714, to M.K.; K08CA172288, to K.E.; and DK60322, to D.Y.R.S.) and from the Packard Foundation (to D.Y.R.S.).
Abstract
Malformations of the intrahepatic biliary structure cause cholestasis, a liver pathology that corresponds to poor bile flow, which leads to inflammation, fibrosis, and cirrhosis. Although the specification of biliary epithelial cells (BECs) that line the bile ducts is fairly well understood, the molecular mechanisms underlying intrahepatic biliary morphogenesis remain largely unknown. Wnt/β-catenin signaling plays multiple roles in liver biology; however, its role in intrahepatic biliary morphogenesis remains unclear. Using pharmacological and genetic tools that allow one to manipulate Wnt/β-catenin signaling, we show that in zebrafish both suppression and overactivation of Wnt/β-catenin signaling impaired intrahepatic biliary morphogenesis. Hepatocytes, but not BECs, exhibited Wnt/β-catenin activity; and the global suppression of Wnt/β-catenin signaling reduced Notch activity in BECs. Hepatocyte-specific suppression of Wnt/β-catenin signaling also reduced Notch activity in BECs, indicating a cell nonautonomous role for Wnt/β-catenin signaling in regulating hepatic Notch activity. Reducing Notch activity to the same level as that observed in Wnt-suppressed livers also impaired biliary morphogenesis. Intriguingly, expression of the Notch ligand genes jag1b and jag2b in hepatocytes was reduced in Wnt-suppressed livers and enhanced in Wnt-overactivated livers, revealing their regulation by Wnt/β-catenin signaling. Importantly, restoring Notch activity rescued the biliary defects observed in Wnt-suppressed livers. Conclusion: Wnt/β-catenin signaling cell nonautonomously controls Notch activity in BECs by regulating the expression of Notch ligand genes in hepatocytes, thereby regulating biliary morphogenesis. (Hepatology 2018;67:2352-2366).
Abbreviations
-
- BEC
-
- biliary epithelial cell
-
- BODIPY C5
-
- 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoic acid
-
- dpf
-
- days postfertilization
-
- F-actin
-
- filamentous actin
-
- GFP
-
- green fluorescent protein
-
- hpf
-
- hours postfertilization
-
- PED6
-
- N-{[6-(2,4-dinitrophenyl)amino] hexanoyl}-2-[4,4-difluoro-5,7-dimethyl-4-bora-3a,-4a-diaza-s-indacene-3-pentanoyl}-1-hexadecanoyl-sn-glycero-3-phosphoethanolamine
-
- WISH
-
- whole-mount in situ hybridization
The intrahepatic biliary network is a complex three-dimensional network of conduits lined by biliary epithelial cells (BECs) and plays a crucial role in transporting bile from hepatocytes to the gallbladder. Hence, malformations of the network can cause bile leakage and lead to inflammation, fibrosis, and cirrhosis, as observed in patients with cholestatic liver diseases. Identification of mutations in JAG1, a Notch ligand gene, in most Alagille syndrome patients1 provoked extensive studies about the role of Notch signaling in intrahepatic biliary development.2 During mammalian biliary development, bipotent liver progenitor cells (i.e., hepatoblasts) can differentiate into either hepatocytes or BECs, the latter forming ductal plates; undergoing extensive morphogenesis, including tubulogenesis; and eventually establishing the intrahepatic biliary network.3, 4
As a key regulator of biliary development, Notch signaling regulates early BEC induction and later morphogenesis processes.2 Hepatoblast-specific deletion of mouse Notch2 completely blocks BEC induction until the perinatal stage.5 Ectopic expression of the intracellular domain of NOTCH1 or NOTCH2 in mouse hepatoblasts initially induces excessive BECs and, later, ectopic biliary tubules.6-8 In another hepatoblast-specific Notch2 deletion setting in mice, BEC induction is unaffected but subsequent morphogenesis is impaired, thereby resulting in the impaired development of biliary tubules.9 In mice knockout of the key Notch target gene, Hes1, also displays the same morphogenesis defect.10 Moreover, Notch signaling regulates BEC proliferation and survival,6, 11 which can contribute to biliary morphogenesis. In mice, Notch signaling regulates the formation of peripheral intrahepatic bile ducts in a dose-dependent manner: higher Notch activity leads to denser intrahepatic bile ducts.11 Besides Notch signaling, which is implicated in both biliary induction and morphogenesis, transforming growth factor beta signaling also regulates biliary induction.12 However, additional factors regulating biliary morphogenesis remain largely unknown.
