New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases
Potential conflict of interest: Neil Kaplowitz consults for Gilead and Ionis Pharmaceuticals.
Supported by R01DK067215 (to N.K.) and the USC Research Center for Liver Disease's Cell Culture, Cell and Tissue Imaging, Histology and Metabolic/Analytical/Instrumentation Cores (P30DK048522; to N.K).
Abstract
The c-Jun-N-terminal-kinase (JNK) family is highly conserved across species such as Drosophila, C. elegans, zebrafish and mammals, and plays a central role in hepatic physiologic and pathophysiologic responses. These responses range from cell death to cell proliferation and carcinogenesis, as well as metabolism and survival, depending on the specific context and duration of activation of the JNK signaling pathway. Recently, several investigators identified the key molecules in the JNK activation loop which include apoptosis signal-regulating kinase (ASK1) and SH3-domain binding protein 5 (Sab) and their involvement in acute or chronic liver disease models. Thus, regulating JNK activation through modulating the JNK activation loop may represent an important new strategy in the prevention and treatment of acute and chronic liver diseases. In this review, we will discuss the molecular pathophysiology of the JNK activation loop and its role in the pathogenesis of liver diseases. (Hepatology 2018;67:2013-2024).
Abbreviations
-
- Akt
-
- AKT serine/threonine kinase 1
-
- ALD
-
- alcoholic liver disease
-
- APAP
-
- acetaminophen
-
- ASK1
-
- apoptosis signal-regulating kinase 1
-
- ATF2
-
- activating transcription factor 2
-
- ATP
-
- adenosine triphosphate
-
- Bcl
-
- B-cell lymphoma
-
- BHA
-
- butylated hydroxyanisole
-
- Bid
-
- BH3 interacting domain death agonist
-
- Ccl2
-
- C-C motif chemokine ligand 2
-
- Ccl5
-
- C-C motif chemokine ligand 5
-
- Cdc42
-
- cell division cycle 42
-
- cFLIP
-
- CASP8- and FADD-like apoptosis regulator
-
- cFos
-
- Fos proto-oncogene
-
- c-Jun
-
- Jun proto-oncogene
-
- c-Myc
-
- MYC proto-oncogene
-
- Con A
-
- concanavalin A
-
- Cre
-
- Cre recombinase
-
- Creg
-
- cellular repressor of E1A-stimulated genes
-
- CXCL10
-
- CXCL10
-
- Cyp2e1
-
- cytochrome P450 2E1
-
- DEN
-
- diethylnitrosamine
-
- DOK-4
-
- docking protein 4
-
- DUSP
-
- dual specificity phosphatase
-
- Elk1
-
- ETS-like gene 1
-
- ER
-
- endoplasmic reticulum
-
- ERK
-
- extracellular signal-regulated kinase
-
- Ets1
-
- ETS proto-oncogene 1
-
- EV
-
- exosome vesicle
-
- FABP4
-
- fatty acid binding protein 4
-
- FAK
-
- focal adhesion kinase
-
- FAS
-
- Fas cell surface death receptor
-
- FASL
-
- Fas ligand
-
- FFA
-
- free fatty acid
-
- FGF21
-
- fibroblast growth factor 21
-
- GalN
-
- N-acetyl-galactosamine
-
- γH2AX
-
- gamma H2A histone family, member X
-
- GSH
-
- glutathione
-
- HFD
-
- high-fat diet
-
- HCC
-
- hepatocellular carcinoma
-
- HFHC
-
- high-fat high-carbohydrate diet
-
- Hspd1
-
- heat shock protein 1
-
- IFNγ
-
- interferon gamma
-
- IL-1
-
- interleukin 1
-
- IL-4
-
- interleukin 4
-
- IL-6
-
- interleukin 6
-
- IRS
-
- insulin receptor substrate 1
-
- ITCH
-
- itchy E3 ubiquitin protein ligase
-
- JIP
-
- JNK-interacting protein
-
- JNK
-
- c-Jun N-terminal kinases
-
- JNK1
-
- mitogen-activated protein kinase 8 (MAPK8)
-
- JNK2
-
- mitogen-activated protein kinase 9 (MAPK9)
-
- KD
-
- knockdown
-
- Keap1
-
- Kelch-like ECH-associated protein 1
-
- KIM
-
- kinase interaction motif
-
- KO
-
- knockout
-
- LPS
-
- lipopolysaccharide
-
- Lyzs
-
- lysozyme 2
-
- MAPK
-
- mitogen-activated protein kinase
-
- MAP2K
-
- mitogen-activated protein kinase kinase
-
- MAP3K
-
- mitogen-activated protein kinase kinase kinase
-
- MKK4
-
- MAPK kinase 4
-
- Mcl-1
-
- myeloid cell leukemia sequence 1
-
- MKP
-
- mitogen-activated protein kinase phosphatase
-
- MLK
-
- mixed lineage kinase
-
- MPT
-
- mitochondrial permeability transition
-
- NAC
-
- N-acetyl cysteine
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAPQI
-
- N-acetyl-p-benzoquinone imine
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NcoR1
-
- nuclear receptor corepressor 1
-
- NcoR2
-
- nuclear receptor corepressor 2
-
- NF-κB
-
- nuclear factor kappa-light-chain-enhancer of activated B cells
-
- Nrf2
-
- nuclear factor erythroid 2-related factor 2
-
- Nrip1
-
- nuclear receptor interacting protein 1
-
- PGC1α
-
- peroxisome proliferative activated receptor, gamma, coactivator 1 alpha
-
- P-JNK
-
- phospho-activated JNK
-
- PKC
-
- protein kinase C
-
- PPARα
-
- peroxisome proliferator-activated receptor-α
-
- PPARγ
-
- peroxisome proliferator-activated receptor-γ
-
- Rac
-
- Rac family small GTPase 1
-
- ROS
-
- reactive oxygen species
-
- Sab (Sh3bp5)
-
- SH3-domain binding protein 5
-
- SabLPC-KO
-
- Sab gene deletion in liver parenchyma cell
-
