Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis
Potential conflict of interest: Nothing to report.
Supported by National Institutes of Health grants 1R01DK62277, 1R01DK100287, 1R01CA204586, R01 HL128297/HL/NHLBI and Endowed Chair for Experimental Pathology (to S.P.M.), NIH-NHLBI 1R01HL128297-01 (PS), NIH-NIDDK-1RO1DK090325(MO) and in part by grant 5T32DK63922-13 (to T.P.-S.).
Abstract
β-Catenin, the downstream effector of the Wnt signaling, plays important roles in hepatic development, regeneration, and tumorigenesis. However, its role at hepatocyte adherens junctions (AJ) is relatively poorly understood, chiefly due to spontaneous compensation by γ-catenin. We simultaneously ablated β- and γ-catenin expression in mouse liver by interbreeding β-catenin–γ-catenin double-floxed mice and Alb-Cre transgenic mice. Double knockout mice show failure to thrive, impaired hepatocyte differentiation, cholemia, ductular reaction, progressive cholestasis, inflammation, fibrosis, and tumorigenesis, which was associated with deregulation of tight junctions (TJ) and bile acid transporters, leading to early morbidity and mortality, a phenotype reminiscent of progressive familial intrahepatic cholestasis (PFIC). To address the mechanism, we specifically and temporally eliminated both catenins from hepatocytes using adeno-associated virus 8 carrying Cre-recombinase under the thyroid-binding globulin promoter (AAV8-TBG-Cre). This led to a time-dependent breach of the blood–biliary barrier associated with sequential disruption of AJ and TJ verified by ultrastructural imaging and intravital microscopy, which revealed unique paracellular leaks around individual hepatocytes, allowing mixing of blood and bile and leakage of blood from one sinusoid to another. Molecular analysis identified sequential losses of E-cadherin, occludin, claudin-3, and claudin-5 due to enhanced proteasomal degradation, and of claudin-2, a β-catenin transcriptional target, which was also validated in vitro. Conclusion: We report partially redundant function of catenins at AJ in regulating TJ and contributing to the blood–biliary barrier. Furthermore, concomitant hepatic loss of β- and γ-catenin disrupts structural and functional integrity of AJ and TJ via transcriptional and posttranslational mechanisms. Mice with dual catenin loss develop progressive intrahepatic cholestasis, providing a unique model to study diseases such as PFIC. (Hepatology 2018;67:2320-2337).
Abbreviations
-
- AJ
-
- adherens junctions
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- BA
-
- bile acid
-
- BSEP
-
- bile salt export pump
-
- CF
-
- fluorogenic carboxyfluorescein
-
- CLD
-
- cholestatic liver disease
-
- DKO
-
- double knockout
-
- dWT
-
- double wild-type
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- IF
-
- immunofluorescence
-
- IHC
-
- immunohistochemical
-
- IP
-
- immunoprecipitation
-
- mRNA
-
- messenger RNA
-
- PCR
-
- polymerase chain reaction
-
- PFIC
-
- progressive familial intrahepatic cholestasis
-
- RT-PCR
-
- reverse-transcription polymerase chain reaction
-
- Sc
-
- scrambled
-
- SiB
-
- silencing of β-catenin
-
- SiBG
-
- silencing of β- and γ-catenin
-
- siRNA
-
- small interfering RNA
-
- TIRF
-
- total internal reflection fluorescence
-
- TJ
-
- tight junctions
-
- TXR
-
- Texas Red
-
- WB
-
- western blot
-
- WT
-
- wild-type
β-Catenin signaling is critical in liver health, where it has been implicated in an array of roles in hepatic development, metabolic zonation, liver regeneration, and xenobiotic metabolism.1 Its aberrant activation is also linked to a subset of hepatocellular carcinoma.1 β-Catenin is also a well-known component of adherens junctions (AJ),2 where it bridges E-cadherin to α-catenin and actin cytoskeleton and facilitates intercellular adhesion.3, 4 Although its contribution to hepatic pathophysiology as a component of the Wnt signaling is relatively well understood, its role as a component of AJ has remained ambiguous. Liver-specific ablation of β-catenin in mice led to loss of the Wnt target genes like glutamine synthetase, cytochrome P450 2E1 (cyp2e1), cyp1a2, lect2, and claudin-2 among others.5, 6 However, there was maintenance of AJ due to compensatory increase in γ-catenin, a normal component of desmosomes, which associated with E-cadherin instead.7, 8 Liver-specific γ-catenin knockout also showed increased β-catenin levels, suggesting a mutually compensatory role of the two catenins at hepatocyte junctions.9