Wnt/β-catenin signaling regulates multiple processes of liver development, including hepatoblast specification and proliferation, hepatocyte maturation and proliferation, and liver zonation.13 It is also implicated in biliary development; however, data supporting the role of Wnt/β-catenin signaling in this process are inconsistent. In liver explant culture studies, WNT3A promotes the differentiation of hepatoblasts into BECs14; β-catenin stabilization with Apc deletion in mouse hepatoblasts induces their premature differentiation into BECs,15 supporting a positive role for Wnt/β-catenin signaling in biliary induction. In contrast, β-catenin deletion in mouse hepatoblasts does not affect their differentiation into BECs,16 suggesting that Wnt/β-catenin signaling is dispensable for biliary induction. This discrepancy makes the role of Wnt/β-catenin signaling in biliary development unclear. Although Wnt5a knockout mice exhibit excessive BECs, indicating a repressive role for Wnt5a in biliary induction, this effect is mediated by calcium/calmodulin-dependent protein kinase II, not by β-catenin.17
We sought to determine the role of Wnt/β-catenin signaling in intrahepatic biliary morphogenesis. Using zebrafish as an animal model, we show that Wnt/β-catenin signaling controls intrahepatic biliary network formation by regulating Notch signaling cell nonautonomously.
Materials and Methods
ZEBRAFISH LINES
Experiments were performed with the approval of the Institutional Animal Care and Use Committee at the University of Pittsburgh. We used the following mutants and transgenic lines: lef1zd11, tcf7nkhg21c, jag1bgd2Gt, Tg(Tp1:GFP)um14, Tg(Tp1:VenusPEST)s940, Tg(Tp1: H2B-mCherry)s939, Tg(Tp1:mCherry)pt611, Tg(Tp1:m Cherry-CAAX)s733, Tg(Tp1:GFP-utrophin)pt612, Tg(hs: Axin1)w35, Tg(hs:wnt2bb)pt603, Tg(hs:N3ICD)co17, Tg(hs:c a-β-catenin)w130, Tg(WRE:d2GFP)kyu1, and Tg(fabp10a: ca-β-catenin)s704. Their full names and references are listed in Supporting Table S1.
CHEMICAL TREATMENT
Stock solutions (1,000×) of XAV939 (Selleckchem, Houston, TX), CHIR99021 (Selleckchem), and LY411575 (Cayman Chemical, Ann Arbor, MI) were prepared in 100% dimethylsulfoxide and diluted to 10, 100, and 1 μM, respectively, with egg water. A 0.1% dimethylsulfoxide solution in egg water was used as a control.
PED6 AND BODIPY C5 ASSAYS
The N-{[6-(2,4-dinitrophenyl) amino]hexanoyl}-2-[4,4-difluoro-5,7-dimethyl-4-bora-3a,-4a-diaza-s-indacene-3-pentanoyl}-1-hexadecanoyl-sn-glycero-3-phosphoethanolamine (PED6) assay was performed by treating larvae with 0.3 μg/mL PED6 (Life Technologies, Grand Island, NY) for 3 hours as described18; the 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoic acid (BODIPY C5) assay was performed with 0.5 μM BODIPY C5 (Life Technologies) for 2 hours as described.19 Images for the PED6 and BODIPY C5 assays were obtained using epifluorescence and confocal microscopes, respectively. BODIPY C5–treated larvae were briefly rinsed, anesthetized with 0.016% tricaine/egg water, and then mounted in 1% low-melting agarose for confocal imaging.
Additional methods are available in Supporting Information.