- SAP1
-
- stress-associated protein 1
-
- SH3
-
- SRC homology 3 domain
-
- Shp1
-
- SH2 phosphatase 1
-
- shRNA
-
- small hairpin RNA
-
- SOCS3
-
- suppressor of cytokine signaling 3
-
- SOD2
-
- superoxide dismutase 2
-
- SP-1
-
- Sp1 transcription factor
-
- Src
-
- SRC proto-oncogene, nonreceptor tyrosine kinase
-
- TAK1
-
- TGF-beta activated kinase 1
-
- Tat
-
- transactivator of transcription of human immunodeficiency virus
-
- TNF
-
- tumor necrosis factor
-
- TNFR
-
- TNF receptor
-
- TRAIL
-
- TNF-related apoptosis inducing ligand
-
- WT
-
- wild type
Mitogen-activated protein kinase (MAPK) signaling can be induced in the liver in response to physical and chemical stress, including alterations in nutrients, growth factors, cytokines, extracellular matrix, DNA damage, drugs, and toxins. This signaling pathway plays a role in liver injury and diseases such as drug-induced hepatotoxicity, viral hepatitis, infection and inflammation, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), alcoholic liver disease (ALD), ischemia/reperfusion, fibrosis, regeneration, and carcinogenesis.1-4 In mammals, three major groups of MAPKs have been identified. Each of these groups of MAPKs is activated by a protein kinase cascade. MAPK signaling cascades consist of at least three components, or tiers: MAPK kinase kinase (MAP3K), MAPK kinase (MAP2K), and MAPK. The MAPK groups are named according to their executing downstream MAPK, such as the extracellular signal-regulated kinase (ERK), p38 kinase, and c-Jun N-terminal kinase (JNK). In the liver, JNK is a dominant effector MAPK, which catalyzes the phosphorylation of numerous substrate proteins, including nuclear AP1 transcription factors (c-Jun, etc.) as well as protein kinases and phosphatases, scaffold proteins, and other functional proteins.4, 5 JNK activation and substrate phosphorylation have two major direct consequences: regulation of gene expression through AP1 transcription factors and direct activation or inhibition of protein targets (Fig. 1). The liver expresses both JNK1 and JNK2. In this review, we will focus on recent studies from many different laboratories in the past 5 years that have improved our understanding of the role of the JNK signaling pathway in the pathogenesis of liver diseases and promise to lead to exciting therapeutic applications. Because of the broad nature of this subject, we have selected specific areas that illustrate important recent conceptual advances.
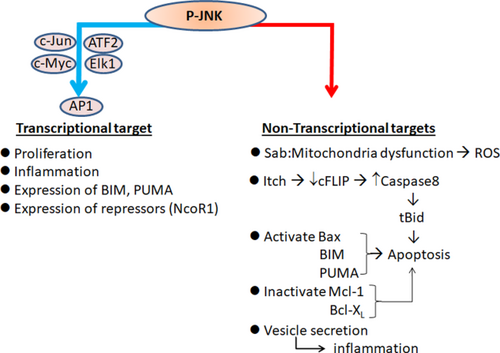
The JNK Signaling Pathway in the Liver: Recent Advances
JNK REGULATES AN INTRAMITOCHONDRIAL SIGNAL TRANSDUCTION PATHWAY
In acute lung injury models where extensive JNK activation has been observed, activated phospho (P)-JNK translocates to mitochondria and binds to the JNK target protein, Sab (Sh3bp5), in hepatic mitochondria.6 Sab is an outer membrane scaffold protein with one hydrophobic transmembrane domain which separates the N-terminal SRC homology 3 domain (SH3) domain facing the intermembrane space and the C-terminal JNK kinase interacting motifs (KIM) facing the cytoplasm.7 The interaction of P-JNK and Sab was first recognized in 2002 by Wiltshire et al.,8 and subsequently confirmed by others.6, 9, 10 In parallel with studies in the liver,6, 7 interaction of JNK and Sab was observed in Hela cells and inhibited by transactivator of transcription of human immunodeficiency virus (Tat)-Sab inhibitory peptide (KIM1 20 amino acid peptide linked to membrane permeant Tat peptide). Tat-Sab inhibitory peptide competitively inhibits JNK interaction with the KIM domain on the Sab platform, and blocks the JNK-induced inhibition of respiration and increased production of reactive oxygen species (ROS).9 The protective role of Tat-Sab inhibitory peptide was studied in heart and brain injury models.11, 12 Further studies using primary mouse hepatocytes and isolated liver mitochondria confirmed the mitochondrial production of ROS as a consequence of the JNK interaction with Sab and the prevention of ROS production by knockdown (KD) of Sab or by Sab blocking peptide.13