To conclusively address the role of β-catenin and γ-catenin in the absence of β-catenin in the liver, we generated β- and γ-catenin double knockout (DKO) mice by interbreeding β- and γ-catenin double floxed and Alb-Cre transgenic mice. The DKO mice showed notable mortality and morbidity associated with failure to thrive, impaired hepatocyte differentiation, cholestasis, cholemia, portal hypertension, ductular reaction, fibrosis, increased serum alkaline phosphatase (ALP), elevated serum bile acid (BA) and bilirubin levels, all reminiscent of early childhood cholestatic liver diseases (CLDs) such as progressive familial intrahepatic cholestasis (PFIC).10, 11 To carefully delineate the mechanism, we next performed temporal and hepatocyte-specific elimination of the two catenins using hepatocyte-specific adeno-associated virus 8 carrying Cre-recombinase expressed under hepatocyte-specific thyroid hormone-binding globulin promoter (AAV8-TBG-Cre). Dual catenin loss from hepatocytes also acutely also led to increased serum bilirubin and ALP, allowing us the opportunity to carefully dissect the molecular basis of observed cholemia. Using intravital microscopy, we show unique para-hepatocyte leaks leading to mixing of blood and bile as well as spillage of blood from one sinusoid to another. Such disruption of hepatic tissue barrier was confirmed by loss of TJ integrity as seen on transmission electron microscopy, lanthanum oxide exclusion, and Evan's blue dye leakage into bile after intravenous injection. There was an associated loss of AJ assembly due to loss of the catenin–cadherin complex in DKO mice that led to enhanced proteasomal degradation of TJ proteins, including the two major tetraspanins, occludin, and plaque-forming claudins; this was further aggravated by loss of claudin-2, a Wnt/β-catenin transcriptional target, which is important in biliary canaliculi formation. These findings identify an important role of β-catenin in regulating TJ function.
Materials and Methods
Animals
Previously reported homozygous β-catenin–floxed mice and γ-catenin–floxed mice were interbred to generate double-floxed mice.6, 9 Conditional deletion of both β- and γ-catenin is described in the Supporting Information. All animal experiments and procedures were performed according to the National Institutes of Health guidelines under an animal protocol approved by the Institutional Animal Use and Care Committee at University of Pittsburgh.
Viruses and Infections
AAV8-TBG-Cre or AAV8-TBG-GFP were obtained from Penn Vector Core at the University of Pennsylvania. Four-week-old or older β- and γ-double–floxed mice were given a single intraperitoneal injection of 2.5 × 1011 genome copies of AAV8-TBG-Cre or AAV8-TBG-GFP. At least three mice per time point ranging from 7 to 14 days were used for the studies.
EVAN'S BLUE VASCULAR PERMEABILITY ASSAY
Next, 0.5% sterile solution of Evans blue was prepared in phosphate-buffered saline. At 10 or 12 days postinjection, 200 μL of Evans blue was injected in the inferior vena cava of double wild-type (dWT) or DKO2 mice. Bile was collected from the gall bladder 15 minutes after inferior vena cava injection to analyze the presence of Evans blue dye using fluorometric measurement.12 All experiments were performed in triplicate.
TRANSMISSION ELECTRON MICROSCOPY
For transmission electron microscopy, whole liver was perfused fixed in glutaraldehyde. The surgical and perfusion techniques have been described previously.5, 9 Slides were examined in a JEM 1011 transmission electron microscope at 80 kV. For lanthanum staining, tissues were incubated with lanthanum hydroxide (pH 7.8) for 3 hours at room temperature.13, 14 Lanthanum hydroxide was prepared by slowly adding drops of 0.01N NaOH to 2% lanthanum nitrate until the solution reached a pH of 7.8.
INTRAVITAL IMAGING OF LIVER
Intravital imaging of liver is described in detail elsewhere.15 Briefly, mice were anesthetized and placed on a heated stage. A catheter was placed into the right carotid artery to enable intravenous delivery of intravascular fluorochromes. Gentle vacuum suction was applied to the abdominal imaging window device to immobilize. FITC dextran (MW:70,000) or Texas Red (TXR) dextran (MW:70,000) was used to visualize the blood flow through liver sinusoids, whereas carboxyfluorescein (MW:367.82) was used to visualize biliary canaliculi.16 After being internalized by hepatocytes, carboxyfluorescein is hydrolyzed by esterase into fluorogenic carboxyfluorescein (CF), which emits green fluorescence at 517 nm. In wild-type (WT) mice, carboxyfluorescein is taken up and hydrolyzed into CF in 1-5 minutes after injection. Videos and images were recorded at 5, 10, and 30 minutes postinjection). Microscopy was performed using a Nikon multiphoton excitation microscope at the imaging service of the Pittsburgh Liver Research Center.