Results
SUPPRESSION OF Wnt/β-CATENIN SIGNALING IMPAIRS INTRAHEPATIC BILIARY NETWORK FORMATION
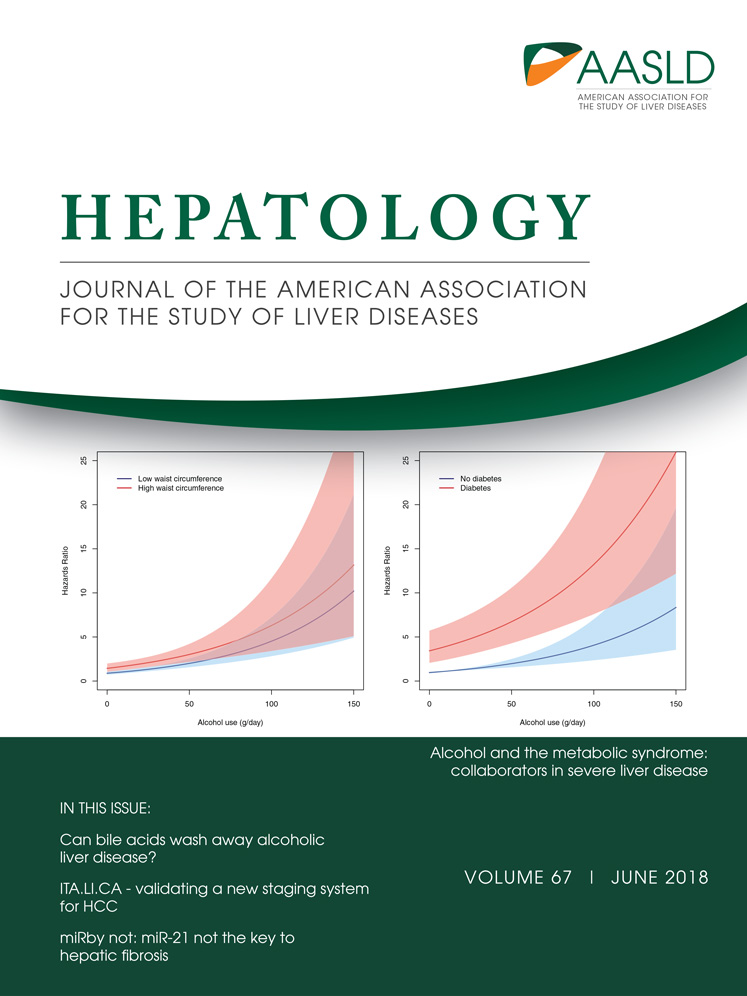
In zebrafish, hepatoblasts form around 22-24 hours postfertilization (hpf)20 and begin differentiating into BECs around 40-44 hpf.21, 22 By 5 days postfertilization (dpf), BECs establish a fully functional, intricate intrahepatic biliary network.23 To visualize BECs in the liver, we used the Tg(Tp1:GFP) line, which expresses green fluorescent protein (GFP) under the control of the promoter containing the Notch-responsive element.22 Using this transgenic line, we and others have reported the intrahepatic biliary morphogenesis process at the cellular level.21, 22 To determine if Wnt/β-catenin signaling regulates this morphogenesis process, we first suppressed Wnt/β-catenin signaling and examined the functionality of the biliary network. To suppress Wnt/β-catenin signaling, we used a Wnt inhibitor, XAV939, which stimulates β-catenin degradation by stabilizing Axin.24 To visualize biliary network functionality, we used a fluorescently labeled fatty acid reporter, PED6, which is metabolized into a component of bile in hepatocytes and accumulates in the gallbladder after biliary secretion.18 PED6 accumulation in the gallbladder was greatly reduced in larvae treated with XAV939 from 48 or 72, but not 96, hpf (Fig. 1A), suggesting the essential role of Wnt/β-catenin signaling in biliary morphogenesis prior to 96 hpf. To avoid impacting early liver development, as Wnt/β-catenin signaling also regulates hepatoblast specification and proliferation,20, 25, 26 we chose 72 hpf as the treatment start time for subsequent analyses.
To reveal bile canaliculi and intrahepatic bile conduits, in addition to the gallbladder, we used the fluorescently labeled lipid reporter BODIPY C5.27 In dimethylsulfoxide-treated control livers, intricate intrahepatic bile conduits were detected, whereas in XAV939-treated livers, the number of conduits was reduced (Fig. 1B, arrows). Bile canaliculi were thin and elongated in controls, whereas they were round and short in XAV939-treated livers (Fig. 1B, arrowheads), which was confirmed by the expression of Abcb11, a bile salt export pump present in the bile canaliculi of hepatocytes28 (Fig. 1C, arrowheads). In addition to the pharmacological inhibition, we suppressed Wnt/β-catenin signaling using a genetic tool, Tg(hs:Axin1), which expresses Axin1 upon heat shock.29 Axin1-overexpressing larvae also exhibited the same biliary defects as observed in XAV939-treated larvae: a poor connection of bile conduits (Supporting Fig. S1A, arrows) and stunted, round bile canaliculi (Supporting Fig. S1A,B, arrowheads).
