Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis
Potential conflict of interest: Dr. Carey consults for NGM and advises Target. Dr. Silveira consults for Conatus.
Abstract
Primary sclerosing cholangitis (PSC) is a risk factor for cholangiocarcinoma (CCA) and gallbladder carcinoma (GBCa). Surveillance for GBCa is recommended, but the clinical utility of surveillance for other hepatobiliary cancers (HBCa) in PSC, namely CCA and hepatocellular carcinoma (HCC), remains unclear. We aimed to determine whether surveillance is associated with better survival after diagnosis of HBCa in patients with PSC. Medical records of PSC patients seen at the Mayo Clinic Rochester from 1995 to 2015 were reviewed. Patients were included if they had ≥1 year of follow-up and developed HBCa. Patients were categorized according to their surveillance status (abdominal imaging, carbohydrate antigen 19-9, and alpha-fetoprotein). The primary endpoints were HBCa recurrence, HBCa-related death, and all-cause mortality. Overall survival was assessed by the Kaplan-Meier survival method; HBCa-related survival was assessed using competing risk regression. Tests of significance were two-tailed, and a P value <0.05 was considered statistically significant. From 1995 to 2015, a total of 79 of 830 PSC patients were diagnosed with HBCa. Cumulative follow-up was 712 and 283 person-years pre- and post-HBCa diagnosis, respectively. Seventy-eight percent of patients (54/79) developed CCA, 21% (17/79) HCC, 6% (5/79) GBCa, 3% (2/79) both CCA and HCC, and 1% (1/79) both HCC and GBCa. Fifty-one percent (40/79) were under HBCa surveillance, and 49% (39/79) were not. Patients in the surveillance group had significantly higher 5-year overall survival (68% versus 20%, respectively; P < 0.001) and significantly lower 5-year probability of experiencing an HBCa-related adverse event (32% versus 75%, respectively; P < 0.001) compared with the no-surveillance group. Conclusion: This study demonstrates that HBCa surveillance significantly improves outcomes, including survival, in patients with PSC. (Hepatology 2018;67:2338-2351).
Abbreviations
-
- CA19-9
-
- carbohydrate antigen 19-9
-
- CCA
-
- cholangiocarcinoma
-
- CI
-
- confidence interval
-
- CT
-
- computed tomography
-
- eCCA
-
- extrahepatic CCA
-
- ERC
-
- endoscopic retrograde cholangiography
-
- FISH
-
- fluorescence in situ hybridization
-
- GBCa
-
- gallbladder carcinoma
-
- HBCa
-
- hepatobiliary cancer
-
- HCC
-
- hepatocellular carcinoma
-
- HR
-
- hazard ratio
-
- iCCA
-
- intrahepatic CCA
-
- LT
-
- liver transplantation
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- MRI
-
- magnetic resonance imaging
-
- pCCA
-
- perihilar CCA
-
- PSC
-
- primary sclerosing cholangitis
-
- US
-
- ultrasound
“Surveillance” is derived from the French compound word surveiller (sur-, “over”; veiller, “to watch”), meaning “to watch over” or “to oversee”; when applied to health, it refers to monitoring for the occurrence of a disease/condition in an at-risk population. Patients with primary sclerosing cholangitis (PSC) are known to be at increased risk for hepatobiliary cancer (HBCa); indeed, cholangiocarcinoma (CCA) and gallbladder carcinoma (GBCa) are feared complications of PSC.1 Hepatocellular carcinoma (HCC) in PSC is reportedly rare,2 although this is controversial. Current guidelines recommend assessing for GBCa in PSC with annual ultrasonography3, 4; however, the clinical utility of surveillance for CCA and HCC in PSC remains unknown.
Treatment options and outcomes of patients with HBCa depend to a large extent on tumor stage. Early diagnosis of HBCa is thus imperative for patients to achieve maximal benefit from existing treatment modalities.5 Because only patients with early-stage CCA are eligible for liver transplantation (LT) or surgical resection,6, 7 experts have advocated for surveillance in patients with PSC.1, 8-11
An HBCa surveillance program was established for PSC patients at the Mayo Clinic beginning in 1995. Our preliminary data demonstrated that HBCa surveillance was associated with a trend toward better survival.12 The present report is a continuation of efforts to determine the clinical utility of HBCa surveillance in PSC. Our main objective was to determine whether HBCa surveillance is associated with better clinical outcomes after diagnosis of HBCa in patients with PSC.
Materials and Methods
This study was approved by the Mayo Clinic institutional review board (application 16-007183).
PATIENT SELECTION
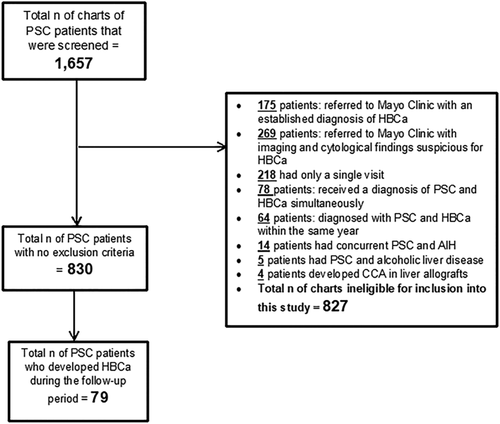
The Mayo Clinic Rochester database was queried for medical records of patients who were diagnosed with PSC between the years 1995 and 2015 based on established criteria.3 Patients were included in this study if they: (1) had ≥1 year of clinical, laboratory, and imaging follow-up from the time of PSC diagnosis or first clinical encounter at Mayo Clinic Rochester,2 developed HBCa during the follow-up period, and3 had ≥1 year of follow-up after receiving a diagnosis of HBCa and undergoing treatment for HBCa (when applicable). Subjects were excluded if any of the following applied: received a new diagnosis of PSC and HBCa simultaneously; referred to the Mayo Clinic with an outside diagnosis of HBCa or with cytology suspicious for malignancy, biliary epithelial fluorescence in situ hybridization (FISH) polysomy, and/or malignant-appearing stricture/mass on imaging; diagnosis of HBCa during the first year after PSC diagnosis; history of LT; and comorbid hepatitis B, C, or D infection, primary biliary cholangitis, autoimmune hepatitis, alcoholic liver disease, nonalcoholic steatohepatitis, Wilson's disease, hemochromatosis, or immunoglobulin G4–associated cholangitis.