TOTAL INTERNAL REFLECTION FLUORESCENCE IMAGING
The method of total internal reflection fluorescence (TIRF) imaging has been described elsewhere.17 Briefly, a frozen section of hepatocytes was fixed on a coverslip. Immunofluorescence (IF) assay was performed using E-cadherin and occludin antibodies as described previously.17 Coverslips were mounted with phosphate-buffered saline and vectashield. Microscopy was performed using a Nikon TIRF microscope.
Additional methods are available in the Supporting Information.
Results
SIMULTANEOUS DELETION OF β-CATENIN AND γ-CATENIN IN THE LIVER DURING DEVELOPMENT RESULTS IN SIGNIFICANT MORTALITY AND MORBIDITY
Liver-specific β- and γ-catenin DKO mice were generated using Alb-Cre as described in the Materials and Methods section and confirmed by genomic DNA polymerase chain reaction (PCR) analysis (Fig. 1A). When compared with WT, increased mortality was evident in DKO with >80% dying by 30 days and no survivors beyond 2.5 months (Fig. 1B). Both male and female DKO mice were smaller in body size compared with WT mice (Fig. 1C). Grossly, livers of DKO mice were yellow, smaller and stiffer as compared with WT mice at 24 days, albeit the liver weight to body weight ratio (LW/BW) was comparable to WT (Fig. 1D,E). DKO mice that survived to 2.5 months had higher LW/BW, and showed yellow and stiffer livers along with splenomegaly, a sign of portal hypertension (Fig. 1D,E). Intriguingly, serum alanine aminotransferase (ALT) levels were unchanged, although ALP and total and direct bilirubin were significantly up-regulated in DKO mice (Fig. 1F,G). Western blot (WB) and immunohistochemical (IHC) assay confirmed loss of both β-catenin and γ-catenin in DKO livers compared with livers from WT mice (Figs. 1H and 2A).
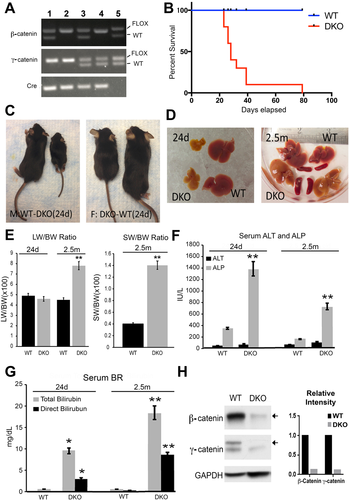
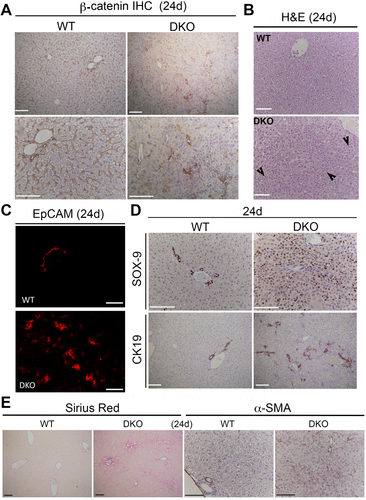
DKO MICE SHOW EXTENSIVE DUCTULAR REACTION, LIVER FIBROSIS, INFLAMMATION, TUMORIGENESIS, AND LOSS OF E-CADHERIN
To further characterize the DKO phenotype displaying notable morbidity in neonatal stages, we assessed histology and performed IHC analysis for injury and inflammation markers in DKO livers 24 days after birth. A notable increase in the presence of smaller ductular hepatocytes, especially in the periportal region, were evident on hematoxylin and eosin staining in DKO livers (Fig. 2B). Enhanced ductular reaction was also evident as seen by a notable increase in the numbers of ductules positive for epithelial cell adhesion molecule and CK-19 (Fig. 2C,D). A profound increase in numbers of ductular hepatocytes was also verified by sox-9 staining, as is often seen in CLD (Fig. 2D). Accompanying the ductular reaction, a noteworthy increase in hepatic fibrosis as seen by Sirius red staining along with increased presence of activated myofibroblasts as evident by α-smooth muscle actin (α-SMA) IHC analysis was also observed in DKO at 24 days (Fig. 2E).