Because bile canalicular defects can block the flow of bile from hepatocytes to bile ductules, bile conduits may not be fully revealed by BODIPY C5 fluorescence in Wnt-compromised livers. To minimize the effect of nonfunctional bile canaliculi on revealing bile conduits by BODIPY C5 fluorescence, we examined BODIPY C5 fluorescence in the Tg(Tp1:mCherry-CAAX) line,30 which expresses a membrane-localized form of mCherry in BECs, allowing for visualization of bile ductules. We quantified the length of bile ductules that exhibited BODIPY C5 fluorescence at both ends because BODIPY C5 must pass through the ductules and reveal them if they are open. Quantification of these ductules revealed that ∼18% of the length of the biliary network was not perfused by BODIPY C5 fluorescence in XAV939-treated livers compared to ∼1.2% in control livers (Fig. 1D, arrows, percentage of C5– bile ductules displaying C5 fluorescence at both ends), suggesting a defect in the formation of open ductules. Given the essential role of filamentous actin (F-actin) assembly in lumen31 and bile conduit formation,32 we examined actin filaments in XAV939-treated livers. Although no difference in BEC morphology (i.e., Tp1:GFP expression) was observed between XAV939-treated and control livers, the F-actin filaments appeared disconnected in XAV939-treated livers (Fig. 1E, arrows). To observe F-actin specifically in the BECs, as F-actin is also present in other cell types in the liver, we generated a Tg(Tp1:GFP-utrophin) line, in which utrophin, fused to GFP, binds to F-actin.33 This transgenic line also revealed disconnected actin filaments in XAV939-treated livers but not in control livers; ∼26% of Alcam+ bile ductules did not contain GFP-utrophin+ actin filaments (Fig. 1F, arrows). Altogether, these data suggest that Wnt/β-catenin signaling regulates intrahepatic biliary network formation.
OVERACTIVATION OF Wnt/β-CATENIN SIGNALING IMPAIRS INTRAHEPATIC BILIARY NETWORK FORMATION
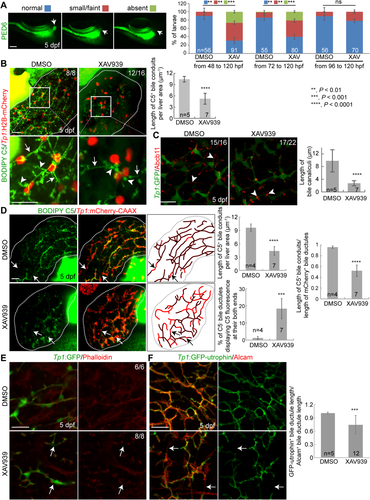
In addition to the suppression of Wnt/β-catenin signaling, we enhanced Wnt/β-catenin signaling using a glycogen synthase kinase 3 inhibitor, CHIR99021.34 Glycogen synthase kinase 3 inhibition leads to the accumulation of β-catenin and its subsequent nuclear translocation, thereby enhancing Wnt/β-catenin signaling. CHIR99021 treatment from 72 hpf impaired PED6 accumulation in the gallbladder (Supporting Fig. S2A) and resulted in a poor connection of bile conduits and stunted, round bile canaliculi (Fig. 2A). These phenotypes are similar to those observed in Wnt-suppressed livers (Fig. 1; Supporting Fig. S1). However, CHIR99021-treated livers had much thicker bile ductules than controls, as clearly revealed by the expression pattern of another BEC marker, Anxa435 (Fig. 2C), whereas the thickness was comparable between XAV939-treated and control livers (Fig. 1C). Consistent with the pharmacological activation results, enhancing Wnt/β-catenin signaling with two different genetic tools, Tg(hs:ca-β-catenin) and Tg(hs:wnt2bb), which upon heat shock express constitutively active β-catenin and a Wnt ligand, Wnt2bb, respectively, also resulted in a poor connection of bile conduits, stunted bile canaliculi, and thickened bile ductules (Fig. 2B,D; Supporting Fig. S2B-E).
