Actin cytoskeleton remodeling drives epithelial-mesenchymal transition for hepatoma invasion and metastasis in mice
Potential conflict of interest: Nothing to report.
Supported by research grants from Chang Gung Memorial Hospital (CMRPG3F0031-3), the National Health Research Institute (NHRI-EX106-10408BI), and Ministry of Science and Technology (MOST103-2314-B-182A-117-MY3), Taiwan.
The microarray data in Hep3B and Huh7 cells with versus without KLHL23 silenced have been deposited in the GEO database under accession number GSE74996.
Abstract
High invasiveness is a hallmark of human hepatocellular carcinoma (HCC). Large tumors predict invasion and metastasis. Epithelial-mesenchymal transition (EMT) is crucial for cancer invasion and metastasis. However, the mechanisms whereby large tumors tend to undergo EMT remain unclear. We conducted a subgenome-wide screen and identified KLHL23 as an HCC invasion suppressor by inhibiting EMT. KLHL23 binds to actin and suppresses actin polymerization. KLHL23 silencing induced filopodium and lamellipodium formation. Moreover, EMT was suppressed by KLHL23 through its action on actin dynamics. Traditionally, actin cytoskeleton remodeling is downstream of EMT reprogramming. It is therefore intriguing to ask why and how KLHL23 inversely regulates EMT. Activation of actin cytoskeleton remodeling by either KLHL23 silencing or treatment with actin cytoskeleton modulators augmented cellular hypoxic responses in a cell-density–dependent manner, resulting in hypoxia-inducible factor (HIF) and Notch signals and subsequent EMT. Environmental hypoxia did not induce EMT unless actin cytoskeleton remodeling was simultaneously activated and only when cells were at high density. The resulting EMT was reversed by either adenosine 5′-triphosphate supplementation or actin polymerization inhibitors. Down-regulation of KLHL23 was associated with invasion, metastasis, and poor prognosis of HCC and pancreatic cancer. Correlations of tumor size with EMT and inverse association of expression of KLHL23 with HIF/Notch signals were further validated in patient-derived xenograft HCCs in mice. Conclusion: Simultaneously activation of actin cytoskeleton remodeling by intrinsic (such as KLHL23 down-regulation) or microenvironment cues is crucial for cell-density–dependent and hypoxia-mediated EMT, providing a mechanistic link between large tumor size and invasion/metastasis. Our findings provide a means of developing the prevention and treatment strategies for tumor invasion and metastasis. (Hepatology 2018;67:2226-2243).
Abbreviations
-
- ATP
-
- 5′-triphosphate
-
- CK 8
-
- cytokeratin 8
-
- CTCD
-
- cytochalasin D
-
- DMSO
-
- dimethyl sulfoxide
-
- EMT
-
- epithelial-mesenchymal transition
-
- F-actin
-
- actin filaments
-
- G-actin
-
- globular actin monomer
-
- HCC
-
- hepatocellular carcinoma
-
- HIF
-
- hypoxia-inducible factor
-
- IHC
-
- immunohistochemistry
-
- IP
-
- immunoprecipitation
-
- Jasp
-
- jasplakinolide
-
- LA
-
- latrunculin A
-
- LB
-
- latrunculin B
-
- NICD
-
- Notch intracellular domain
-
- RFP
-
- red fluorescent protein
-
- rKLHL23
-
- recombinant KLHL23
-
- RNAi
-
- RNA interference
-
- ROS
-
- reactive oxygen species
-
- shRNAs
-
- short hairpin RNAs
-
- α-SMA
-
- alpha-smooth muscle actin
-
- TGF-β
-
- transforming growth factor beta
-
- UTMDACC
-
- The University of Texas MD Anderson Cancer Center
-
- UTR
-
- untranslated region
High invasiveness with subsequent recurrence and metastasis is a hallmark of human hepatocellular carcinoma (HCC).1 Large tumor size predicts tumor invasion and metastasis,2-4 which are the primary causes of cancer-related mortality. Tumor metastasis is a multistep cascade.5 Epithelial-mesenchymal transition (EMT), characterized by loss of apicobasal polarity and intercellular adhesion and acquisition of mesenchymal cell morphology and motility by cytoskeleton remodeling, is the initial stage and a necessary step of the metastasis cascade.6-9 EMT is usually initiated and controlled by cellular signals that respond to extracellular cues, such as transforming growth factor beta (TGF-β), Wnt, Notch, and Hedgehog signaling pathways. However, the mechanism by which large tumors induce EMT to facilitate tumor invasion and metastasis remains largely unknown.
Hypoxia usually develops in large tumors, and is thought to trigger EMT programming, which promotes tumor malignancy, and increases resistance to chemotherapy.10, 11 Tumors hypoxia usually leads to massive cell necrosis and is not usually associated with tumor invasion or metastasis. Instead, additional microenvironmental cues, such as inflammatory cytokines and growth factors derived from immune cells, mesenchymal cells, senescence, or necrotic cells are required for EMT and subsequent tumor invasion and metastasis.12-14 It is unclear how cellular hypoxic responses and microenvironmental cues interact with each other in EMT induction resulting in tumor invasion and metastasis.