Because 10% of mice survived to around 2.5 months, we assessed the phenotype of DKO livers at this stage. A notable increase in CD45-positive inflammatory cell infiltrate was evident in DKO at this time (Supporting Fig. S1A). Sirius red and α-SMA staining showed extensive collagen deposition and activated myofibroblasts, respectively, in DKO mice (Supporting Fig. S1A). Enhanced fibrosis was also confirmed by increased expression of profibrogenic markers like col1a1, Pdgfra, and Tgfb1 by reverse-transcription PCR (RT-PCR) analysis (Supporting Fig. S1B).
Any compensatory changes in ductular and hepatocyte proliferation were assessed next by IHC analysis for Ki-67 at 24 days and 2.5 months (Supporting Fig. S2A,B). Careful quantification showed greater baseline Ki-67 staining in hepatocytes in WT livers at 24 days due to known postnatal hepatic growth; however, DKO mice showed significantly fewer Ki-67–positive hepatocytes (Supporting Fig. S2C). Intriguingly, a significant increase in Ki-67–positive ductular cells was evident in DKO mice (Supporting Fig. 2C). At 2.5 months, both hepatocytes and ductular cells showed significantly more Ki-67 positivity in DKO mice compared with WT mice (Supporting Fig. S2D).
As a result of the chronic injury and regeneration, dysplastic nodules were apparent especially at 2.5 months in DKO mice (Supporting Fig. S1A). A subset of such nodules progressed to frank hepatocellular carcinoma (Supporting Fig. S2E).
LOSS OF β- AND γ-CATENIN LEADS TO IMPAIRED BILE FLOW AND ALTERED EXPRESSION OF SEVERAL JUNCTIONAL PROTEINS AND BILIARY TRANSPORTERS
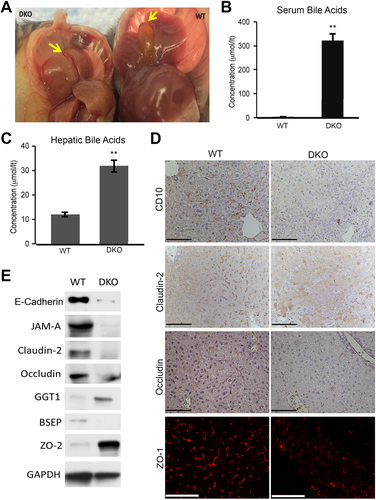
Elevated serum ALP and bilirubin, extensive ductular reaction and increased biliary markers in hepatocytes, all indicated development of CLD in DKO mice. Indeed, in WT mice where gall bladders were intact and filled with bile, in DKO these structures were involuted and without bile (Fig. 3A). We next assessed whether cholestasis is leading to altered levels of BA in the liver and blood to also validate the presence of cholemia. DKO showed around three-fold higher hepatic BA levels and almost 300-fold higher serum BA levels (Fig. 3B,C). Associated with increased BA levels, we observed notable ductular reaction at this stage (Supporting Fig. S3A). Also, increased TUNEL-positive apoptotic nuclei were evident in the livers of DKO mice (Supporting Fig. S3B).
It was intriguing that despite increased hepatocyte injury, serum ALT levels remained unchanged. We hypothesized this to be due to inappropriate hepatocyte maturation since DKO livers showed several sox-9–positive ductular hepatocytes (Fig. 2D). Indeed, RT-PCR for targets of a major hepatocyte transcriptional regulator HNF4α18 showed significant decreases in its positive targets, including Acot3, Ces3, Ugt2b1, and Dio1, and increased expression of negative targets, including Akr1b7, Ect2, Egr1, and Myc, in DKO livers (Supporting Fig. S3C). Expression of Foxa2, another early hepatic transcription factor was also reduced (Supporting Fig. S3D). Likewise, expression of the ALT gene was significantly reduced in DKO livers (Supporting Fig. S3E), suggesting lack of increased serum ALT despite increased hepatocyte injury is due to improper hepatocyte maturation.
We next assessed localization of biliary canalicular markers to address the structure and viability of intrahepatic biliary canaliculi. IHC and IF verified decreased levels and mislocalization of CD10, occludin, claudin-2, and ZO-1 in DKO livers, suggesting perturbations in biliary canaliculi (Fig. 3D).