Wnt/β-CATENIN SIGNALING IN HEPATOCYTES REGULATES INTRAHEPATIC NETWORK FORMATION
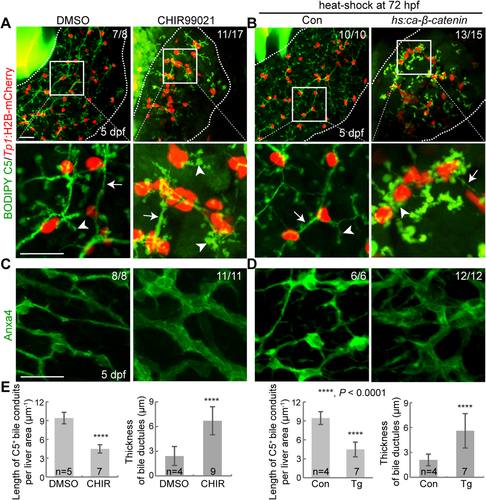
Using a Wnt/β-catenin signaling reporter line, Tg(WRE:d2GFP),36 we next determined hepatic cell types that exhibit active Wnt/β-catenin signaling. WRE:d2GFP was expressed in hepatocytes, but not BECs, of the liver at 3 dpf (Fig. 3A). At 4 dpf, although WRE:d2GFP expression was still restricted to the hepatocytes, fewer hepatocytes exhibited Wnt activity and the d2GFP intensity was much weaker than at 3 dpf (Fig. 3A). This hepatocyte-specific Wnt/β-catenin activity suggests a cell nonautonomous effect of Wnt/β-catenin signaling on BECs. To test this nonautonomous effect, we injected the fabp10a:mCherry-T2A-Axin1 or Tp1:mCherry-T2A-Axin1 DNA construct to drive Axin1 expression and suppress Wnt/β-catenin signaling in either hepatocytes or BECs, respectively. All Axin1-overexpressing BECs had well-connected actin filaments; however, ∼40% of bile ductules that contacted Axin1-overexpressing hepatocytes exhibited disconnected or absent actin filaments (Fig. 3B, arrows), confirming the cell nonautonomous role of Wnt/β-catenin signaling in biliary morphogenesis. Supporting this nonautonomous effect, hepatocyte-specific overactivation of Wnt/β-catenin signaling using the Tg(fabp10a:ca-β-catenin) line,37 which expresses constitutively active β-catenin under the hepatocyte-specific fabp10a promoter, resulted in a poor connection of bile conduits, stunted bile canaliculi, and thickened bile ductules (Fig. 3C,D), as observed upon the global overactivation of Wnt/β-catenin signaling (Fig. 2; Supporting Fig. S2C-E). Moreover, Tg(fabp10a:ca-β-catenin) livers exhibited disconnected actin filaments in bile ductules (Fig. 3E,F, arrows).
Wnt/β-CATENIN SIGNALING REGULATES NOTCH ACTIVITY IN BECs
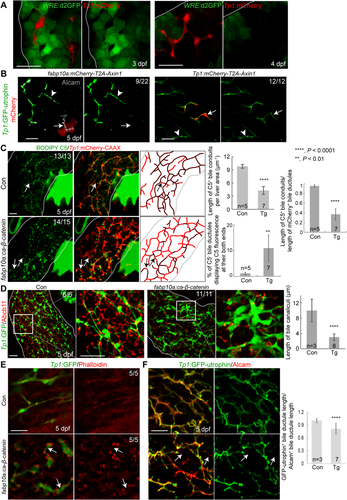
Given the role of Notch signaling in BEC induction and proliferation and biliary morphogenesis,2 we investigated whether Notch signaling was compromised in Wnt-suppressed livers. To reveal the strength of active Notch signaling, we used the Notch reporter line Tg(Tp1:VenusPEST), which expresses Venus fluorescent proteins fused with a short-lived PEST domain under the promoter containing the Notch-responsive element.38 Tp1:VenusPEST expression was greatly reduced in XAV939-treated and Axin1-overexpressing livers compared with their controls (Fig. 4A), indicating that Wnt/β-catenin signaling positively regulates Notch activity in the developing liver. Tp1:H2B-mCherry expression also revealed such a difference, but the difference was not apparent as with VenusPEST because H2B-mCherry is a very stable protein. In addition to the global suppression of Wnt/β-catenin signaling, the mosaic suppression of Wnt/β-catenin signaling in hepatocytes reduced Notch activity in BECs that contacted Axin1-overexpressing hepatocytes (Fig. 4B, arrows). However, Notch activity was not reduced in Axin1-overexpressing BECs (Fig. 4B), indicating the cell nonautonomous role of Wnt/β-catenin signaling in controlling hepatic Notch activity.
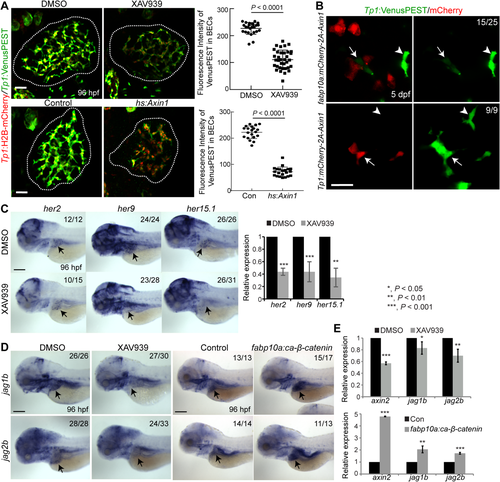
In addition to the Notch reporter lines, we examined the expression of Hes genes, well-known targets of Notch signaling. By whole-mount in situ hybridization (WISH) analysis, we first identified Hes genes that are restrictively expressed in BECs in the liver: her2, her9, and her15.1 (Supporting Fig. S3A, arrows). Their hepatic expression was significantly reduced in XAV939-treated larvae compared with controls (Fig. 4C), further supporting the positive role of Wnt/β-catenin signaling in controlling hepatic Notch signaling.