JNK is not taken up by mitochondria. Therefore, a key question was how the interaction of JNK with an outer membrane platform on the surface of the mitochondria can exert effects inside the mitochondria. Recently, a JNK/Sab-mediated intramitochondrial signaling pathway was elucidated.7 Briefly, P-JNK binding to Sab initiates intramitochondrial release of tyrosine phosphatase SH2 phosphatase 1 (Shp1) from Sab leading to dephosphorylation of activated-Src (P-419-Src), which occurs on and requires the platform, docking protein 4 (DOK4), located on the mitochondrial inner membrane. P-Src is required to maintain electron transport. Decreased P-Src inhibits mitochondrial respiration and enhances ROS production. Using mitochondria after in vivo knockdown of Shp1 or DOK-4, P-JNK-mediated inhibition of mitochondrial respiration was abrogated. After knockdown of DOK4, cell death in vitro or in vivo was inhibited in several acute injury models.7 Of note, P-MKK4 translocates to mitochondria along with JNK only when Sab is expressed.6, 14 The requirement of adenosine triphosphate (ATP) for P-JNK to inhibit mitochondrial respiration7, 13 supports earlier evidence that Sab is a JNK substrate. Interestingly, P-JNK1 or P-JNK2 with ATP equally inhibits mitochondrial respiration and only when Sab is expressed in mitochondria.7 Further studies are required to determine the JNK phosphorylation site(s) on Sab, and whether MAP3K is also associated with the Sab signalosome. However, these recent findings support the overall concept that Sab functions like a plasma membrane receptor, which is activated by binding of its ligand and its phosphorylation leading to a transmembrane signal transduction pathway.
THE SIGNIFICANCE OF THE SUSTAINED JNK ACTIVATION LOOP
In the context of receptor signaling, such as TNFR1, JNK activation is transient due to dampening by the concomitant activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) responsive survival genes. However, sustained JNK activation directly and positively correlates with liver injury (cell death) and liver metabolic stress/dysfunction.2, 3 A feed-forward self-sustaining signaling pathway has been elucidated in liver models, which we refer to as the JNK amplification loop (P-JNK → Sab → Intramitochondrial pathway → ↑ROS → ↑P-ASK1 → ↑P-MKK4 → ↑P-JNK).6, 7, 14 In normal liver, JNK is minimally or transiently activated and is not detectably associated with mitochondria. P-JNK translocates to mitochondria and binds with mitochondrial outer membrane protein Sab (Sh3bp5).6, 7, 10 Depletion of Sab or the presence of inhibitory Sab peptide completely prevents/blocks translocation of P-JNK to mitochondria.6, 7, 9 As a consequence of the binding of P-JNK to Sab, the above-described intramitochondrial signaling pathway leads to inhibition of mitochondrial respiration and electron transport, leading to ROS (presumably H2O2) release from mitochondria and activation of the MAP3K/MAP2K pathway sustaining JNK activation. Increased ROS also feeds back to inhibit JNK phosphatase, such as MKP1 (DUSP1), which contributes to the sustained level of JNK activation.15 Sustained JNK activation then mediates phosphorylation of transcription factors or directly regulates cell death, for example, phosphorylation of the B-cell lymphoma (Bcl) family. Interruption of JNK activation at multiple sites in the loop can dampen the sustained activation of JNK and protect against cell death or metabolic consequences. The initiation of the MAPK cascade can occur through cell membrane receptor or intracellular inflammasomes (by TGF-β activated kinase 1 [TAK1], mixed lineage kinase [MLK]2/3, and ASK1) or through organelle stress, such as mitochondria, endoplasmic reticulum (ER), or nuclear DNA. However, for the activation to be sustained usually requires the participation of ASK1. ASK1 is normally held in an inactivated state bound to various inhibitors such as thioredoxin or glutathione (GSH) S-transferases, which are redox sensitive so that ROS can release ASK1 from inhibition.3, 16, 17 In addition, CASP8- and FADD-like apoptosis regulator (cFLIP) and cellular repressor of E1A-stimulated genes (CREG) have been shown to interfere with the dimerization of ASK1, preventing activation.18, 19 Therefore, conditions in which the expression of cFLIP or CREG is inhibited can lead to enhanced ASK1 activation. The importance of ASK1 in cell death has been confirmed by knockout (KO) and specific inhibitors.20, 21 Other approaches that confirm the importance of the JNK-mitochondrial-ROS activation loop include specific inhibition of interaction of P-JNK with Sab using peptides,9, 11-13 small molecules,22 or KD and KO of Sab,6, 7 as well as the direct suppression of mitochondrial ROS with antioxidants such as butylated hydroxyanisole (BHA)15 and N-acetyl cysteine (NAC),18, 23 the increased expression of superoxide dismutase 2 (SOD2),24 mitochondrial targeted SOD mimetics,25, 26 and Kelch-like ECH-associated protein 1 (Keap1) deletion to enhance nuclear factor erythroid 2-related factor 2 (Nrf2) regulated antioxidant defense.27 Importantly, inhibition of the feed-forward JNK activation loop spares the transient JNK-dependent signal transduction to the nucleus and gene regulation, thereby not interfering with the prosurvival functions of the MAPK and JNK signaling pathways. Figure 2 summarizes the role of the feed-forward loop and the integration of mitochondria and ER in sustained JNK activation.