To further address any molecular aberrations that may be contributing to cholestasis and cholemia, expression of various biliary transporters, BA metabolism genes, and TJ proteins were assessed. WB from whole liver lysates showed decreases in several junctional proteins such as occludin, claudin-2, JAM-A, and E-cadherin in DKO mice, whereas ZO-2 (TJP2) expression was increased (Fig. 3E). E-cadherin decrease was also verified by way of IHC analysis in DKO (Supporting Fig. S4A). Bile salt export pump (BSEP) protein levels were decreased (Fig. 3E) while its messenger RNA (mRNA) expression was unchanged (Supporting Fig.4B). RT-PCR also revealed decreases in the mRNA expression of NTCP, Cyp7a1, and MRP2 in DKO (Supporting Fig. S4B).
Thus overall, DKO mice exhibit a phenotype that is truly reminiscent of PFIC patients showing a plethora of aberrations including defects in expression of biliary transporter BSEP and deregulation of a host of hepatic junctional proteins.
SPECIFIC DELETION OF β- AND γ-CATENIN ONLY FROM HEPATOCYTES LEADS TO TIME-DEPENDENT CHOLEMIA
To identify the primary molecular basis and sequence of the phenotype evident in DKO mice, we conditionally deleted β- and γ-catenin from the hepatocytes only by injecting AAV8-TBG-Cre in 4-week-old β- and γ-catenin double-floxed mice (DKO2) and sacrificing at 7-14 days for evaluation (Supporting Fig. S5A).19 As a control, β- and γ-catenin double-floxed mice were injected with AAV8-TBG-GFP (dWT). To show the robustness and hepatocyte selectivity, IHC for GFP was performed at 7 days postinjection of AAV8-TBG-GFP. Almost 100% of hepatocytes and no cholangiocytes were GFP-positive (Supporting Fig. S5B). Efficacy of AAV8-TBG-Cre was also evident by a significant and sustained decrease in the protein and mRNA expression both catenins in DKO2 livers at 7-14 days postinjection compared with dWT (Supporting Fig. S5C-E). Because we posit that loss of both catenins simultaneously from hepatocytes affects junctional stability, we assessed serum from DKO2 mice at various times. Although serum bilirubin (both total and direct) and ALP were unremarkable at day 7 and day 10, a significant and progressive increase in their levels was evident at days 12-14 (Supporting Fig. S5F). An increase in ductular reaction was also observed at day 14 in DKO2 livers (Supporting Fig. S5G). Thus, an acute and hepatocyte-specific loss of β- and γ-catenin resulted in an acute onset of cholemia similar to DKO and provided a unique opportunity to examine sequentially both cellular and molecular basis of the observed pathology.
UNIQUE PARA-HEPATOCYTE LEAKS LEAD TO ADMIXING OF BLOOD AND BILE AND CHANNELING OF BLOOD FROM ONE SINUSOID TO ANOTHER IN DKO LIVERS AS SHOWN BY MULTIPHOTON INTRAVITAL MICROSCOPY
To investigate the structural basis of observed cholemia at the earliest time in DKO2 mice, we used multiphoton excitation–enabled in vivo real-time fluorescence microscopy of intact liver in live mice as described previously.15 FITC-dextran was intravascularly administered to visualize the blood flow in liver sinusoids. Blood flow (green) was restricted and linear within sinusoids in dWT mice (Fig. 4A; Supporting Movies S1 and S2). However, thin conduits reminiscent of para-hepatocyte leaks carrying cell-free plasma (green) were observed connecting adjacent sinusoids outlining single hepatocytes in DKO2 livers at day 12 (Fig. 4A; Supporting Movies S3 and S4).