DATA COLLECTION
The following data were collected: age at time of PSC and HBCa diagnosis; gender; HBCa surveillance status (yes/no); presence of inflammatory bowel disease; type of inflammatory bowel disease; type of HBCa (extrahepatic CCA [eCCA, which includes perihilar as well as distal CCA], intrahepatic CCA [iCCA], GBCa, or HCC); stage of PSC at the time of PSC diagnosis/initial encounter and HBCa treatment; lymph node status at time of HBCa diagnosis; HBCa staging according to the tumor–node–metastasis classification system; FISH status; imaging modality used for detecting HBCa; symptoms at time of HBCa diagnosis; serum liver biochemistries, carbohydrate antigen 19-9 (CA19-9) level, and alpha-fetoprotein level at time of HBCa diagnosis; whether patient underwent chemotherapy, radiation therapy, brachytherapy, transarterial chemoembolization, radiofrequency ablation, surgical resection, or LT; death; cause of death; HBCa recurrence; re-LT; and reason for re-LT.
HBCa SURVEILLANCE PROGRAM
HBCa surveillance in PSC patients began at the Mayo Clinic Rochester in 1995. Not all patients and their providers chose to participate; thus, a natural experimental group occurred. Surveillance for CCA consisted of annual imaging with abdominal ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI)/cholangiopancreatography (MRCP) plus CA19-9. Surveillance for GBCa consisted of imaging, usually US, every 6-12 months. Surveillance for HCC began once PSC progressed to cirrhosis and consisted of imaging (US, MRI/MRCP [contrast-enhanced unless contraindicated], or CT) every 6-12 months plus alpha-fetoprotein. The imaging modality used was based on patient factors and physician preference.
In general, new dilatation and/or thickening of the bile ducts on imaging or the presence of elevated CA19-9 prompted MRI/MRCP and/or endoscopic retrograde cholangiography (ERC) to evaluate the biliary tree and obtain brushings for cytological and FISH analyses. Repeat MRI/MRCP and/or ERC with CA19-9 was usually performed every 3-6 months when the initial cytology/FISH results were inconclusive. If a mass was detected by US, an MRI or a multiphasic CT was performed. If a gallbladder mass/polyp was detected by cross-sectional imaging, either it was imaged more frequently (usually every 3-6 months) or surgery was consulted for cholecystectomy.
DEFINITIONS
HBCa
CCA was diagnosed by a combination of imaging, cholangiography, CA19-9, pathology, cytology, digital imaging analysis, and/or FISH techniques.13-15 For the purposes of this study, CA19-9 >100 U/mL (without alternative etiology) was the cutoff for diagnosis of CCA,16 and confirmation was by histopathology in selected cases. HCC was diagnosed by published criteria,17 with biopsy in selected cases. GBCa was diagnosed using published criteria18 and confirmed histopathologically.
Severity of Liver Disease
Liver biopsies were obtained and staged according to the criteria of Ludwig et al.19 Cirrhosis was diagnosed by liver biopsy, endoscopy confirming portal hypertension, and/or radiological evidence of cirrhosis and/or portal hypertension. For analysis, patients were grouped into “early stage” (I, I-II, and II) and “late stage” (III, III-IV, and IV) PSC.
Treatment Protocols
Treatment protocols for HBCa (perihilar CCA [pCCA], iCCA, distal CCA, HCC, and GBCa) are presented in Supporting Table S1.20, 21
STATISTICAL ANALYSIS
Continuous data were expressed as median with interquartile range or minimum and maximum. Categorical data were expressed as frequency and percentage. Chi-squared and Fisher's exact tests were used to compare categorical variables between the HBCa surveillance and no-surveillance groups. The Wilcoxon rank-sum and Student t test were used to compare means between the surveillance and no-surveillance groups.
Survival analyses were used to compare long-term outcomes between the HBCa surveillance and no-surveillance groups. The primary endpoint was time from HBCa detection to first occurrence of any of the following: HBCa recurrence, HBCa-related death, all-cause mortality, or last known clinic follow-up.
When analyzing HBCa-related survival, we estimated cumulative incidence based on a competing risks framework.22 Herein, HBCa-related adverse events (HBCa recurrence and HBCa-related death) were treated as failures, whereas death from any other cause was treated as a “competing event,” and patients who were HBCa-free at last follow-up were censored. We used the competing-risks regression model23 to examine the association between variables of interest and HBCa-related outcomes.
When analyzing overall survival, all-cause death (including HBCa-related death) was treated as a failure, whereas patients who were alive at last follow-up were censored. Survival in the HBCa surveillance and no-surveillance groups was computed using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards regression was used to determine independent predictors of HBCa-related adverse events and all-cause mortality.
Survival analyses were also used to compare long-term outcomes based on the imaging modality used for HBCa detection (US, CT, or MRI/MRCP). For all three imaging groups, the competing risks framework was used to analyze HBCa-related survival, and the Kaplan-Meier method was used for overall survival. Analyses were conducted using STATA v12.1 (StataCorp LP, College Station, TX); P < 0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS
From 1995 to 2015, a total of 79 of 830 patients with PSC developed HBCa (Fig. 1 and Table 1) meeting inclusion criteria. Sixty-eight percent (54/79) developed CCA, 21% (17/79) HCC, 6% (5/79) GBCa, 3% (2/79) both CCA and HCC, and 1% (1/79) developed both HCC and GBCa. Severity of liver disease and symptoms at the time of HBCa diagnosis are presented in Supporting Tables S2 and S3, respectively. The median time from PSC diagnosis until HBCa diagnosis was 8 years (range, 1-27 years), and the median time from HBCa diagnosis until HBCa recurrence, death, or last clinic visit was 2 years (range, 1-20). The cumulative follow-up was 712 and 283 person-years pre-HBCa diagnosis and post-HBCa diagnosis, respectively.