In colorectal cancer cells, Wnt/β-catenin signaling regulates Jag1 expression and thereby controls Notch activity.39 We investigated if such regulation also occurred in the developing liver. Jag1 is a key Notch ligand gene in the liver: mouse Jag1 mutants exhibit bile duct paucity,40 and human JAG1 mutation leads to Allagile syndrome.1 In zebrafish, although four jagged genes (jag1a, jag1b, jag2a, and jag2b) are expressed in the developing liver, jag1b and jag2b appear to be required for biliary formation.41 By WISH, we detected clear hepatic expression of all four jagged genes at 72 hpf (Supporting Fig. S3B, arrows). The overall staining in the entire liver suggests their expression in hepatocytes. Using a gene trap line that expresses GFP under the endogenous jag1b promoter,42 we confirmed jag1b expression in hepatocytes (Supporting Fig. S3C). Moreover, we observed the localized expression of jag1b around BECs at 60 and 72, but not 96, hpf (Supporting Fig. S3D). A subset of BECs at these earlier stages also expressed jag1b (Supporting Fig. S3D, arrows). Intriguingly, expression of both jag1b and jag2b was reduced in XAV939-treated livers and enhanced in ca-β-catenin-overexpressing livers compared with their controls (Fig. 4D), which was further confirmed by quantitative RT-PCR (Fig. 4E). Using the jag1b:GFP line, we additionally revealed that the change of jag1b expression occurred in hepatocytes but not in BECs (Supporting Fig. S3E,F). Three of the four Notch receptor genes in zebrafish, notch1a, notch1b, and notch2, were broadly expressed in the liver, as reported,41 whereas notch3 was restrictively expressed in BECs (Supporting Fig. S4A). This BEC-restricted expression was confirmed by coexpression of notch3 with Tp1:GFP (Supporting Fig. S4B). Unlike jag1b and jag2b, notch3 expression was not significantly changed in Wnt-suppressed livers compared with controls (Supporting Fig. S4C).
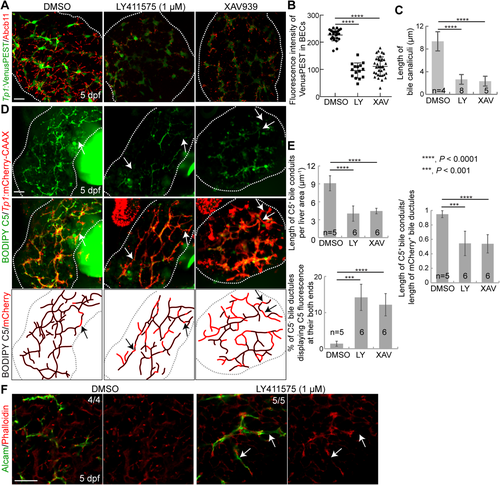
RESTORING NOTCH ACTIVITY RESCUES INTRAHEPATIC BILIARY DEFECTS OBSERVED IN XAV939-TREATED LIVERS
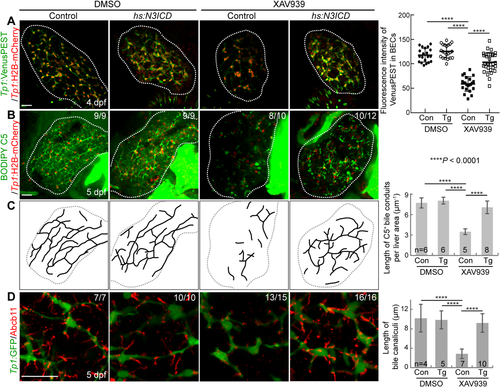
We next determined if the repressed Notch activity in XAV939-treated livers caused the intrahepatic biliary defects. If this were the case, reducing Notch activity to the same level as that detected in XAV939-treated livers should reproduce the biliary defects observed in XAV939-treated livers. First, we determined the dosage of a Notch inhibitor, LY411575, at which hepatic Notch activity is reduced to the level observed in XAV939-treated livers. Livers treated with 10 or 5 μM LY411575 exhibited almost no Notch activity (data not shown), whereas livers treated with 1 μM LY411575 exhibited a similar level of Notch activity to XAV939-treated livers (Fig. 5A,B). Importantly, 1 μM LY411575 treatment resulted in stunted bile canaliculi (Fig. 5C), a poor connection of bile conduits (Fig. 5D,E), and disconnected actin filaments (Fig. 5F, arrows), as XAV933 treatment did. To further prove that repressed Notch activity in XAV939-treated livers caused the intrahepatic biliary defects, we performed a rescue experiment using the Tg(hs:N3ICD) line that upon heat shock expresses the intracellular domain of Notch3.43 Intriguingly, upon the expression of N3ICD through a single heat shock at 72 hpf, the biliary defects observed in XAV939-treated livers were significantly rescued. Notch activity, which was assessed by Tp1:VenusPEST expression, was greatly increased at 4 dpf in N3ICD-overexpressing, XAV939-treated livers compared with only XAV939-treated livers (Fig. 6A), validating the efficacy of the Tg(hs:N3ICD) line. N3ICD overexpression significantly improved the connection of bile conduits (Fig. 6B,C) and the morphology of bile canaliculi (Fig. 6D) in XAV939-treated livers. These data clearly indicate that Wnt/β-catenin signaling controls intrahepatic biliary morphogenesis by regulating Notch signaling.