The actin cytoskeleton is formed by polymerization of actin monomers (globular or G-actin) to form microfilaments (F-actin) and is a highly dynamic structure that involves continuous assembly and turnover of essential cellular processes, including endocytosis, intracellular transport, cell morphogenesis, and cell motility.15, 16 Actin cytoskeleton remodeling is a very late step in the EMT signaling cascade for cell morphogenesis and motility and is in the downstream of EMT.16 Actin cytoskeleton remodeling leads to adenosine 5′-triphosphate (ATP) and oxygen consumption, which might augment cellular hypoxic stress.17, 18 However, the biological significance of cellular hypoxic stress induced by activation of actin cytoskeleton remodeling in cells under stress has never been investigated.
To identify genes and underlying mechanisms orchestrating the high invasiveness of HCC, we performed a subgenome-wide RNA interference (RNAi) screen and identified KLHL23. KLHL23 suppresses EMT, thereby inhibiting tumor invasion/metastasis. Surprisingly, KLHL23 is an actin-binding protein that inhibits actin polymerization. We thus investigated how KLHL23, an actin modulator, inversely regulates EMT and found that simultaneous activation of actin cytoskeleton remodeling was crucial for induction of cell-density–dependent, hypoxia-mediated EMT. Our findings provide a mechanistic link between large tumor size and metastasis and suggest promise by intervention to this signaling in prevention and treatment of HCC invasion and metastasis.
Materials and Methods
CELL CULTURE, PLASMIDS, AND SHORT HAIRPIN RNAs
The sources of the human hepatoma cell lines (HepG2, Hep3B, Huh7, Sk-Hep1, and Mahlavu) used in this study were described.19 HeLa cells, a cervical cancer cell line, were purchased from American Type Culture Collection (https://www.atcc.org/en/). Plasmids, including pKLHL23-Flag, pKLHL23-RFP, and pGST-KLHL23, contain KLHL23 complementary DNA with fusion to a flag tag, RFP (red fluorescent protein), and glutathione S-transferase, respectively. pLv-Tet-off-KLHL23 contains KLHL23 with a promoter terminated by doxycycline treatment (Clontech 632163). The three clones of short hairpin RNAs (shRNAs) targeting KLHL23 mRNA (shKLHL23-1, -2, and -3) and shRNA targeting luciferase (shLuc) or containing an empty vector (shEV) were obtained from National RNAi Core, Taiwan.
shRNA LIBRARY AND SCREENING
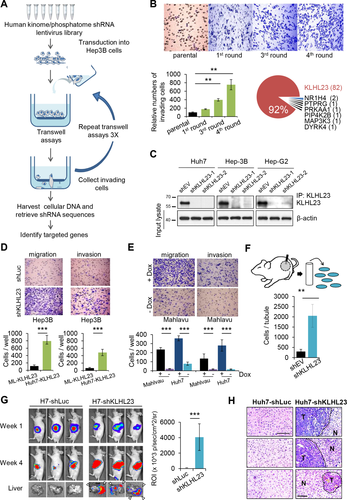
A lentivirus-based shRNA library containing approximately 7,000 shRNA clones targeting 1,228 human kinases and phosphatases was divided into seven pools, which were separately transduced into Hep3B cells for four rounds of selection of highly invasive cells (the schematic shown in Fig. 1A).19
ANIMAL EXPERIMENTS
All procedures and experimental protocols involving mice were approved by the Animal Care and Use Committee of Chang Gung Memorial Hospital (Taiwan) and developed in accord with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, USA, 1985).
PATIENTS AND TISSUE ARRAYS
All procedures for collecting HCC and pancreatic cancer specimens were approved by the Internal Review Board of Medical Ethics of Chang Gung Memorial Hospital and The University of Texas MD Anderson Cancer Center (UTMDACC), respectively. HCC tissue arrays were obtained from the Taiwan Liver Cancer Network, and tissue arrays containing pancreatic cancer and matched non-tumor pancreatic tissues were collected at the UTMDACC. Informed consent documents were collected at the same time as tissue sample collection and those used for tissue microarrays were recorded in the Taiwan Liver Cancer Network and the UTMDACC (see Supporting Methods for patient inclusion details). A tissue microarray containing 100 HCCs, 40 intrahepatic cholangiocarcinomas, 10 metastatic HCCs, 40 chronic hepatitis tissues (matched or unmatched), eight adjacent normal tissues, and four normal liver tissues was purchased (LV2018; Biomax US) to validate clinical relevance of KLHL23 in human HCC metastasis.
PATIENT-DERIVED HCC XENOGRAFTS IN NUDE MICE
Human HCC samples were obtained at the time of surgery. Fragments (1 × 1 mm) were grafted subcutaneously into 6- to 9-week-old male nude mice under isoflurane anesthesia. Mice were maintained in specific pathogen-free animal housing until the xenografts developed.
Statistical analysis
Student t tests were used for comparison between variables and paired samples, respectively. Kaplan-Meier analysis and the log-rank test were used to illustrate differences between each potential risk factor in recurrence-free and overall survival probability after patients received primary curative hepatectomy. In our analysis of the probability that patients would remain free of hepatoma recurrence, we defined recurrence as the first event in treatment failure; data on all other patients were censored on the date of the last follow-up visit, death from causes other than hepatoma, and any subsequent recurrences of hepatoma. Data on patients were analyzed from the date of surgery to the time of the first event or to the date on which data were censored (according to Kaplan-Meier method), and the curves were compared using the log-rank test.