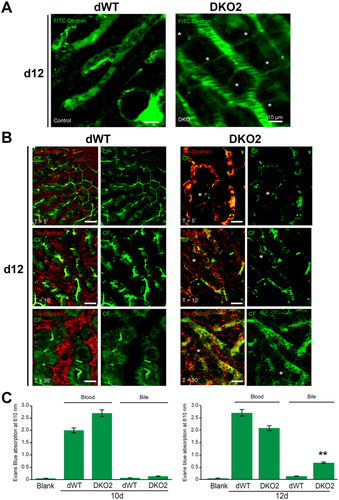
To visualize both blood and bile flow using multiphoton excitation–enabled intravital microscopy, we next used TXR-dextran (red) to label blood flow through liver sinusoids and CF (green) to envision bile flow through biliary canaliculi.20 In dWT mice, the uptake of CF in hepatocytes occurred within 1-5 minutes after injection and CF appeared as a linear green streak highlighting its secretion into biliary canaliculi (Fig. 4B; Supporting Movie S5). Red fluorescence indicated normal blood flow in sinusoids. At 10 minutes, CF prominently localized to biliary canaliculi (Fig. 4B; Supporting Movie S6). By 30 minutes, CF decreased considerably in biliary canaliculi, suggesting its clearance from the intrahepatic biliary compartment (Fig. 4B; Supporting Movie S7). DKO2 at day 12 showed a peculiar and unique phenotype in which TXR-dextran–positive red spots along with CF were found in punctate patterns within sinusoids along with absence of green linear biliary canalicular presence at 5, 10, and 30 minutes (Fig. 4B; Supporting Movies S8-S10). Furthermore, thin conduits reminiscent of para-hepatocyte leaks, outlining hepatocytes and interconnecting sinusoids through which CF and TXR-dextran flowed freely, were evident in DKO2 mice at all times (Supporting Movies S8-S10).
In vivo vascular permeability assay using Evans blue vascular dye was performed next.12 Complete absence of Evans blue in the bile was observed after a single intravenous injection of Evans blue to DKO2 mice at day 10 (Fig. 4C). However, at day 12, Evans blue was significantly increased in bile in DKO2 but not dWT mice (Fig. 4C).
Altogether, these observations allowed a direct visualization of structural perturbations in the form of para-hepatocyte leaks connecting adjacent sinusoids and sinusoids to biliary canaliculi, demonstrating the basis of cholemia in DKO2 mice at 12 days.
DUAL LOSS OF β- AND γ-CATENIN LEADS TO THE DISRUPTION OF HEPATOCYTE JUNCTIONS
Because β-catenin—and, in its stead, γ-catenin—is a structural component of AJ, to understand the primary mechanism that led to disruption of the blood–biliary barrier following the loss of both, we next assessed the structure of AJ and the barrier forming TJ in DKO2 and dWT livers. Transmission electron microscopy of TJ and AJ in dWT liver sections at any stage (shown for day 7) revealed TJ as electron-dense structures in the space between cells near the bile canaliculi abutted by AJ, which is evident as thin double-stranded structure of defined diameter (Fig. 5A). In DKO2 livers, the electron dense TJ structure appeared normal at day 7; however, at days 10 and 14, TJ contained less electron-dense material, resulting in a dilated structure (Fig. 5A). AJ in DKO2 livers showed increased intercellular distance at day 7, which were further dilated by day 10, and their structure was lost by day 14 (Fig. 5A).
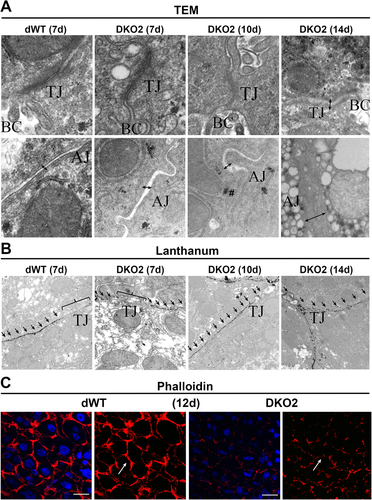
TJ integrity was tested next using lanthanum hydroxide permeability.13 Intact TJs surrounding biliary canaliculi shown by lanthanum hydroxide exclusion from TJ showing only its extracellular distribution signified an intact barrier in the dWT livers (Fig. 5B). In DKO2 livers, such exclusion was apparent at day 7; however, by day 10 and 14, lanthanum was seen accumulating in the TJ, indicating loss of structural integrity (Fig. 5B).
DUAL LOSS OF β- AND γ-CATENIN LEADS TO DISTORTION OF BILIARY CANALICULAR NETWORK
Biliary canalicular networks, which are lined by the apical surfaces of hepatocytes and flanked by TJ proteins, allow unidirectional flow of bile secreted by hepatocytes toward the portal triad. Because the simultaneous loss of the two catenins disrupted TJ, we next investigated whether this results in any disruption of the structural integrity and patency of biliary canalicular networks. We stained liver sections from DKO2 and dWT mice with TRITC-phalloidin to visualize F-actin, which normally marks the pericanalicular outline of hepatocytes. dWT livers at d12 exhibited interconnected, evenly spaced “train track” bile canalicular structures (Fig. 5C). In contrast, DKO2 livers at day 12 showed pronounced distortion of bile canaliculi observed as misshapen, collapsed, and incomplete network structures (Fig. 5C).