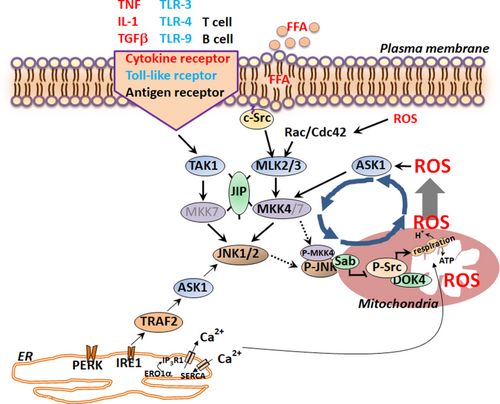
Role of JNK in Liver Disease Models: Recent Advances
ACUTE LIVER INJURY MODELS: ROLE OF JNK IN CELL DEATH
Death of the hepatocyte mainly occurs by apoptosis or necrosis. Hepatocytes express death receptors, including tumor necrosis factor (TNF) receptor (TNFR), Fas cell surface death receptor (FAS), and TNF-related apoptosis inducing ligand (TRAIL). Death receptor-mediated acute liver injury is mediated by inflammatory cell membrane-bound or -secreted death receptor ligands in infections/sepsis, viral hepatitis, acute alcoholic hepatitis, and NASH. Lipopolysaccharide (LPS) from gut microbiome or infection activates T cells, natural killer (NK) T cells, macrophages/Kupffer cells to express soluble or membrane bound cytokines (TNFα, Fas ligand [FASL], interleukin 1 [IL-1], and interferon gamma [IFNγ]). Cytokines activate death receptor signaling in hepatocytes. TNFα/GalN (N-acetyl-galactosamine) and Fas agonist monoclonal antibody (Jo2) induce apoptosis of hepatocytes and are widely used models to study the death receptor-mediated acute liver injury. Sustained JNK activation precedes apoptotic hepatic injury in both models. In the FasL-induced model, TRAIL has been shown to amplify Fas-mediated cell death through activation of JNK.2, 3
In addition to death receptor-initiated JNK activation in hepatocytes, intrinsic cellular stress induced by acetaminophen (APAP), fatty acids, and bile acids leads to the sustained JNK activation loop.28-30 This loop has been extensively studied in the APAP model. A fraction of an APAP dose is converted to an electrophilic reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), mainly mediated by cytochrome P450 2E1 (Cyp2e1). When sufficient NAPQI is produced, GSH in the cytoplasm and mitochondria is depleted. After marked GSH depletion, NAPQI covalently binds to protein thiols throughout the cells, but binding in mitochondria is critical. The depleted mitochondria GSH level and covalent binding cause moderate mitochondrial dysfunction and ROS release, activating the MAPK cascade.28 It is possible that covalent binding-induced ER stress may also contribute to the initial MAPK activation.31 The magnitude and duration of JNK activation is a major determinant of acute injury from APAP. The extent of APAP-induced JNK activation and translocation is dose dependent and correlates well with subsequent liver necrosis: minimal JNK activation but no translocation and no injury at the 100mg/kg nontoxic dose, whereas JNK activation and translocation to mitochondria increase with APAP doses from 150 to 300 mg/kg and this dose relationship is inversely related to SRC proto-oncogene, nonreceptor tyrosine kinase (Src) activity in mitochondria.7 Previous studies showed that female mice are resistant to APAP-induced liver injury compared to age-matched male mice. We found that Sab protein expression was markedly lower in female than in male liver mitochondria, which may contribute to less liver injury in female mice,32 suggesting that the level of expression of Sab is an important determinant of the level of sustained JNK activation loop. Intriguingly, in Sab KD or KO mice, mitochondrial translocation of P-JNK is completely inhibited irrespective of various isoforms of JNK.6, 7 Therefore, in acute APAP liver injury, mitochondrial Sab is pivotal in leading to sustained JNK activation loop. In the APAP model, the sustained JNK activation loop does not cause apoptosis because caspases are inactivated. In this special case, the marked amplification of mitochondrial ROS attributed to the JNK activation loop overwhelms mitochondria leading to mitochondrial permeability transition (MPT)-mediated necrosis. Figure 3 provides a comparison of the role of signaling pathways in APAP necrosis and TNF/GalN apoptosis.