| Variable | Descriptive Statistics (n = 79) |
|---|---|
| Age at time of PSC diagnosis, yearsa | 47 (37-52) |
| Age at time of HBCa diagnosis, yearsa | 56 (49-62) |
| Male gender, % (n) | 65% (51) |
| IBD, % (n) | 92% (71) |
| Type of IBD, % (n) | |
| UC | 88% (63) |
| CD | 11% (8) |
| Indeterminate | 1% (1) |
| Severity of PSC at time of PSC diagnosis/initial encounter by combined clinical data | |
| Early-stage PSC | 53% (42/79) |
| Late-stage PSC | 29% (23/79) |
| Unknown | 18% (14/79) |
| Method of cirrhosis/portal hypertension assessment | |
| Cirrhotic morphology on imaging | 5% (6/65) |
| Varices | 15% (10/65) |
| Ascites | 5% (3/65) |
| Splenomegaly | 9% (6/65) |
| Histologyb | 10% (4/40) |
| Severity of PSC at time of LT or resection by combined clinical data | |
| Early-stage PSC | 8% (6/79) |
| Late-stage PSC | 75% (59/79) |
| Unknown | 17% (14/79) |
| Method of cirrhosis/portal hypertension assessment | |
| Morphology of cirrhosis on imaging | 63% (41/65) |
| Varices | 49% (32/65) |
| Ascites | 46% (30/65) |
| Splenomegaly | 48% (31/65) |
| Histologyc | 69% (34/49) |
| Patients with HBCa symptoms, % (n) | 60% (47/79) |
| Diagnosis of CCA (n = 56) | |
| Mass on cross-sectional imaging, % (n) | 52% (29/56) |
| Stricture on cholangiography, % (n) | 88% (43/49) |
| FISH polysomy, % (n) | 51% (25/49) |
| Positive DIA, % (n) | 53% (10/19) |
| Positive cytology, % (n) | 26% (11/43) |
| Suspicious cytology, % (n) | 28% (12/43) |
| Biopsy, % (n) | 34% (19/56) |
| Found CCA at LT, surgical resection, or diagnostic laparoscopy, % (n) | 75% (42/56) |
| Diagnosis of HCC (n = 20) | |
| By imaging, % (n) | 95% (19/20) |
| Biopsy, % (n) | 40% (8/20) |
| Found at time of LT, % (n) | 100% (13/13) |
| Diagnosis of GBCa | |
| By imaging, % (n) | 67% (4/6) |
| Biopsy, % (n) | 100% (6/6) |
| Laboratory values at time of HBCa diagnosisa | |
| CA19-9 (U/mL) | 59 (18-865) |
| ALP (U/L) | 413 (233-810) |
| AST (U/L) | 91 (58-126) |
| ALT (U/L) | 84 (44-147) |
| T. bili. (mg/dL) | 2.4 (1-6.9) |
| Type of HBCa, % (n) | |
| CCA | 68% (54) |
| CCA and HCC | 3% (2) |
| HCC | 22% (17) |
| GBCa | 6% (5) |
| GBCa and HCC | 1% (1) |
| Lymph node involvement, % (n) | 36% (26/73) |
| Intrahepatic metastases, % (n) | 22% (16/73) |
| Extrahepatic metastases, % (n) | 19% (14/73) |
| Treatment | |
| Surgical | |
| LT | 46% (36/79) |
| Resection | 39% (7/18) |
| Pancreaticoduodenectomy | 6% (5/79) |
| Palliative | 9% (7/79) |
| Otherd | 15% (12/79) |
- a Values expressed as median (interquartile range).
- b PSC histological stage data at the time of PSC diagnosis/initial encounter were available for 40 patients (20 had PSC stage I-II, 15 had stage II-III, and 5 had stage III-IV).
- c PSC histological stage data at the time of HBCa diagnosis/LT/resection were available for 49 patients (3 had PSC stage I-II, 1 had stage II-III, and 45 had stage III-IV).
- d Twelve patients underwent transarterial chemoembolization, of whom 1 also underwent radiofrequency ablation and 1 cyberknife treatment.
- Abbreviations and reference ranges: ALP, alkaline phosphatase (normal range, 45-115); ALT, alanine aminotransferase (normal range, 7-55); AST, aspartate aminotransferase (normal range, 8-48); CA19-9 (normal, <55); CD, Crohn's disease; DIA, digital imaging analysis; IBD, inflammatory bowel disease; T. bili., total bilirubin (normal range, 0.1-1); UC, ulcerative colitis.
CONFIRMATION OF HBCa
HBCa diagnoses were established/confirmed pretreatment and/or posttreatment histopathologically and/or cytopathologically in 86% (68/79) of the study population. Diagnostic specimens were obtained from the following interventions: 38% (26/68) liver explants, 26% (18/68) percutaneous biopsies of the liver (n = 15) or metastatic lesions (n = 3), 18% (12/68) surgical specimens (partial hepatectomy [n = 6], gallbladder [n = 6]), and 7% (5/68) in brushings/biopsies obtained by ERC/endoscopic US. Of the patients without tissue diagnosis (n = 11), 5 had evidence of malignancy by a visible mass on imaging, and 6 were diagnosed with pCCA on the basis of a malignant-appearing stricture on cholangiography, CA19-9 >100 U/mL (n = 2), polysomy on FISH (n = 3), and/or positive digital imaging analysis (n = 3).
SURVIVAL ANALYSIS IN THE ENTIRE STUDY POPULATION BASED ON SURVEILLANCE STATUS
Impact of HBCa Surveillance on HBCa-Related Survival
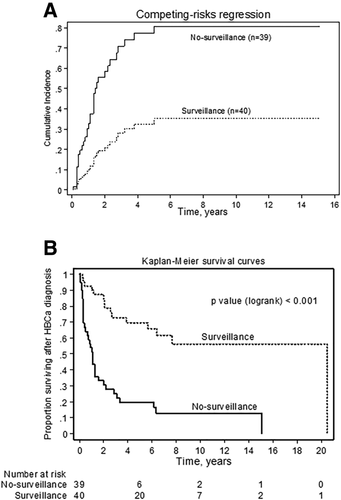
The clinicodemographic and biochemical features of our study population and tumor staging according to the tumor–node–metastasis classification system are summarized in Table 2 and Supporting Table S4, respectively. Lymph node-positive HBCa and HBCa with intrahepatic and extrahepatic metastasis were significantly more frequent in the no-surveillance group than in the surveillance group (54% versus 17%, P = 0.001; 35% versus 10%, P = 0.012; and 31% versus 8%, P = 0.016, respectively). Moreover, patients in the surveillance group were more likely to receive LT as curative treatment for HBCa compared to those in the no-surveillance group (65% versus 23%, P < 0.001). The probability of an HBCA-related event (recurrence or HBCa-related death) within 5 years was 32% with surveillance versus 75% without surveillance (Fig. 2A).