Results
SUBGENOME-WIDE RNAi SCREENING IDENTIFIED KLHL23 AS A TUMOR INVASION SUPPRESSION GENE OF HCC
To identify genes that suppress tumor invasion, we conducted an RNAi screen of the entire human kinome/phosphatome in Hep3B HCC cells, which have low motility. After four rounds of transwell selection (Fig. 1A), 89 clones of transduced Hep3B cells with high motility were retrieved, 82 of which were identified as PHOSPHO2-KLHL23 (Fig. 1B; Supporting Table S1). PHOSPHO2-KLHL23 is located on chromosome 2q31 and is transcribed as a single transcript. However, PHOSPHO2 (phosphatase orphan 2) is a noncoding orphan gene, and KLHL23 is translated into a protein with unknown function. We therefore selected KLHL23 for further investigation.
We knocked down KLHL23 expression in different HCC cells by using two clones of shRNA targeting KLHL23 mRNA (shKLHL23; Fig. 1C) and found that cell migration and invasion were 10- and 7-fold, respectively, higher than those of control (shLuc, cells transfected with shRNA targeting luciferase; Fig. 1D) in Hep3B, Huh7, and HepG2 cells (Supporting Fig. S1A). These findings were consistent with those of a wound healing assay (Supporting Fig. S1B). In contrast, ectopic expression of KLHL23 (−Dox) in two highly invasive HCC cell lines, Mahlavu and Huh7, resulted in a 15- and 12-fold reduction in cell migration and invasion, respectively, as compared to control (+Dox; Fig. 1E). Additionally, ectopic expression of KLHL23 in Huh7 and Mahlavu cells suppressed proliferation and tumor-sphere formation in soft agars (Supporting Fig. S1C,D).
Furthermore, we performed an in vivo invasion assay by inserting Matrigel-filled intravenous catheters into three empty-vector–transduced Huh7 (Huh7-shEV) and three shKLHL23-transduced Huh7 (Huh7-shKLHL23) xenograft tumors. We found an 8-fold increase in the number of Huh7-shKLHL23 cells migrating into the tubes as compared to the Huh7-shEV control (Fig. 1F), which was consistent with our in vitro results. Then, using a xenograft liver metastasis assay, we injected tumor cells into spleens of nude mice,20 which yield many more metastatic tumors of Huh7-shKLHL23 cells than of Huh7-shLuc cells in mouse livers (Fig. 1G). Metastatic tumor growth in mouse livers was confirmed by histopathological examination (Fig. 1H). Together, these data confirm that KLHL23 suppresses invasion and metastasis of HCC cells in vitro and in vivo.
DOWN-REGULATION OF KLHL23 IN HUMAN LIVER AND PANCREATIC CANCERS IS ASSOCIATED WITH WORSE CLINICAL OUTCOME
To examine the role of KLHL23 in HCC development and progression, we correlated KLHL23 expression with the clinical manifestations of patients with HCC by using immunohistochemistry (IHC) of tissue microarrays containing paired HCC and para-tumor liver tissues from 89 HCC patients. KLHL23 intensity was significantly lower in HCC (Fig. 2A; P < 0.001, paired t test). Lower KLHL23 level in tumor was significantly associated with younger age (55.6 vs. 64.1 years; P = 0.003), hepatitis B and non-C infection (P = 0.009 and 0.001, respectively), and invasion into large vessels (P = 0.013; Supporting Table S2). Cox regression analysis revealed that relatively high KLHL23 was associated with lower mortality (hazard ratio for overall mortality = 0.42; 95% confidence interval = 0.23-0.079; P = 0.007), which suggests that KLHL23 protects against HCC progression (Supporting Table S3). Kaplan-Meier survival curves and the log-rank test revealed lower cumulative disease-free survival after initial curative therapy in patients with lower KLHL23 in their HCCs (P = 0.0052; Fig. 2B).
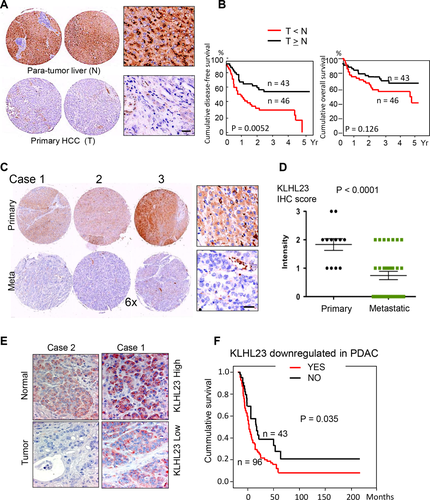
We further compared relative KLHL23 levels in primary and metastatic HCCs from 12 patients with extrahepatic metastasis and found that KLHL23 was significantly down-regulated in tumors at metastatic sites (Fig. 2C,D; P < 0.0001, paired t test). We further validated the suppressive role of KLHL23 in HCC metastasis by using IHC on a commercially available tissue microarray (LC2081, Biomax US) containing 100 tissue cores of HCC, 10 tissue cores of metastatic HCC, and 12 normal liver tissues (IHC scores: normal livers > HCCs at the primary site, P < 0.0001; HCCs at the primary site > HCCs at metastatic sites, P = 0.0067; Supporting Fig. S2).