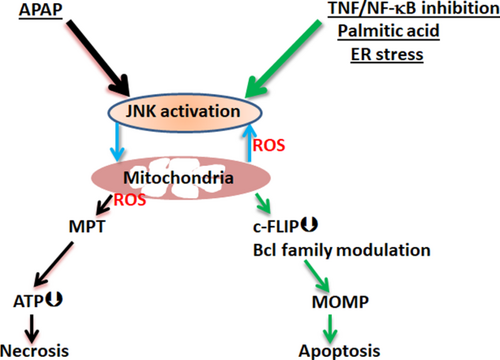
Despite the many independent studies6, 7, 14, 20-23, 26-28, 33, 34 demonstrating the importance of MAPK and JNK signaling in APAP toxicity, one study reported the apparent opposite, namely embryonic liver-specific JNK1 and 2 double KO (Jnk2–/– global KO crossed with Jnk1f/f mice and liver-specific Cre recombinase [Cre] transgenic mice) increased the severity of APAP toxicity.35 However, the sensitization to APAP toxicity seemed to be attributed to enhanced mitochondrial oxidative stress under basal conditions, suggesting a developmental impairment in antioxidant defense, the nature of which is presently unknown but potentially related to long-term suppression of AP-1.36, 37 Study of APAP in adult inducible double JNK1 and JNK2 floxed KO mice is needed for further clarification.
When the magnitude of JNK activation reaches a threshold to translocate to mitochondria and interact with mitochondrial outer membrane protein Sab,6, 7 this results in amplification of oxidative stress in the mitochondria.7, 24 Thus, in primary mouse hepatocytes, chemical ER stressor (tunicamycin, brefeldin A)13 or saturated free fatty acids (FFAs; palmitic acid)29, 38 activate JNK and the sustained JNK activation loop. In these models, membrane permeable Sab peptide blocks the binding of P-JNK and prevents sustained JNK activation and thereby inhibits apoptotic cell death.13, 38 Thus, Sab is a common binding target of isoforms of P-JNK for both apoptosis and necrosis signal transduction pathways in many liver injury scenarios. However, there are exceptions: for example, hepatocellular JNK/Sab does not appear to play a role in furosemide39 or CCL4-induced acute liver injury.23 Other forms of cell death have been identified, such as necroptosis, pyroptosis, and ferroptosis, but their contribution to liver injury or the role of JNK is either unknown or controversial.
NAFLD/NASH AND METABOLIC DISEASE MODELS
The role of the MAPK signaling pathway in the development of NAFLD, NASH, fibrosis, obesity, and insulin resistance has been extensively discussed.40-43 Past research using global KOs in these models has led to conflicting and inconclusive results. Recently, however, cell type-specific Cre-mediated JNK1 and/or JNK2 conditional KO mice have become available to study the role of JNK in different liver cell types and has improved our understanding. Still, there remain concerns about potential confounding effects of embryonic conditional KOs with respect to compensatory adaptive responses, which may be obviated, to some extent, by use of inducible cell type-specific KOs in adult mice.
Role of Hepatic JNK in Fatty Liver Disease and Metabolic Syndrome
A number of past studies have linked sustained JNK activation to ER stress and insulin resistance in the liver. More recently, the role of JNK activation in hepatocytes has been linked to the peroxisome proliferator-activated receptor-α (PPARα)/fibroblast growth factor 21 (FGF21) hepatokine axis.44 FGF21 regulates adipose tissue metabolism, in part, by reducing peroxisome proliferator-activated receptor-γ (PPARγ) inhibitory sumoylation and increasing PPARγ, coactivator 1 alpha (PGC1α) expression.45 Thus, hepatic deletion of JNK1+2 mice decreased adipocyte hypertrophy and macrophage infiltration of adipose tissue induced by HFD44 (Fig. 4). Sustained hepatic JNK activity in high-fat diet (HFD)-fed mice potently repressed the nuclear hormone receptor, PPARα, responsive genes through increased expression of PPARα corepressor nuclear receptor corepressor 1 (NcoR1). Therefore, hepatic P-JNK caused decreased expression of PPARα target genes that increase fatty acid β-oxidation, ketogenesis, and FGF21 expression and promoted development of insulin resistance. FGF21 is produced preferentially in the liver as a hepatokine, which possesses potent regulatory effects on adipose tissue, liver, and brain, such as glucose and lipid metabolism, insulin sensitivity, and adaptive starvation response in animal models.45-47 In addition, FGF21 regulates Nrf2 expression and enhances antioxidant defense, which could dampen the JNK activation loop.48 Hepatocyte deletion of JNK2, but not JNK1, alone only slightly increased hepatocyte expression of Fgf21 mRNA and decreased body weight gain induced by HFD. Combined JNK1+2 hepatocyte-specific deletion markedly increased hepatic expression of Fgf21 mRNA, circulating amounts of FGF21 in the blood, and was associated with reduced fatty liver and body weight gain by HFD, thus indicating the synergistic effects of JNK1 and 2 in hepatocytes.44 Though JNK2 appeared to have a greater role than JNK1 in this study, several other recent reports suggest that hepatocyte JNK1 plays the dominant role in mediating the hepatic effects of HFD models.18, 19 The relative contribution of JNK1, 2, or both remain unresolved and the effects of knocking out one isoform on the expression and activation of the other complicates these types of studies. Conditional JNK1/JNK2 double KO appears to provide the most interpretable results.