| Variable | Overall (n = 79) | Surveillance (n = 40) | No-surveillance (n = 39) | P |
|---|---|---|---|---|
| Male | 65% (51) | 60% (24) | 69% (27) | 0.48 |
| Age at time of PSC diagnosis, yearsa | 47 (37-52) | 46 (37-50) | 47 (37-54) | 0.45 |
| Age at time of HBCa diagnosis, yearsa | 56 (49-62) | 55 (49-61) | 56 (49-63) | 0.62 |
| Diagnosis of PSC, year | ||||
| 1980-1984 | 5% (4) | 5% (2) | 5% (2) | |
| 1985-1989 | 4% (3) | 5% (2) | 3% (1) | |
| 1990-1994 | 20% (16) | 18% (7) | 23% (9) | 0.86 |
| 1995-1999 | 28% (22) | 32% (13) | 23% (9) | |
| 2000-2004 | 23% (18) | 18% (7) | 28% (11) | |
| 2005-2009 | 15% (12) | 18% (7) | 13% (5) | |
| 2010-2013 | 5% (4) | 5% (2) | 5% (2) | |
| Time from PSC to HBCa diagnosis (years)b | 8 (1-27) | 10 (1-27) | 7 (1-26) | 0.61 |
| Severity of PSC at time of PSC diagnosis/initial encounter by combined clinical data | ||||
| Early-stage | 53% (42/79) | 48% (19/40) | 59% (23/39) | |
| Late-stage | 29% (23/79) | 35% (14/40) | 23% (9/39) | 0.48 |
| Unknown | 18% (14/79) | 17% (7/40) | 18% (7/39) | |
| Method of cirrhosis/portal hypertension assessment | ||||
| Cirrhotic on imaging | 5% (3/65) | 3% (1/33) | 6% (2/32) | |
| Varices | 15% (10/65) | 18% (6/33) | 13% (4/32) | |
| Ascites | 5% (3/65) | 6% (2/33) | 3% (1/32) | |
| Splenomegaly | 9% (6/65) | 12% (4/33) | 6% (2/32) | |
| Histology | 6% (4/65) | 6% (2/33) | 6% (2/32) | |
| Severity of PSC at time of LT or resection by combined clinical data | ||||
| Early-stage | 8% (6/79) | 10% (4/40) | 5% (2/39) | |
| Late-stage | 75% (59/79) | 85% (34/40) | 64% (25/39) | 0.06 |
| Unknown | 17% (14/79) | 5% (2/40) | 31% (12/39) | |
| Method of cirrhosis/portal hypertension assessment | ||||
| Cirrhotic on imaging | 63% (41/65) | 66% (25/38) | 59% (16/27) | |
| Varices | 49% (32/65) | 53% (20/38) | 44% (12/27) | |
| Ascites | 46% (30/65) | 45% (17/38) | 48% (13/27) | |
| Splenomegaly | 48% (31/65) | 47% (18/38) | 48% (13/27) | |
| Histology | 69% (34/49) | 47% (23/49) | 22% (11/49) | |
| Patients with HBCa symptoms, % (n) | 60% (47/79) | 23% (9/40) | 97% (38/39) | <0.001 |
| HBCa type | ||||
| pCCA+dCCA | 43% (34) | 33% (13) | 53% (21) | |
| pCCA + HCC | 2% (2) | 2% (1) | 3% (1) | |
| iCCA | 23% (18) | 15% (6) | 32% (12) | |
| dCCA | 4% (3) | 0% (0) | 7% (3) | |
| HCC | 22% (17) | 35% (14) | 7% (3) | |
| GBCa | 6% (5) | 12% (5) | 0% (0) | |
| GBCa + HCC | 1% (1) | 3% (1) | 0% (0) | |
| Type of IBD | ||||
| UC | 88% (63/72) | 89% (32/36) | 86% (31/36) | |
| CD | 11% (8/72) | 11% (4/36) | 11% (4/36) | 1 |
| Indeterminate | 1% (1/72) | 0% (0/36) | 3% (1/36) | |
| Histological stage of PSC at time of LT or resection, % (n) | ||||
| Biopsy not done | 38% (30/79) | 22% (9/40) | 54% (21/39) | |
| I – II | 8% (4/49) | 9% (3/32) | 6% (1/17) | 1 |
| III – IV | 92% (45/49) | 91% (29/32) | 94% (16/17) | |
| Laboratory values at time of HBCa diagnosis | ||||
| CA 19-9 (U/mL)c | 301 (34-1,643) | 90 (23-1,082) | 392 (42-2,604) | 0.22 |
| AFP (ng/mL)d | 5.5 (3-7.1) | 5.4 (2.4-7) | 17,567(7-35,126) | 0.072 |
| ALP (U/L) | 413(233-810) | 393(191-711) | 486 (298-819) | 0.12 |
| AST (U/L) | 91 (58-126) | 89 (58-110) | 95 (58-132) | 0.41 |
| ALT (U/L) | 84 (44-147) | 77 (40-115) | 102 (45-181) | 0.068 |
| T. bili. (mg/dL) | 2.4 (1-6.9) | 1.8 (0.9-3.1) | 3.2 (1.3-10.4) | 0.057 |
| Imaging used as diagnostic tool, % (n)e | ||||
| US | 24% (19/78) | 35% (14/40) | 13% (5/38) | |
| MRI/MRCP | 40% (31/78) | 35% (14/40) | 45% (17/38) | 0.19 |
| CT | 28% (22/78) | 23% (9/40) | 34% (13/38) | |
| ERC | 8% (6/78) | 7% (3/40) | 8% (3/38) | |
| Lymph node involvement, % (n) | 36% (26/73) | 17% (6/36) | 54% (20/37) | 0.001 |
| Intrahepatic metastases, % (n) | 22% (16/73) | 10% (4/39) | 35% (12/34) | 0.012 |
| Extrahepatic metastasis, % (n) | 19% (14/73) | 8% (3/38) | 31% (11/35) | 0.016 |
| Treatment | ||||
| LT | 44% (35/79) | 65% (26/40) | 23% (9/39) | <0.001 |
| Palliative | 9% (7/79) | 0% (0/40) | 18% (7/39) | 0.021 |
- a Median (interquartile range).
- b Median (range).
- c Included only patients with CCA (n = 56).
- d Alpha-fetoprotein levels were only for those with HCC (n = 20).