Because of the highly invasive property of pancreatic cancer, we examined KLHL23 levels in 136 patients with pancreatic ductal carcinoma. KLHL23 levels were significantly lower in the tumors as compared with corresponding nontumor pancreatic tissues (Fig. 2E; P < 0.0001). Kaplan-Meier survival curve and the log-rank test revealed that lower KLHL23 in pancreatic cancer was associated with lower overall survival (Fig. 2F; P = 0.035).
KLHL23 SUPPRESSES EMT TRANSCRIPTIONAL REPROGRAMMING
Because EMT is crucial for invasion and metastasis of cancer cells, we examined whether KLHL23 suppressed tumor cell invasion by inhibiting EMT. We found that KLHL23 silencing changed cell morphology from a cuboid shape with cell-cell adhesion to a spindle shape and dispersed Hep3B and Huh7 cells (Fig. 3A). Immunofluorescence showed that E-cadherin level was lower in the membrane of Huh7-shKLHL23 cells than in control (Fig. 3B, upper panel), which was further confirmed by an immunoblotting assay (Fig. 3B, lower panel). Induction of EMT by KLHL23 silencing was further demonstrated by down-regulation of epithelial markers, such as E-cadherin, occludin, and claudin-1, and up-regulation of mesenchymal markers, such as N-cadherin, vimentin, and fibronectin, and EMT transcriptional factors, including Snail and Twist, in HepG2 and Hep3B cells (both were relatively epithelioid) compared to controls (shLuc; Fig. 3C).
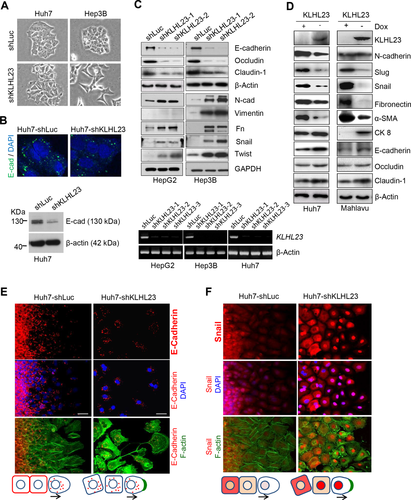
By contrast, ectopic expression (−Dox) of KLHL23 in Huh7 and Mahlavu cells (which are mesenchymal in nature) reversed mesenchymal traits, as evidenced by down-regulation of N-cadherin, Slug, Snail, fibronectin, and alpha-smooth muscle actin (α-SMA) and up-regulation of cytokeratin 8 (CK 8), E-cadherin, occludin, and claudin 1 (Fig. 3D). Together, these data suggest that KLHL23 regulates tumor cell invasion by modulating cell transition between epithelial and mesenchymal traits.
To further examine spatiotemporal regulation of EMT by KLHL23 silencing, we used wound healing assays. KLHL23 silencing (Huh7-shKLHL23) down-regulated E-cadherin and disrupted cell-cell contact (Fig. 3E), whereas up-regulating and activating Snail (nuclear translocation; Fig. 3F). Interestingly, at the moving front, Snail rapidly decreased in conjunction with stress F-actin formation in controls (Huh7-shLuc; Fig. 3F, left panel), whereas Snail up-regulation and activation (nuclear localization) were maintained, along with enhanced lamellipodia and filopodia, when KLHL23 had been silenced (Fig. 3F, right panel). These results suggest that KLHL23 silencing induces EMT and enables cells to sustain mesenchymal traits during migration.
KLHL23 IS AN ACTIN-BINDING PROTEIN
To determine how KLHL23 suppresses EMT, we used coimmunoprecipitation to identify the proteins that interact with KLHL23 by ectopically expressing a flag-tagged KLHL23 in Mahlavu cells, followed by a flag pull-down and mass spectrometry. Actin was thus identified (Supporting Fig. S3A). Interaction between actin and endogenous KLHL23 was shown by immunoprecipitation (IP) with an anti-KLHL23 antibody in Huh7, Hep3B, and HepG2 cells, with and without silencing of KLHL23 expression (Supporting Fig. S3B). An in situ proximity ligation assay confirmed the direct interaction between actin and endogenous KLHL23 in cytoplasm of Tong, HepG2, and Huh7 cells (Supporting Fig. S3C).19
In vitro binding assays followed by differential sedimentation further showed that recombinant KLHL23 (rKLHL23) was coprecipitated in actin polymer (P: precipitate) fractions, as was α-actinin (a positive control), but not albumin (a negative control; Supporting Fig. S3D). Binding of rKLHL23 to actin polymers was dose dependent (Supporting Fig. S3E). Together, these findings indicate that KLHL23 is an actin-binding protein.
Ectopically expressed KLHL23-RFP (fused with red fluorescent protein) was localized to the cytoplasm and only partially colocalized with G-actin (Supporting Fig. S3F). Interestingly, the total amount of F-actin in cells positive for ectopic expression of KLHL23-RFP was greatly reduced (Supporting Fig. S3G, arrowhead), raising an interesting possibility that KLHL23 may suppress F-actin formation.