Discussion
In this study, we provide several lines of evidence supporting the role of Wnt/β-catenin signaling in intrahepatic biliary morphogenesis. First, suppressing or enhancing Wnt/β-catenin signaling with pharmacological or genetic tools impaired intrahepatic biliary morphogenesis. Second, the suppression of Wnt/β-catenin signaling greatly reduced Notch activity in BECs. Third, Wnt/β-catenin signaling in hepatocytes regulated expression of the Notch ligand genes jag1b and jag2b, thereby controlling Notch activity. Last, restoring Notch activity rescued intrahepatic biliary defects observed in Wnt-suppressed livers. Altogether, these data demonstrate a role for Wnt/β-catenin signaling in intrahepatic biliary network formation as the upstream regulator of Notch signaling.
Notch signaling is a key regulator of biliary development: it regulates biliary specification and intrahepatic biliary morphogenesis.2 Despite these key roles, the upstream regulator of Notch signaling in the liver remains unknown. Our finding that Wnt/β-catenin signaling regulates Notch activity by promoting the expression of Notch ligand genes provides an example of such an upstream regulator in the developing liver. Wnt/β-catenin signaling has been known to regulate Notch activity in other tissues. Wnt/β-catenin signaling directly regulates Jag1 expression, thereby inducing Notch signaling in colorectal cancer cells39; it also regulates the expression of Dll1, another Notch ligand gene, during somitogenesis, thereby inducing Notch activity in the presomitic mesoderm.44 In addition, activated β-catenin stimulates Jag1 expression in osteoblasts, thereby inducing Notch activity in hematopoietic stem cell progenitors, which can lead to leukemia.45 Likewise, we found that in the developing zebrafish liver Wnt/β-catenin signaling in hepatocytes regulates jag1b and jag2b expression, thereby controlling Notch activity in BECs.
Although suppressing and overactivating Wnt/β-catenin signaling both impaired biliary morphogenesis, the biliary defects observed in Wnt-overactivated livers did not appear to be due to abnormal Notch activity because Notch activity did not appear to be affected in Wnt-overactivated livers (Supporting Fig. S5). Moreover, overactivation of Notch signaling through N3ICD expression did not impair biliary morphogenesis (Fig. 6B), suggesting that increased Notch activity does not compromise biliary morphogenesis. N3ICD expression also failed to rescue biliary defects in ca-β-catenin-expressing livers (Supporting Fig. S6), unlike in XAV939-treated livers (Fig. 6), suggesting that Notch activity was not reduced in Wnt-overactivated livers. Although Wnt overactivation increased hepatic expression of jag1b and jag2b, it appears to affect biliary morphogenesis through a mediator other than Notch signaling.
During zebrafish biliary morphogenesis, Wnt and Notch activities were observed only in hepatocytes and BECs, respectively. However, global suppression of Notch signaling affected not only bile conduits but also the bile canaliculi of hepatocytes. In addition, global activation of Notch signaling rescued the defects in the morphology of bile canaliculi in Wnt-suppressed livers. These data raise the possibility that the formation of bile canaliculi and bile ductules is interdependent. Supporting this possibility, blocking the formation of bile canaliculi impairs intrahepatic biliary network formation in mice.3, 46 This interdependence makes it very difficult to determine whether the suppression of Wnt/β-catenin signaling primarily affects bile canaliculi or bile ductules.