- e Diagnosis of HBCa was established in one case by biopsy of metastatic nodule in the lungs (found to be HCC).
- Abbreviations and reference ranges: AFP, alpha-fetoprotein (normal, <6); ALP, alkaline phosphatase (normal range, 45-115); ALT, alanine aminotransferase (normal range, 7-55); AST, aspartate aminotransferase (normal range, 8-48); CA19-9 (normal, <55); CD, Crohn's disease; dCCA, distal cholangiocarcinoma; IBD, inflammatory bowel disease; T. bili., total bilirubin (normal range, 0.1-1); UC, ulcerative colitis.
The incidence of HBCa-related adverse events was 8.5 times greater in the no-surveillance group than in the surveillance group (3.4; 95% confidence interval [CI], 2.35-4.82; versus 0.4; 95% CI, 0.21-0.76 events per 10 person-years). Univariate analysis using competing-risks regression demonstrated that surveillance was associated with better overall outcomes (hazard ratio [HR], 0.18; P < 0.001; 95% CI, 0.09-0.37). Higher CA19-9 at the time of CCA diagnosis was associated with worse outcomes in the CCA group (HR, 1.26; P < 0.001; 95% CI, 1.14-1.39). In a multivariable competing-risks regression model of patients who developed CCA (n = 56), surveillance status (HR, 0.26; P = 0.002; 95% CI, 0.11-0.6) and CA19-9 at the time of CCA diagnosis (HR, 1.24; P < 0.001; 95% CI, 1.12-1.37) were the only independent predictors of CCA-related adverse outcomes.
Impact of HBCa Surveillance on Overall Survival
The incidence of all-cause mortality was 5.5 times greater in the no-surveillance group than in the surveillance group (3.8; 95% CI, 2.67-5.24; versus 0.7; 95% CI, 0.39-1.08 deaths per 10 person-years). Sixty-two percent (49/79) of patients died during follow-up. The 5-year and 10-year overall survival rates were significantly higher in the surveillance group versus the no-surveillance group (68% and 56% versus 20% and 13%, respectively; P < 0.001; Fig. 2B).
Surveillance status for all HBCa patients (n = 79) and CA19-9 level at the time of diagnosis for CCA patients (n = 56) were the only independent predictors of all-cause mortality by univariate analysis (surveillance status: HR, 0.22; P < 0.001; 95% CI, 0.12-0.41; CA19-9: HR, 1.2; P < 0.001; 95% CI, 1.08-1.3). In multivariate analysis, surveillance status and CA19-9 levels at diagnosis were once again the only independent predictors of all-cause mortality but only in CCA patients (surveillance status: HR, 0.3; P = 0.002; 95% CI, 0.12-0.61; CA19-9: HR, 1.18; P = 0.001; 95% CI, 1.07-1.3).
Impact of HBCa Surveillance on HBCa-Related and Overall Survival, Excluding Patients Without Pathological Evidence of Tumor in Posttreatment Biopsy Specimens
After excluding the 6/79 patients who had no evidence of residual HBCa (pCCA) in their liver explants, the 5-year and 10-year overall survival rates in the study sample (n = 73) remained significantly higher in the surveillance group versus the no-surveillance group (65% and 50% versus 14% and 11%, respectively; P < 0.001). Univariate Cox proportional hazards analysis revealed that surveillance status (HR, 0.22; P <0.001; 95% CI, 0.12-0.43) and, for patients who developed CCA (n = 50), CA19-9 at the time of CCA diagnosis (HR, 1.18; P <0.001; 95% CI, 1.1-1.3) were independent predictors of all-cause mortality. Multivariate Cox proportional hazards analysis including only patients with CCA (n = 50) showed surveillance status (HR, 0.35; P = 0.012; 95% CI, 0.16-0.79) and CA19-9 at the time of CCA diagnosis (HR, 1.17; P = 0.002; 95% CI, 1.1-1.28) to be independent predictors of all-cause mortality. Similarly, the probability of experiencing a CCA-related adverse event (CCA recurrence or CCA-related death) in the CCA group (n = 50) within 5 years was 42% in the surveillance group compared to 81% in the no-surveillance group. Multivariable competing-risks regression including only CCA patients showed that surveillance status (HR, 0.36; P = 0.008; 95% CI, 0.14-0.75) and CA19-9 at the time of CCA diagnosis (HR, 1.22; P < 0.001; 95% CI, 1.1-.35) were the only independent predictors of CCA-related adverse events.
SURVIVAL ANALYSIS BASED ON SURVEILLANCE STATUS AFTER EXCLUDING PATIENTS WHO RECEIVED LT AS TREATMENT FOR pCCA OR HCC
LT for pCCA remains a subject of debate in many countries; thus, we sought to determine if surveillance had an impact on the outcomes and survival of patients who did not receive LT. After excluding patients who received LT for either pCCA or HCC (n = 35; Supporting Table S5), we found that the 5-year probability of an HBCa-related adverse event was 45% with surveillance versus 85% without surveillance (Supporting Fig. S1); indeed, surveillance was a significant predictor of HBCa-related adverse events (HR, 0.33; P = 0.009; 95% CI, 0.13-0.76). Similarly, 5-year overall survival was significantly higher in the surveillance group versus the no-surveillance group (40% versus 7%, respectively; P = 0.003; Supporting Fig. S2). Using Cox proportional hazards, surveillance remained associated with better overall survival (HR, 0.3; P = 0.002; 95% CI, 0.13-0.71).
IMPACT OF SURVEILLANCE ON OUTCOMES BASED ON TYPE OF HBCa
The clinical characteristics, treatment, and outcomes of patients according to their HBCa type are presented in Supporting Table S6.
eCCA
In this group, 38% (14/37) underwent routine surveillance (Supporting Tables S7 and S8). Patients in the surveillance group were more likely to present with early-stage eCCA compared to patients in the no-surveillance group. In addition, patients in the surveillance group were more likely to receive LT as curative treatment for pCCA compared to the no-surveillance group (93% versus 45%, P = 0.004; Supporting Table S7). There was a trend toward lower frequency of eCCA recurrence in the surveillance group compared with the no-surveillance group (7% versus 31%, P = 0.17; Supporting Table S7).