KLHL23 SUPPRESSES F-actin, FILOPODIUM, AND LAMELLIPODIUM FORMATION
Actin cytoskeleton remodeling underlies the formation of lamellipodia and filopodia in cell migration.8 KLHL23 silencing by different clones of shRNA (shKLHL23) led to a 4- to 9-fold increase in total F-actin intensity (Fig. 4A) and a 4- to 6-fold increase in the number of filopodia (Fig. 4B). Conversely, ectopic expression of KLHL23 (−Dox) caused a 4-fold decrease in total F-actin intensity and a marked decrease in filopodia, as compared to control (+Dox; Fig. 4C). These findings indicate that KLHL23 suppresses F-actin formation, including filopodium and lamellipodium development.
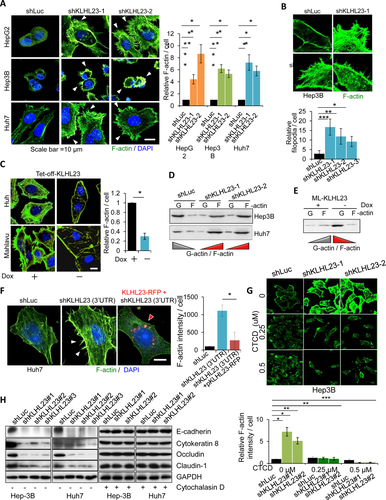
Suppression of F-actin formation by KLHL23 was further confirmed by examining changes in the ratio of globular actin (G)- to filament (F)-actin levels in the differential sedimentation assays. KLHL23 silencing reversed the ratio of G- versus F-actin from >1 to <1 in Hep3B and Huh7 cells (cells with epithelial traits; Fig. 4D). By contrast, ectopic expression of KLHL23 (−Dox) reversed the G- to F-actin ratio from <1 to >1 in Mahlavu cells, a cell line with mesenchymal traits (Fig. 4E).
Moreover, enhancement of F-actin formation by KLHL23 silencing (Fig. 4F, left and middle panels) was reversed (Fig. 4F, middle and right panels) by transfection of KLHL23-RFP in Huh7-shKLHL23 (3′-UTR [untranslated region]) cells, in which KLHL23 expression had been silenced by an shRNA targeting the 3′-UTR of the KLHL23 mRNA.
Suppression of F-actin polymerization by KLHL23 was further validated by using in vitro actin polymerization assays. Treatment with the lysate of Huh7-shKLHL23 enhanced actin polymerization (Supporting Fig. S4; red, left panel). By contrast, ectopic expression of KLHL23 suppressed actin polymerization (blue, left panel), as compared to control (black; shLuc, lysate of cells transfected with shLuc). Moreover, enhancement of actin polymerization by KLHL23 silencing was attenuated by adding purified recombinant KLHL23 to the reaction (brown vs. red; Supporting Fig. S4, right panel).
In sum, we conclude that KLHL23 blocks F-actin formation by direct binding to F-actin and that KLHL23 silencing enhances actin polymerization and subsequent formation of lamellipodia and filopodia.
KLHL23 SUPPRESSES EMT BY ITS SUPPRESSION ON ACTIN POLYMERIZATION
Actin cytoskeleton remodeling is generally regarded as a downstream event of EMT. It is intriguing to ask how KLHL23, an actin cytoskeleton modulator, inversely regulates EMT. To test whether EMT induced by KLHL23 silencing is dependent on the action of KLHL23 in actin cytoskeleton remodeling, we used cytochalasin D (CTCD), a fungal metabolite that binds to actin and blocks actin polymerization.21 We found that enhancement of F-actin, lamellipodia, and filopodia formation by KLHL23 silencing was reversed by CTCD treatment (Fig. 4G for Hep3B; Supporting Fig. S5 for Huh7 cells). Moreover, CTCD reversed the suppression of epithelial markers, including E-cadherin, cytokeratin 8, occludin, and claudin 1, by KLHL23 silencing (Fig. 4H). Collectively, KLHL23-mediated EMT suppression depends on the function of KLHL23 in suppressing actin cytoskeleton remodeling.
KLHL23 SILENCING INDUCES EMT BY ACTIVATION OF HIF AND NOTCH SIGNALING
To determine how actin cytoskeleton remodeling activated by KLHL23 silencing induces EMT, we used microarray assays and MetaCore pathway analysis (http://lsresearch.thomsonreuters.com/) to reconstruct the biological networks that cause KLHL23 silencing in Huh7 and Hep3B cells. We found that Notch signaling pathways may be activated when KLHL23 expression was silenced (Fig. 5A). We then used qRT-PCR and confirmed that KLHL23 silencing (Supporting Fig. S6) up-regulated Notch ligands (JAGGED1 and JAGGED2), Notch receptors (NTOCH1 and NOTCH2), and Notch-specific targets (HES1 and HEY1; Fig. 5B).