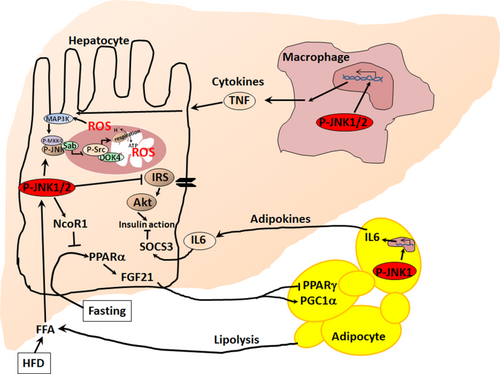
Role of Extrahepatic JNK in Fatty Liver Disease and Metabolic Syndrome
JNK is activated in the adipose tissue, macrophage, and striated muscle of HFD-fed mice. This was associated with decreased whole-body insulin sensitivity43 as a consequence of JNK inhibiting insulin-stimulated AKT serine/threonine kinase 1 (Akt) activation.49 Interestingly, insulin resistance and hepatic steatosis were suppressed by JNK1 deficiency in adipose tissue of Fabp4-Cre+ Jnk1f/– mice. Lack of JNK1 in adipose tissue prevented the secretion of inflammatory cytokine interleukin 6 (IL-6) by adipose tissue in response to HFD feeding.49 IL-6 causes increased expression of hepatic suppressor of cytokine signaling 3 (SOCS3), a protein that induces hepatic insulin resistance by inhibiting insulin receptor and by targeting insulin receptor substrate 1 (IRS) degradation.49 However, the role of IL-6 in whole-body insulin resistance has been debated because of tissue-specific signaling differences of IL-6. Nevertheless, JNK1 activation in adipose tissue can cause insulin resistance in the liver,49 but JNK2 in adipose tissue may be dispensable. However, direct comparison of JNK1 deletion versus JNK2 deletion or JNK1+2 deletions in adipose tissue is required for further clarification.
Tissue infiltration by proinflammatory macrophages (M1) is a major contributor to inflammation and insulin resistance.50 In an earlier study, transfer of bone marrow (myeloid cells) from global Jnk1–/- mice to irradiated wild-type (WT) mice fed with HFD improved the liver and peripheral tissue insulin sensitivity. In this model, most immune cells, including Kupffer cells, are replaced by transferred Jnk1–/– myeloid cells.51 However, JNK1 deficiency in macrophages of Lyzs-Cre+ Jnk1f/f mice did not suppress HFD-induced hepatic insulin resistance, suggesting that JNK2 is the relevant isoform.49 In both models, JNK1 depletion in macrophages did not suppress macrophage infiltration of the liver and adipose tissue. A recent study using JNK1+2 conditional deletion in macrophages (Lyzs-Cre+ Jnk1f/f Jnk2f/f) showed that JNK is required for polarization of proinflammatory macrophages to promote infiltration of tissue and suppression of insulin sensitivity in the liver, muscle, and peripheral tissues in HFD-fed mice. However, HFD-induced obesity was not prevented by deleting JNK1+2 in macrophages.50
Role of MAP3K Upstream of JNK in Metabolic Syndrome
Two MAP3Ks, MLK3 and ASK1, have been implicated in diet-induced regulation of hepatic JNK. Past work has shown that global KO of MLK3 prevents JNK activation in high-fat high-carbohydrate (HFHC) diet-fed mice.52, 53 Despite a comparable increase in weight gain, hepatic steatosis by histological examination and hepatic triglyceride quantification were reduced in HFHC diet-fed Mlk3–/– mice compared to WT mice. The ubiquitously expressed MLK2 and MLK3 protein kinases have partially redundant functions. Therefore, in compound mutant global Mlk2–/– and Mlk3–/– mice JNK activation in liver was inhibited and the mice were protected against HFD-induced insulin resistance and obesity.54
More recently, ASK1 has been the focus of attention. cFLIP directly targets ASK1 and interrupts its N-terminus-mediated dimerization, thereby blocking signaling involving ASK1 to JNK1. Activation of JNK in HFD feeding may activate the E3 ligase, itchy E3 ubiquitin protein ligase (ITCH), and then destabilize cFLIP by proteosomal degradation. Decreased cFLIP expression has been observed in human liver samples from NAFLD/NASH patients.18 Interestingly, liver-specific ASK1 deletion protected against HFD-induced insulin resistance, fatty liver, and obesity.18 In addition, the activation of ASK1 is inhibited by CREG. CREG expression is decreased in NAFLD patients and liver-specific CREG deletion increases ASK1-JNK axis in HFD mice and worsens fatty liver and insulin resistance.19 Of note, small-molecule ASK1 inhibitor (GS-4997) reduces hepatic steatosis and fibrosis in the diet-induced NASH model.55
Hepatocyte apoptosis plays a critical role in the progression of NASH to cirrhosis and HCC. JNK activation is crucial in the development of hepatocyte apoptosis accompanying NASH.29 MLK3 activates JNK during lipotoxic insults.53 JNK activation is critical in mediating saturated fatty-acid–induced apoptosis by modulating the activity and expression of Bcl family proteins.56 In addition, JNK inhibition results in retention of chemokine, C-X-C motif chemokine ligand 10 (CXCL10), in hepatocytes and decreases CXCL10 trafficking into the exosome vesicles (EVs), suggesting a regulatory role of hepatocyte JNK in liver inflammation.57 Although strong evidence has been provided for a key role for both MLK3 and ASK1 in lipotoxicity, fatty liver, and insulin resistance, a key question has been the mechanism of their sustained activation of the MAPK cascade.