The probability of an eCCA-related adverse event within 5 years was 64% and 14% in the no-surveillance and surveillance groups, respectively (Fig. 3). Univariate analysis using competing-risks regression identified surveillance status (HR, 0.13; P = 0.007; 95% CI, 0.03-0.58) and CA19-9 at the time of eCCA diagnosis (HR, 1.3; P < 0.001; 95% CI, 1.15-1.47) as independent predictors of eCCA-related adverse events. In multivariable competing-risks regression, CA19-9 at the time of eCCA diagnosis (HR, 1.22; P = 0.003; 95% CI, 1.07-1.4) and surveillance status (HR, 0.19; P = 0.038; 95% CI, 0.04-0.91) were both significantly associated with eCCA-related adverse outcomes.
iCCA
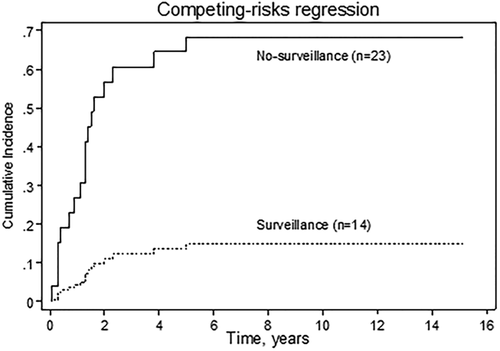
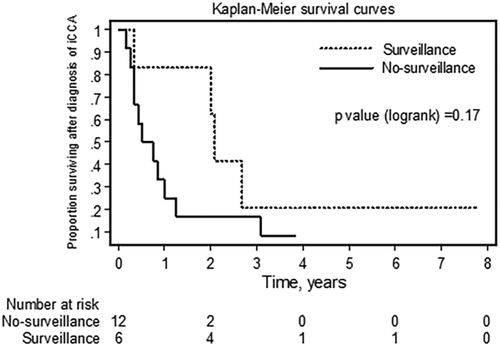
Thirty-two percent (6/19) of patients in the iCCA group underwent surveillance (Supporting Tables S7 and S9). There was a trend toward fewer cases with positive lymph nodes and intrahepatic metastasis in the surveillance group compared to the no-surveillance group as well as a trend toward longer survival in the surveillance group compared to the no-surveillance group (median time until death 24.5 months [range, 4-93 months] versus 7.5 months [range, 2-46 months], respectively; P = 0.074). There was also a trend toward higher 5-year iCCA-related survival in the surveillance group compared to the no-surveillance group (21% versus 8%, P = 0.17; Fig. 4). Univariate analysis using Cox proportional hazards demonstrated only age at the time of iCCA diagnosis to be an independent predictor of iCCA recurrence and iCCA-related death (HR, 0.94; P = 0.03; 95% CI, 0.89-0.99).
HCC
Eighty percent (16/20) of patients in the HCC group underwent surveillance (Supporting Table S10). Seventy-five percent (3/4) of patients who did not undergo routine HBCa surveillance died, 2 of whom died of HCC (median time from HCC diagnosis until death 0.3 years [range 0.2-3.3 years]). There was a trend toward more frequent lymph node–positive disease (33% [1/3] versus 7% [1/15]; P = 0.31) and extrahepatic metastases (33% [1/3] versus 7% [1/15]; P = 0.31) in the no-surveillance group compared to the surveillance group.
GBCa
All patients in this group were under surveillance (Supporting Table S11). All cases were diagnosed by US and later confirmed by histopathology.
SURVIVAL ANALYSIS IN THE ENTIRE STUDY POPULATION BASED ON THE IMAGING MODALITY USED FOR HBCa DETECTION
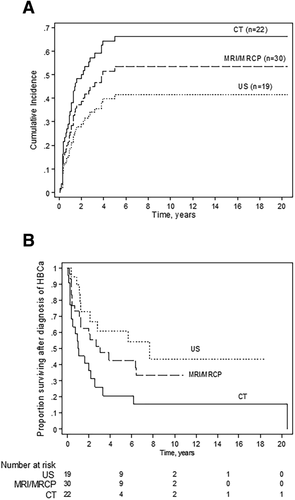
Patients who underwent HBCa surveillance had annual evaluations by one or more imaging modality plus CA19-9. Patients who progressed to cirrhotic stage PSC underwent surveillance with imaging (US, CT, or MRI/MRCP) plus CA19-9 and alpha-fetoprotein every 6-12 months. MRI/MRCP was the modality which most commonly prompted the clinician to rule out HBCa (40% [32/80]), followed by CT (27% [21/80]), US (25% [20/80]), and ERC (8% [6/80]); Table 3). The probability of an HBCa-related adverse event at 5 years was highest when HBCa was diagnosed by CT (65%) and lowest when diagnosed by US (40%) (Fig. 5A). Likewise, 5-year and 10-year overall survival rates were highest in the group that had HBCa diagnosed by US (61% and 43%, respectively) and lowest in the group that had HBCa diagnosed by CT (21% and 16%, respectively) (Fig. 5B). The difference in overall survival between the three imaging groups was statistically significant (P = 0.02), with the US and CT groups significantly different (P = 0.008), but the US and MRI/MRCP groups being similar (P = 0.3).
| Type of HBCa | Imaging Modality | |||
|---|---|---|---|---|
| US | MRI/MRCP | CT | ERC | |
| pCCA | 25% (9/36) | 42% (15/36) | 17% (6/36) | 17% (6/36) |
| Surveillance (yes) | 43% (6/14) | 36% (5/14) | 0% (0/14) | 21% (3/14) |
| LT (yes) | 30% (7/23) | 44% (10/23) | 4% (1/23) | 22% (5/23) |
| Endpointc (yes) | 24% (4/17) | 47% (8/17) | 24% (4/17) | 5% (1/17) |
| Death from all-cause (yes) | 23% (5/22) | 41% (9/22) | 27% (6/22) | 9% (2/22) |
| iCCA | 17% (3/18) | 50% (9/18) | 33% (6/18) | 0 |
| Surveillance (yes) | 17% (1/6) | 50% (3/6) | 33% (2/6) | 0 |
| LT (yes) | — | — | — | — |
| Endpointc (yes) | 20% (3/15) | 40% (6/15) | 40% (6/15) | 0 |
| Death from all-cause (yes) | 20% (3/15) | 40% (6/15) | 40% (6/15) | |
| HCCa | 10% (2/20) | 40% (8/20) | 45% (9/20) | 0 |
| Surveillance (yes) | 12% (2/16) | 44% (7/16) | 44% (7/16) | 0 |
| LT (yes) | 7% (1/14) | 57% (8/14) | 36% (5/14) | 0 |
| Endpointc (yes)b | 0% (0/6) | 50% (3/6) | 33% (2/6) | 0 |
| Death from all-cause(yes)b | 0% (0/10) | 30% (3/10) | 60% (6/10) | |
| GBCa | 100% (6/6) | 0% (0/6) | 0% (0/6) | |
| LT (yes) | — | 0 | 0 | |
| Endpointc (yes) | 0% (0/6) | 0 | 0 | |
| Death from all-cause (yes) | 17% (1/6) | 0 | 0 | |
| Frequency of imaging modality used for HBCa diagnosis | 25% (20/80) | 40% (32/80) | 27%(21/80) | 8% (6/80) |
- a Diagnosis of HCC was established in one case (1/20 = 5%) by biopsy of metastatic nodule in the lungs.