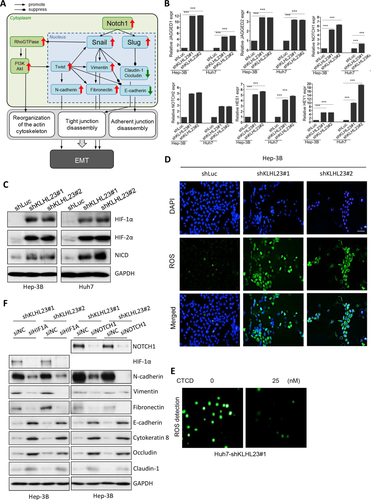
Because EMT and Notch signaling could be activated by hypoxia,22-25 we hypothesized that KLHL23 silencing induces cellular hypoxic responses. Indeed, we found increased levels of HIF-1α, HIF-2α, and Notch intracellular domain (NICD) in shKLHL23-transduced Hep3B and Huh7 cells (Fig. 5C). Because hypoxia is always associated with mitochondrial oxidative stress and generation of reactive oxygen species (ROS), we assayed intracellular ROS and found greater ROS accumulation in shKLHL23-transduced Hep3B and Huh7 cells than in control (Fig. 5D). Interestingly, ROS accumulation in Huh7-shKLHL23 cells was reversed by CTCD treatment (Fig. 5E and Supporting Fig. S7), which suggests that hypoxia-induced oxidative stress induced by KLHL23 silencing was attributable to enhanced actin cytoskeleton remodeling. Together, these findings indicate that KLHL23 silencing enhances actin cytoskeleton remodeling, which then induces intracellular hypoxic stress that culminates in ROS accumulation and activation of HIF and Notch signaling.
We then examined whether EMT induced by KLHL23 silencing was dependent on HIF and Notch signals. In Hep3B-shKLHL23 cells, the induced mesenchymal markers, including N-cadherin, vimentin, and fibronectin, and the suppressed epithelial markers, including E-cadherin, cytokeratin 8, occludin, and claudin-1 were reversed when HIF1A or NOTCH1 was silenced (by siHIF1A and siNOTCH1, respectively), as compared to control (siNC, small interfering RNA containing scrambled sequences; Fig. 5F). We conclude that EMT induced by KLHL23 silencing is attributable to the following signaling cascade: KLHL23 silencing → actin cytoskeleton remodeling → intracellular hypoxic stress → HIF/Notch signals → EMT.
ACTIN CYTOSKELETON REMODELING DRIVES HYPOXIA-MEDIATED, CELL-DENSITY–DEPENDENT HIF AND Notch SIGNALING AND SUBSEQUENT EMT
To determine whether induction of actin cytoskeleton remodeling by cues other than KLHL23 silencing would still activate HIF and Notch signals and then EMT, we used jasplakinolide (Jasp), which induces actin polymerization, and latrunculin A and B (LA and LB; F-actin disruptors), which disrupt actin polymers and then enhance F-actin turnover.26 CTCD suppresses actin polymerization and was used as a control. Induction of HIF and Notch signals and EMT by KLHL23 silencing or treatment with LA was observed when cells were at high density (H: >140% confluence), but not at low density (L: <70% confluence; Fig. 6A, left and middle panels). Combining KLHL23 silencing and high cell density further enhanced HIF and Notch signaling and EMT induction (Fig. 6A, right panel). Interestingly, in the absence of KLHL23 silencing, activation of actin cytoskeleton remodeling by LA, LB, or Jasp treatment also induced HIF and Notch signals and EMT, when cells were at high density (Fig. 6B). By contrast, HIF and Notch signals and EMT were not induced by CTCD treatment, even when the cells were at high density (Fig. 6B). Together, these findings suggest that actin cytoskeleton remodeling by either KLHL23 silencing or treatment with F-actin inducers or disruptors induces HIF and Notch signaling and EMT in a cell-density–dependent manner.

Large tumor size and high cell density usually represent a relative hypoxia in the tumor microenvironment. We examined the effect of microenvironment hypoxia on induction of HIF and Notch signaling and EMT. Surprisingly, hypoxic microenvironment (0.5%-1.0% of oxygen for up to 48 hours) alone was not sufficient to induce HIF or Notch signaling or EMT (Fig. 6C; leftmost two lanes). However, Notch and HIF signaling and EMT was incrementally induced when high cell density (H), KLHL23 silencing, and high cell density + KLHL23 silencing were added to the underlying hypoxic environment (Fig. 6C). The strongest induction of HIF and Notch signaling and EMT occurred when cancer cells at high cell density were under hypoxic conditions and actin cytoskeleton remodeling was evoked, for example, by KLHL23 silencing (Fig. 6C). This finding suggests a synergistic effect of microenvironment hypoxia, high cell density, and actin cytoskeleton remodeling on the induction of HIF and Notch signaling and subsequent EMT.
To directly inspect spatiotemporal regulation of EMT by activation of actin cytoskeleton remodeling when cells were at high density, we used wound healing assays (Fig. 6D). As expected, both LA and LB treatment enabled moving cells to maintain their mesenchymal state (represented by sustained high expression and nuclear localization of Snail along with prominent lamellipodia and filopodia formation) at the moving front and a greater distance of movement, as compared with the rapid decrease and inactivation of Snail and limited distance of movement in control cells (treated with dimethyl sulfoxide [DMSO]; Fig. 6D). These findings are consistent with those observed when KLHL23 was silenced (Figs. 3E,F and 4A,B). In contrast, CTCD decreased total F-actin level, suppressed Snail expression and activation, and arrested cell motion (Fig. 6D, left, lower panel). Collectively, actin cytoskeleton remodeling induced by either intrinsic (such as KLHL23 silencing) or extrinsic (such as treated with F-actin modulators) cues could induce hypoxia-mediated EMT in a cell-density–dependent manner.