Intrahepatic biliary morphogenesis continues between 4 and 5 dpf: bile ductules further elongate and become thinner.21, 22, 32 However, suppressing Wnt/β-catenin signaling during this period did not impair intrahepatic biliary morphogenesis or reduce Notch activity (Supporting Fig. S7). In contrast, the complete inhibition of Notch signaling during this period impairs biliary morphogenesis,22 indicating the continuous role of Notch signaling in biliary morphogenesis between 4 and 5 dpf. Given that hepatic Wnt activity is very weak at 96 hpf and only a small subset of hepatocytes exhibit the weak Wnt activity (Fig. 3A), Wnt/β-catenin signaling does not appear to regulate hepatic Notch activity from 4 dpf. It will be interesting to identify another upstream regulator of Notch signaling in the developing liver.
We sought to identify key component genes of the Wnt/β-catenin and Notch signaling pathways that are essential for intrahepatic biliary morphogenesis. For Wnt/β-catenin signaling, we examined biliary functions and morphology in lef1 and tcf7 single and double mutants, given their relatively high expression in the developing liver compared with three other Tcf/Lef family member genes, tcf7l1a, tcf7l1b, and tcf7l2 (Supporting Fig. S8C). However, there was no biliary defect in these mutants (data not shown), suggesting that the three other genes may compensate for the absence of lef1 and tcf7 in the developing liver. We also examined the expression of Wnt receptor fzd and coreceptor lrp genes and found that six fzd genes and both lrp5 and lrp6 were expressed in the developing liver (Supporting Fig. S8A,B, arrows). For Notch signaling, we examined biliary functions and morphology in jag1b mutants, given its positive regulation by Wnt/β-catenin signaling (Fig. 4E; Supporting Fig. S3D). However, there was no biliary defect in these mutants (data not shown), suggesting the involvement of other Notch ligand genes, such as jag1a, jag2a, and jag2b, in biliary morphogenesis. Given published morpholino-mediated jagged knockdown studies41 and the positive regulation of jag2b in the developing liver by Wnt/β-catenin signaling (Fig. 4E), jag2b together with jag1b may regulate intrahepatic biliary morphogenesis in zebrafish. Although our mutant analyses failed to reveal a key component of the Wnt/β-catenin or Notch signaling pathway that is essential for biliary morphogenesis, these negative data suggest high redundancy among the Tcf/Lef family member and Notch ligand genes.
In the zebrafish liver most BECs directly contact surrounding hepatocytes,47 whereas in the mammalian liver only BECs that reside in intrahepatic bile ductules directly contact hepatocytes. Although in the healthy mouse liver BECs in bile ductules are a small portion of the total BECs, in the injured liver bile ductules elongate through the proliferation of BECs, making longer and denser bile ductules.48, 49 These extended ductules appear to replace collapsed bile canaliculi between damaged hepatocytes, thereby restoring bile drain. This temporarily established intrahepatic biliary network is very similar to the intrahepatic biliary network in zebrafish because BECs in the network directly contact hepatocytes. In the healthy adult liver Wnt activity is restricted to hepatocytes near the central veins (i.e., pericentral hepatocytes); however, upon liver injury, Wnt activity is induced in other hepatocytes.13 Intriguingly, in β-catenin conditional knockout mice which lack β-catenin in both hepatocytes and BECs, the number of activated BECs, also called “oval cells,” induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine treatment was dramatically reduced compared with wild-type controls.50 Because the reduced BEC number should cause shorter and sparser bile ductules, the mutant data suggest the involvement of Wnt/β-catenin signaling in intrahepatic biliary morphogenesis during liver injury. In the future, it will be interesting to test whether the Wnt/β-catenin and Notch crosstalk observed during zebrafish liver development also regulates intrahepatic biliary morphogenesis following liver injury.
Acknowledgment
We thank Bruce Appel for Tg(hs: N3ICD), Jian Zhang for jag1b, Richard Dorsky for lef1, and Koichi Kawakami for tcf7 fish. We also thank Jinrong Peng for anti-Bhmt antibodies, Beth Roman for in situ probes, and Neil Hukriede and Michael Tsang for discussion.
REFERENCES
Author names in bold designate shared co-first authorship.