- b One patient had diagnosis established by biopsy of metastatic nodule in the lungs.
- c Refers to HBCa-related adverse events (recurrence and HBCa-related death).
Discussion
In this study examining the impact of HBCa surveillance in a PSC patient cohort, we found that: (1) cancers diagnosed in patients undergoing HBCa surveillance are smaller in size and less often have nodal involvement and/or metastases, (2) patients undergoing HBCa surveillance are more likely to benefit from available treatment modalities (e.g., LT) for HBCa, (3) the probability of an HBCa-related adverse event is significantly higher without surveillance, (4) 5-year and 10-year overall survival rates are significantly higher among patients undergoing HBCa surveillance, and (5) HBCa surveillance with US is associated with similar outcomes as MRI/MRCP and better outcomes as compared to CT.
CCA
HBCa surveillance was associated with various beneficial outcomes depending on the site of CCA. In the subset of PSC patients who developed eCCA, HBCa surveillance had a significant protective impact on multiple clinical outcomes. It was associated with earlier detection of cancer and greater frequency of LT as curative treatment (for pCCA). In addition, the probability of an eCCA-related adverse event was 4.6 times higher within 5 years not undergoing HBCa surveillance.
In the subset of PSC patients who developed iCCA, surveillance was associated with a trend toward less lymph node–positive iCCA and intrahepatic metastasis, fewer iCCA-related and all-cause deaths, and a trend toward higher 5-year iCCA-related survival compared to no surveillance. Furthermore, the median time to death tended to be longer in the surveillance group compared to the no-surveillance group. These differences did not reach statistical significance, which could be due to (1) the small sample size of the group with iCCA, (2) advanced stage at presentation, and/or (3) the poor prognosis of iCCA compared to pCCA.24 We found that 63% of patients who were diagnosed with iCCA were not surgical candidates due to large tumor burden and locoregional and/or distant metastases. We observed recurrence in 16% (3/19) of patients with iCCA; the distribution of recurrence was similar in the surveillance and no-surveillance groups. The 5-year overall survival of our patients with iCCA was 12.1%. These data are consistent with the known lethality associated with iCCA despite curative-intent surgical resection.
While the rate of resection was slightly higher in the surveillance group (50% [3/6] in the surveillance group versus 31% [4/13] in the no-surveillance group), it did not reach statistical significance. This study does not address whether patients in the iCCA group would have benefited from “more aggressive” surveillance.
HCC and GBCa
The 20 HCC cases in the current study all occurred in patients with cirrhotic stage PSC and represent the largest number (and proportion) of cases reported in a PSC cohort. Most studies to date have been small with limited follow-up, and at the other end of the spectrum are studies with no or remarkably low HCC incidence (Supporting Table S12).25
The reason for the high number of HCC cases in our cohort is unclear, though several potential explanations may be considered. Cirrhosis, irrespective of etiology, is an important risk factor for HCC; in our study, a large proportion of patients had late-stage PSC by the time of HBCa diagnosis. Alternatively, this may be a reflection of the increasing national incidence of HCC.26 Another possibility is that the short survival after diagnosis of advanced-stage CCA or GBCa may preclude the diagnosis/development of HCC in patients with PSC. Interestingly, two different types of HBCa were diagnosed concurrently in 4% (3/79) of our study cohort (2 patients had HCC and CCA, and 1 had HCC and GBCa). In our study, surveillance was associated with a trend toward less frequent lymph node–positive disease, a trend toward less frequent extrahepatic metastasis, and a trend toward more frequent LT as curative treatment for PSC complicated by HCC. The differences between the comparison groups did not reach statistical significance, likely due to insufficient sample size.
Eight percent (6/79) of the study sample developed GBCa, all of whom underwent routine surveillance. Sixty-seven percent of patients who developed GBCa were still alive at the time of most recent follow-up, and none developed recurrent GBCa. Our data show excellent outcomes for PSC patients who developed GBCa during surveillance.
IMAGING AND CA19-9 FOR HBCa SURVEILLANCE IN PSC
MRI/MRCP was the surveillance imaging modality whose findings most commonly prompted further investigation for HBCa. US, however, was associated with comparable overall survival and was superior to CT. It is important to emphasize that the first-line imaging modality for HBCa surveillance/diagnosis was determined based on clinician preference,27, 28 and thus, high-risk patients with PSC were not routinely surveilled with a set of imaging modality. Some hepatologists in our institution perform annual US combined with CA19-9 for HBCa surveillance.27 If US is reported as abnormal/suspicious (with or without CA19-9 elevation), a recommendation is typically made for MRI/MRCP (preferably with contrast, as this enhances the imaging study and decreases the risk of underdiagnosis, particularly for subtle periductal tumors), ERC, or both.1 Other clinicians use MRI/MRCP combined with CA19-9 as the first line for HBCa surveillance in patients with PSC.28 Additional studies to define the role of specific imaging methods for HBCa surveillance are needed and underway.