DUAL LOSS OF β- AND γ-CATENIN LEADS TO DECREASED LEVELS OF KEY JUNCTIONAL PROTEINS
To address the temporal deregulation of various junctional proteins following loss of β- and γ-catenin, we next investigated the protein and mRNA expression of these proteins in DKO2 and dWT livers. Decrease in E-cadherin protein was observed at days 10, 12, and 14 (Fig. 6A). This was also visible by IF, which not only displayed E-cadherin decrease at hepatocyte membrane but also showed its redistribution as punctate cytoplasmic staining in the DKO2 livers at day 12, suggestive of proteasomal degradation (Fig. 6B). Indeed, mRNA expression of E-cadherin was unchanged until day 14 (Fig. 6C). Next, we assessed claudins, which are tetraspanins and key components of TJ in DKO2 liver lysates. Claudin-3 and -5, which are plaque-forming, were decreased at day 12-14 (Fig. 6A). IF staining verified a notable decrease in surface localization of claudin-3 and -5 at hepatocyte surface at day 12 as well (Fig. 6D). Claudin-2, a known Wnt/β-catenin target in the liver, was significantly decreased at day 7-14 in DKO2 livers by both WB and RT-PCR (Fig. 6A,C). Occludin, another TJ tetraspanin protein, was also decreased at days 12 and 14, whereas its mRNA expression was unaffected until day 14 (Fig. 6A,C). Occludin staining at the TJ in hepatocytes was decreased in DKO2 livers compared with dWT at day 12, whereas ZO-1 remained unchanged (data not shown).
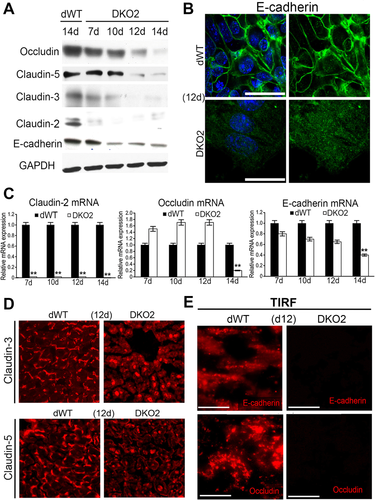
To unequivocally demonstrate the lack of localization of E-cadherin and occludin at cell surface in the DKO2 livers, we used TIRF microscopy. TIRF revealed E-cadherin and occludin localizing at hepatocyte membrane in dWT livers, which was completely lost in DKO2 livers at 12d (Fig. 6E).
Thus, dual loss of catenins triggers deregulation of E-cadherin, along with of key TJ proteins including occludin and claudin-2, -3, and -5 in the DKO2 livers.
ABSENCE OF β- AND γ-CATENIN LEADS TO ENHANCED DEGRADATION OF E-CADHERIN AND KEY TJ PROTEINS, WHICH IS ALLEVIATED BY PROTEASOME INHIBITOR WHEREAS CLAUDIN-2 LOSS IS UNAFFECTED
Next, we addressed the basis of decrease in E-cadherin, occluding, and claudins following loss of both catenins in vitro and in vivo. First, we used human Hep3B cells to determine whether TJ proteins such as occludin coprecipitate with AJ proteins. Immunoprecipitation (IP) studies in scrambled small interfering RNA (siRNA) (scrambled [Sc])-transfected Hep3B cells showed association of occludin with E-cadherin, β-catenin, and γ-catenin (Fig. 7A). Dual silencing of β- and γ-catenin (SiBG) led to abrogation of this association (Fig. 7A). SiBG transfection in Hep3B cells also led to notable decreases in total E-cadherin, occludin, claudin-2, and claudin-3 (Fig. 7B,C). Blocking proteasomal degradation by MG132 in SiBG-transfected cultures rescued E-cadherin, occluding, and claudin-3 but not claudin-2 (Fig. 7B,C). Similarly, increased E-cadherin ubiquitination was observed upon SiBG transfection in Hep3B cells (Fig. 7D). Blocking lysosomal degradation by cyclohexamide did not influence stabilization of any of these proteins, and no advantage of combining MG132 and cyclohexamide was observed (Fig. 7B).