Role of Sustained-JNK Activation Loop in Metabolic Syndrome
Activating signals from Rac family small GTPase 1 (Rac)/cell division cycle 42 (Cdc42), Src, and protein kinase C (PKC) have been proposed for MLK3 activation based on cell studies,58, 59 but reports of in vivo confirmation are sparse. The effect of liver-specific MLK2/3 deletion on the sustained JNK activation loop and the relative contribution of hepatic MLK2/3 versus ASK1 are needed. The involvement of the JNK/mitochondria/ROS feed-forward loop has been examined in diet-induced NAFLD/NASH and obesity by targeting Sab. HFHC diet was fed to Sabf/f and SabLPC-KO mice for 16 weeks and inducible deletion of hepatic Sab prevented sustained JNK activation, suppressed insulin resistance and consequently prevented NAFLD/NASH and obesity.60 Thus, the sustained activation of hepatic JNK in diet-induced obesity models can be explained by the Sab-dependent activation loop in which JNK induces mitochondrial dysfunction and ROS release in a Sab-dependent fashion and the ROS production enhances MAP3K. The relative contribution of ASK1 and MLK2/3 in this loop needs additional study. Ultimately, signaling through cytokine receptors and intrinsic stress (ER and mitochondria) converge on the interaction of JNK and Sab, which continues to stimulate the JNK loop and the metabolic and apoptotic consequences of sustained JNK activation.
JNK IN LIVER CANCER MODELS
Liver cancer has been studied mainly using two approaches: carcinogen (diethylnitrosamine; DEN) induced and genetic models (liver-specific conditional KO of myeloid cell leukemia sequence 1 [Mcl-1], TAK-1, or heat shock protein 1 [Hspd-1]). Past studies in the DEN model have been performed using embryonic Jnk1–/– or Jnk2–/– or liver-specific JNK1 and 2 double KO (Jnk2–/– global KO crossed with Jnk1f/f mice and liver-specific Alb-Cre or liver + lymphocyte specific Mx1-Cre transgenic mice) mice to test whether JNK in hepatocyte or nonparenchymal cells is required for HCC development. Conflicting results on DEN-induced hepatocyte apoptosis and HCC formation in embryonic JNK KO mice were discussed in an earlier review.2 Recently, mice with JNK1+2 conditional deletions in myeloid cells (Lyzs-Cre+ Jnk1f/f Jnk2f/f), such as Kupffer cells, macrophages, and neutrophils, were treated with DEN 25 mg/kg single intraperitoneal injection. Indeed, myeloid cell infiltration and cytokine expression, including IL-6, TNFα, IFNγ, and IL-1β in the liver and blood, were significantly decreased by JNK1+2 deletion in myeloid cells. JNK deficiency in myeloid cells significantly decreased liver mass and tumor size, but tumor number was not different.61 Further evaluation of DEN-induced liver tumor development in hepatic conditional deletion of JNK1, JNK2, or JNK1+2 mice is required to examine the role of JNK in DEN-induced hepatocyte apoptosis and compensatory proliferation.
In most genetic models, HCC develops in response to liver cell death and compensatory replication proliferation. For example, more than 50% of mice with liver-specific conditional deletion of Mcl-1 (Alb-Cre Mcl1f/f) develop spontaneous HCC within 8 months.62 Similarly, liver-specific TAK-1 deleted mice develop spontaneous apoptosis and HCC.63 In these models, HCC develops in the absence of overt inflammation. Recently, the signaling pathway in hepatocytes mediating the progression of tumor development of Alb-Cre Mcl1f/f mice has been further elucidated. Cross-breeding of liver Mcl-1 KO mice or TAK 1 KO mice with global Tnfr1–/– mice prevented spontaneous apoptosis and decreased expression of inflammatory cytokines IL-6, IL-33, and IFNγ, indicating the involvement of TNFR1-dependent apoptosis and downstream signaling, TNFR-caspase-8-BID (BH3 interacting domain death agonist)/tBID-Mcl-1.64 The role of JNK in HCC development in the apoptosis-mediated pathway is complicated by the promotion of apoptosis versus the potential role in DNA repair. JNK plays a pivotal role in apoptosis, but also appears to regulate DNA repair through a novel platform consisting of nonenzymatic caspase-8 in association with RIPK1 and cFLIP leading to gamma H2A histone family, member X (γH2AX) phosphorylation, which activates its role in DNA repair. These apparent contradictory roles of JNK need to be further studied, particularly to understand the impact of abrogation of the JNK activation loop during various stages of the progression from chronic apoptosis to HCC.