CA19-9 has been extensively studied as a tumor marker for hepatopancreatobiliary malignancies.29 In PSC, it has been shown to be of clinical utility in distinguishing between CCA and benign strictures; CA19-9 is higher in patients with CCA than in patients without CCA.16, 29-31 A recent meta-analysis on the utility of CA19-9 as a serum marker for CCA demonstrated that CA19-9 is a useful tumor maker for the diagnosis of CCA, with a reported overall sensitivity and specificity of 72% and 84%, respectively.32 In our study, CCA appeared to be of additive value. Eleven percent (6/56) of all CCA patients had no visible mass on imaging, 3 of whom had no cancer symptoms. All six of these patients had elevated CA19-9 levels (range 288-3,074 U/mL). The rise in CA19-9, in conjunction with the development of symptoms in 5 patients, was the main reason for cancer workup. Of note, none of these patients had bacterial cholangitis at the time of CCA diagnosis and had persistently elevated CA19-9 levels despite endoscopic stricture management (even though liver biochemistries had nearly normalized). These data suggest that a persistent/continued rise in CA19-9 in patients with PSC in the absence of bacterial cholangitis should prompt CCA workup.9
There are shortcomings to the use of CA19-9 as a biomarker for CCA in patients with PSC.9-11 For example, there is no agreement on a cutoff value for CCA diagnosis. In our study cohort, at least 16% of all CCA cases could have been missed if we used CA19-9 cutoff values proposed by others (Supporting Table S13). In addition, elevated CA19-9 has been reported in patients without CCA, such as in the setting of bacterial cholangitis and biliary obstruction. Conversely, patients with CCA may have normal CA19-99, 33, 34; in the current study, one third of all CCA patients had CA19-9 levels below the reference value in our laboratory (<55 U/mL). Wannhoff and colleagues35 showed that profiling patients with PSC according to their fucosyltransferase-2 and fucosyltransferase-3 genotypic statuses improves the diagnostic performance of CA19-9 as a marker for CCA; though this work is preliminary, it appears promising.
TIMING OF HBCa SURVEILLANCE IN PATIENTS WITH PSC
Two thirds of patients with available imaging/endoscopic/histological data and one half of the entire study population developed CCA in the setting of late-stage PSC (Supporting Table S2), which is in agreement with previous studies.36, 38 However, we and others have found that CCA can also occur in the setting of early-stage PSC (Supporting Table S2).36, 38 These data further demonstrate confirm that patients with PSC are at risk for CCA at any time during their disease course, thus suggesting that HBCa surveillance in patients with PSC should begin as soon as the diagnosis of PSC is established.37, 39
STRENGTHS, LIMITATIONS, AND FURTHER CONSIDERATIONS
Our study is unique in that we examined the clinical course and outcomes of a well-defined PSC/HBCa cohort, with and without routine HBCa surveillance, and with long follow-up postdiagnosis of HBCa (Supporting Table S14). In addition, participation in HBCa surveillance was a shared clinical decision; though not having the benefits of randomization, this helped us examine the outcomes and role of HBCa surveillance in a heterogenous group of patients in real-life clinical scenarios. We acknowledge that there is a possibility of selection bias; however, we believe that the surveilled group would have been the more clinically high-risk group (i.e., if the provider had discretion over assigning patients, he or she would have assigned PSC patients perceived to be at highest risk to the surveillance group); therefore, one would expect more HBCa-related adverse events and death from any cause in the surveillance group compared with the no-surveillance group.
Several limitations should be acknowledged. The retrospective nature of this study is a limitation; the ideal study to examine the impact of HBCa surveillance on the outcomes of PSC patients would likely include randomization of a very large number of patients with PSC into imaging groups (with or without tumor markers) and following them for many years. Implementing such a clinical trial, although ideal, would be extremely challenging due to the rarity of PSC, the number of years required for recruitment and adequate follow-up to have a sufficient number of endpoints, and the prohibitive costs, among other issues (as noted below). Our study did not have sufficient power to compare the efficacy of surveillance in improving the outcomes of patients with iCCA and HCC, and the lack of a comparator arm in the GBCa group precluded examining the effects of surveillance on the outcomes of GBCa. Cost analysis is essential to assess and potentially improve the performance/utility of HBCa surveillance in patients with PSC; though we did not address this directly, our results suggest that surveillance with US (when performed by skilled staff with expertise in hepatobiliary imaging) may have similar outcomes as MRI/MRCP and better outcomes than CT (both of which are more costly than US). The mean age of patients at the time of PSC and HBCa diagnosis was somewhat older than what is typical in some PSC cohorts, but we do not believe this represents a clinically significant difference. Lastly, the results of this study may be difficult to apply widely in clinical practice, in part because expertise and available treatment modalities vary in different centers.
It should be noted that although randomized clinical trials hold many advantages over observational studies, many important questions in medicine are not amenable to them, owing to feasibility and ethical considerations, among others. We observed an excess rate of adverse events in the no-surveillance group compared to the surveillance group. Hence, we believe that a randomized clinical trial designed to assess the efficacy of surveillance (in this setting, the active arm being “surveillance” and the placebo arm being “no-surveillance or diagnosis by symptoms”) for HBCa in PSC may lead to randomizing PSC patients who are at substantial risk for HBCa to no surveillance; this may expose these patients to the harm of HBCa diagnosis at a late stage, which would be unethical. Hence, when randomized clinical trials are not feasible, studies such as ours can provide useful data. Indeed, survival benefit data regarding HCC surveillance in patients with other chronic liver diseases have been largely derived from studies like ours.40
Despite the positive results of this study, the survival of patients with HBCa complicating PSC remains subsatisfactory, and better methods for earlier diagnosis of HBCa are needed. The role of tumor markers, cytological techniques, as well as cross-sectional imaging modalities should be better defined in a large-scale study including PSC patients with and without HBCa. Such a study can generate more data regarding the diagnostic performance and accuracy of the surveillance tools used in PSC populations, better target surveillance, and identify high-risk patients.
Conclusions
In summary, we identified 79 of 830 PSC patients who developed HBCa during extended follow-up. Among these patients, HBCa surveillance was associated with numerous clinical benefits, including earlier HBCa detection, higher likelihood of undergoing LT as curative treatment for pCCA, and significantly greater survival among those diagnosed with pCCA as compared to no HBCa surveillance. The incidence of HBCa-related adverse events (recurrence and death) was 8.5 times greater among patients not undergoing surveillance program compared to those who were undergoing HBCa surveillance. Similarly, the incidence of all-cause mortality was 5.5 times greater in those who were not undergoing HBCa surveillance compared to those who were undergoing surveillance. Our study also reports the highest number of HCCs detected in a well-defined cohort of PSC patients. Our findings establish the role of surveillance for detecting CCA, HCC, and GBCa in patients with PSC and will hopefully lay the basis for further studies.