EMT DRIVEN BY ACTIN CYTOSKELETON MODELING IS THROUGH ATP AND OXYGEN OVERCONSUMPTION AND CAN BE REVERSED BY ATP SUPPLEMENTATION
One ATP molecule is required for incorporation of each actin monomer during actin polymerization. We thus hypothesized that evoking actin cytoskeleton remodeling would increase ATP consumption and oxygen demand, thereby augmenting cellular hypoxic stress. Indeed, KLHL23 silencing (Fig. 6E) and treatment with LA and LB (Fig. 6F) increased ATP consumption and decreased intracellular residual oxygen content (Fig. 6G). Interestingly, consumptions of ATP and oxygen after KLHL23 silencing were exponentially positively correlated with cell density (Fig. 6E,G). By contrast, CTCD did not result in an obvious increase in ATP consumption (Fig. 6F). Importantly, ATP supplementation reversed the induction of HIF-1α, HIF-2α, NOTCH1, N-cadherin, and Snail by KLHL23 silencing and LB treatment (Fig. 6H). Together, high cell density and actin cytoskeleton remodeling synergistically promote hypoxia-mediated HIF and Notch signaling and subsequent EMT, at least in part, by increasing intracellular hypoxic stress.
INVERSE CORRELATION OF TUMOR SIZE WITH HYPOXIC STRESS, EMT, AND KLHL23 DOWN-REGULATION IN PATIENT-DERIVED HCC XENOGRAFTS
Cell density is usually greater in large tumors. Clinically, large tumors are more invasive and more likely to metastasize. We thus compared cellular responses to hypoxic stress (HIF-1α level) and EMT transcriptional reprogramming (Snail level) between small (<200 μM) and large (>3 mM) xenograft tumors derived from the same clinical HCC samples in nude mice (patient-derived HCC xenografts). HIF-1α and Snail levels were high in large tumors (lower panel), but not in small tumors (upper panel; Fig. 7A). Moreover, KLHL23 and HIF-1α had a mutually exclusive expression in sections of large HCC xenografts (Fig. 7B), consistent with our hypothesis that KLHL23 down-regulation is associated with augmented cellular hypoxic stress and HIF-mediated signaling.
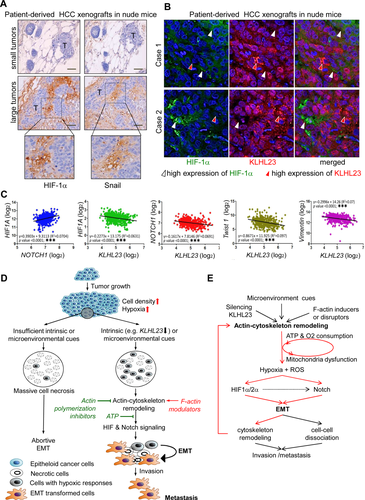
To confirm the role of KLHL23 in hypoxic stress and EMT, we used a cohort of 268 HCC cases from a public data set (Gene Expression Omnibus, GSE25097). We found a positive correlation between HIF1A and NOTCH1 expression and inverse correlations of KLHL23 with HIF1A, NOTCH1, TWIST1, and VIM expressions (all with P < 0.0001; Fig. 7C).
Interestingly, Fig. 7B shows the heterogenous KLHL23 expression in tumor sections and its inverse correlation with HIF-1α, suggesting that the KLHL23 level is regulated by microenvironmental cues mainly at the protein turnover. To verify this, we examined the turnover rate of KLHL23 protein and found that its half-life was as short as 2.5 hours (Supporting Fig. S8). Indeed, the rapid protein turnover rate of KLHL23 was further supported by the rapid change of KLHL23 level at different steps during cell migration in a wound healing assay. We found that KLHL23 level was decreased in cells in response to a migration induction (state 1 to state 2 of Supporting Fig. S9A). By contrast, the KLHL23 protein was rapidly increased and colocalized with the nascent stress F-actin fibers in the cells at the moving front in the absence of further stimuli (such as growth factors; state 2 to state 3 of Supporting Fig. S9A,B). Notably, KLHL23 down-regulation would induce filopodium and lamellipodium formation and cell migration (Figs. 3E,F and 4A,B), whereas binding of KLHL23 to F-actin suppressed F-actin polymerization and lamellipodium and filopodium formation, thereby arresting cell migration (Fig. 4C and Supporting Fig. S4).
In sum, the hypoxic microenvironment created by increasing tumor size is not sufficient to induce EMT. Without sufficient intrinsic or microenvironment cues, hypoxia leads to massive cell necrosis (Fig. 7D, left arm). By contrast, simultaneous activation of actin cytoskeleton remodeling by microenvironmental cues, treatment with F-actin modulators, or KLHL23 silencing synergistically induces HIF and Notch signaling and ultimately EMT in a cell-density–dependent manner cells (Fig. 7D, right arm). Notably, actin cytoskeleton remodeling can be further induced by EMT, thereby forming a positive feedback loop that maintains the resulting mesenchymal state of cancer cells during migration and metastasis (Fig. 7E and Supporting Fig. S10).