In hepatocyte-specific Hspd1–/– mice lacking mitochondrial chaperone Hsp60, mitochondrial oxidative stress leads to sustained JNK activation in hepatocytes. P-JNK in hepatocytes increased the production and release of cytokines C-C motif chemokine ligand 2 (Ccl2), C-C motif chemokine ligand 5 (Ccl5), and IL-1β, which then activated Kupffer cells, leading to release of TNF. TNF then activated JNK-dependent cholangiocyte proliferation and development of intrahepatic cholangiocarcinoma.65 These recent insights indicate that the complexity of the interplay of effects of JNK signaling in hepatocytes, cholangiocytes, and nonparenchymal cell (e.g., Kupffer cells) required for development and progression of various types of liver cancer, which can be prevented by inhibiting sustained JNK-mediated chronic apoptosis and proliferation-replication–associated DNA damage, as well as JNK-dependent proliferative signals from parenchymal and nonparenchymal cells.
Therapeutic Targets in the JNK Activation Loop
The magnitude and duration of JNK activation determine its physiological and pathological consequences. Many important physiological processes are regulated by transcription factors, which are activated by JNK phosphorylation. These processes are largely mediated by receptor signaling and are typically transient, lasting minutes. However, sustained high-level JNK activation in the pathological process lasts hours or more. Direct inhibition of JNK inhibition is generally considered an unfavorable target given that it would interfere with the physiological aspects of JNK signaling. In contrast, the JNK activation loop mainly regulates metabolism and cell death. Therefore, the JNK/ROS feed-forward loop could be a prime target for therapeutic intervention.
ASK1, a key MAP3K in the activation loop, is a very promising target. Recently, ASK1 inhibitors (GS-4997, GS-444217) have been examined in diet-induced NASH, and encouraging results have been obtained in preclinical and clinical studies. A phase 2 study has reported that the ASK1 inhibitor reverses liver fibrosis in humans with NASH.55 As noted above, ASK1 inhibitors or liver-specific ASK1 KO exhibit striking protection against liver injury in mouse models of APAP and NAFLD/NASH.18, 20, 21 Detailed study of mechanism and target cells of ASK1 inhibitors need to be established. Given that ROS activates ASK1, up-regulation of antioxidant genes or antioxidant supplementation is another strategy for inhibiting the sustained JNK activation loop by dampening the release of ROS from mitochondria.24-27
Recently, Antcin H, an herbal chemical, was discovered to selectively block the JNK binding to Sab and thereby inhibit the JNK/ROS feed-forward loop by preventing JNK inhibition of mitochondrial respiration and release of ROS. Antcin H protected against APAP necrosis and TNF/GalN apoptosis.22 However, further detailed studies of the mechanism of binding inhibition and possible off-targets of Antcin H are required. Another way to block JNK binding to Sab is the use of membrane permeable (Tat) peptide corresponding to 20 amino acids of the docking site on Sab (KIM 1 peptides). KIM1 peptide blocks the interaction of JNK and Sab and has shown efficacy in preventing cardiac and brain injury in ischemia/reperfusion.11, 12 Thus, in principle, approaches to blocking the interaction of JNK and Sab show promise. Alternatively, decreasing Sab expression is a potential strategy. Approaches to repressing Sab expression at the transcriptional or posttranscriptional level may be possible using antisense oligonucleotides to target hepatic Sab. Moreover, an estrogen-receptor-α agonist repressed Sab expression and protected against acute liver injury. Thus, elucidation of the mechanism of estrogen receptor target genes involved in Sab repression may provide an approach to targeted therapy.32 Clearly, specifically targeting the individual steps in the JNK activation loop offers considerable promise as a therapeutic strategy in acute and chronic liver diseases in which JNK is a key mediator.
Conclusions
The role of the MAPK cascade in liver injury is complex with effects on survival, proliferation, metabolism, and cell death in cell type-specific fashion. Among the MAPKs, JNK in hepatocytes has been shown to play significant roles in acute and chronic liver injury by regulating cell death and metabolism in the liver. A key aspect of JNK effects is determined by the duration and level of sustained JNK activation, which is influenced by the mitochondrial Sab/ROS signaling loop. The level of activation of this signaling loop is critical in determining the pathophysiological consequences. Targeting the steps involved in the sustained JNK activation loop may have therapeutic potential.
REFERENCES
Author names in bold designate shared co-first authorship.