Discussion
It is generally believed that hypoxia is greater in larger tumors and that it promotes EMT and thus tumor metastasis. However, accumulating evidence suggests that hypoxia is not sufficient to induce EMT.27, 28 Instead, hypoxia usually leads to massive tumor necrosis. Microenvironmental cues, such as inflammatory chemokines and cytokines, growth factors and extracellular matrix components, or cellular mesenchymal nature play important roles in EMT induction.29 However, the mechanism by which EMT is synergistically induced by these intrinsic and extrinsic cues in cancer cells under the hypoxic microenvironment remains unclear. Interestingly, a convergent result of these EMT-inducing intrinsic and extrinsic cues is activation of cytoskeleton remodeling for cell morphological or functional change. In this study, we unexpectedly found that activation of actin cytoskeleton remodeling is critical in cell-density–dependent induction of EMT by HIF and Notch signaling in cancer cells. Although hypoxia might induce HIF signaling, it is not sufficient to activate Notch signaling and EMT, unless actin cytoskeleton remodeling is simultaneously provoked. Our findings are clinically relevant because targeting the signals that activate actin cytoskeleton remodeling or actin polymerization could suppress EMT and thus prevent or treat tumor invasion and metastasis. Indeed, previous studies reported that targeting microenvironment factors, such as neutrophils, prevented tumor metastasis.30, 31
This actin cytoskeleton remodeling-induced EMT is, at least in part, caused by the enhanced cellular hypoxic responses attributed to increased cellular ATP and oxygen demand. Interestingly, the resulting EMT was reversed by ATP supplementation and treatment with inhibitors of actin polymerization, such as CTCD. Our findings suggest that tumor invasion and metastasis could be prevented or treated by an intervention that targets this linkage. However, our results indicate that caution is warranted when using actin cytoskeleton disruptors, which interfere with but simultaneously enhance actin cytoskeleton remodeling, because of their potential to increase tumor invasion and metastasis in human cancers.32, 33
Previously, Scheel et al. identified an autocrine mechanism involving the TGF-β and canonical and noncanonical Wnt signaling pathways that maintain the resulting mesenchymal traits of cells.34 Herein, we report on another mechanism by which the resulting mesenchymal trait is maintained by the involvement of actin cytoskeleton remodeling-induced HIF and Notch signaling. Induction of EMT by HIF and Notch signaling has been thoroughly studied.24, 35-39 “Actin cytoskeleton remodeling → HIF/Notch signals → EMT → actin cytoskeleton remodeling” might form a positive-feedback loop that makes disseminating tumor cells less vulnerable to the absence of or insufficiency of EMT-inducing cues during microenvironmental shifts in metastasis.
Clinically, we found that KLHL23 down-regulation frequently occurs in HCC and pancreatic cancer and is associated with vascular invasion, early recurrence, and poor prognosis. Moreover, KLHL23 down-regulation in HCC is greater at the metastasis site than at the primary site. Our findings indicate that KLHL23 regulates the cellular transition between epithelial and mesenchymal phenotypes during cancer metastasis. Thus, KLHL23 might serve as a marker of cancer recurrence and metastasis. An intervention targeting KLHL23-regulated EMT might be beneficial in preventing and treating metastatic cancers.
Recently, Choi et al. reported on the expression of read-through transcription of PHOSPHO2-KLHL23 in human gastric cancers.40 They found that ectopic expression of this fusion transcript in gastric cancer cells led to increasing in cell proliferation in vitro, functioning as an oncogene. Clinically, however, low expression of KLHL23 in gastric cancer is significantly associated with tumor invasiveness, including Lauren Classification of diffuse versus intestinal type (2.56 vs. 6.03; P = 0.040), presence versus no presence of perineural invasion (3.21 vs. 6.57; P = 0.037), Bormann 4 versus Bormann 2 (0.84 vs. 10.39), and lymph node metastasis (N > 0) versus no metastasis (N = 0; 2.19-5.43 vs. 6.90; Table 2 of Choi et al.40). Their findings suggest that KLHL23 suppresses tumor invasion, which is consistent with our findings in this study.
It seems intuitive that cellular structures are regulated by corresponding cellular signaling, and there are only a few examples of structural changes inversely regulating cell signaling.41, 42 Herein, we identified an instance of how cancer cells utilize cytoskeleton remodeling to drive EMT, so as to coordinate cytoskeleton remodeling and EMT transcription reprogramming for invasion and metastasis. Our findings are an instance of how structural changes in cells inversely induce signaling and offer the promise that this crucial process can be utilized for prevention and treatment of cancer metastasis.
Acknowledgments
We are grateful to the Microscopy Core Lab, Center for Advanced Molecular Imaging and Translation, Laboratory Animal Center, and DNA Sequencing Core Lab of the Chang Gung Memorial Hospital for their technical assistance and to the Taiwan Liver Cancer Network for the HCC tissue arrays and National RNAi Core of Taiwan for the lentivirus-based shRNA clones.
REFERENCES
Author names in bold designate shared co-first authorship.