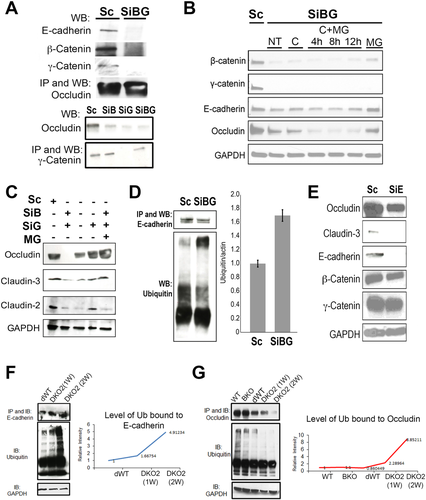
Because a decrease in AJ component E-cadherin due to proteasomal degradation, preceded a loss of TJ proteins both in vitro and in vivo, we next silenced E-cadherin in Hep3B cells. SiBG transfection in Hep3B cells led to notable decreases in occludin and claudin-3 but did not affect β- or γ-catenin levels (Fig. 7E).
To confirm that the mechanism of E-cadherin and occludin loss in vivo in DKO2 livers was indeed evidence of proteasomal degradation, we immunoprecipitated E-cadherin or occludin and performed WB analysis for poly- and mono-ubiquitin. A notable and time-dependent increase in ubiquitination of both proteins was observed at days 10-12 in DKO2 livers (Fig. 7F,G).
Discussion
Liver-specific β-catenin knockout mice have been instrumental in divulging the role of β-catenin in development, regeneration, zonation, and tumorigenesis.1, 6, 21 However, its relative contribution at the hepatocyte junctions has remained poorly understood due to compensation by γ-catenin, which prompted us to generate DKO mice.7, 8, 22 Loss of the two catenins was fatal during the first postnatal month and led to severe and progressive CLD. More controlled deletion of catenins from hepatocytes to address mechanism revealed appearance of unique paracellular leaks that disrupted the blood–biliary barrier and also allowed blood from one sinusoid to leak into another (Fig. 8A). At a molecular level, these phenomena were associated with disruption of the catenin–cadherin complex, prompting proteasome-mediated degradation of E-cadherin and occludin and plaque-forming claudin-3 and -5. Simultaneously, decreased gene expression of claudin-2 was evident. Concomitant loss of occludin and claudins, the two major families of tetraspanins at TJ, led to disintegration of TJ, disruption of the blood–biliary barrier, and cholemia. Thus, we show that β-catenin regulates tissue barrier function through regulation of TJ components through distinct mechanisms (Fig. 8B). We also show that TJ enable orderly and unidirectional flow of sinusoidal blood from portal triad toward central vein within a hepatic lobule (Fig. 8A).

CLD comprises a heterogeneous group of disorders characterized by impaired bile flow, biliary stasis, spillage of bile into blood and hepatic parenchyma, tissue injury, inflammation, fibrosis, and sequelae such as biliary cirrhosis and cancer. Impaired bile flow through intrahepatic biliary canaliculi can be due to bile flow obstruction, defects in hepatocyte secretory function arising from anomalies in expression and function of various transporters, or defects in hepatocyte polarity.23-25 Loss-of-function mutations in the components of tight junctions (TJ), the chief regulator of barrier functions in a tissue, have also been implicated in these diseases. In fact, a subset of patients with PFIC, a group of disorders responsible for 10%-15% of neonatal CLD in the United States and traditionally associated with loss-of-function mutations in transporters essential for optimal bile secretion (PFIC-1:A TP8B1; PFIC-2:BSEP/ABCB11; PFIC-3:MD R3),26-30 have been now identified with mutations in ZO-2, a TJ protein.31 Based on our findings in DKO mice, we demonstrated that loss of β- and γ-catenin led to a host of changes in levels and distribution of both bile acid transporters and tight junctional proteins to yield an early postnatal phenotype reminiscent of PFIC. Specifically, we observed a notable decrease in BSEP, ZO1, occludin, claudin-2, and JAM-A, an up-regulation in ZO-2, and no change in claudin-7. All symptomatic, histological, and biochemical attributes of the disease were evident in the DKO mice, including failure to thrive (which was likely due to impaired BA secretion, failure of bile flow, and fat malabsorption); jaundice and cholemia; progressive intrahepatic cholestatic injury associated with enhanced BA accumulation, inflammation, portal fibrosis, portal hypertension and cirrhosis; and progression to hepatocellular carcinoma in a subset of animals. Thus, the disease in DKO very closely recapitulates PFIC, especially PFIC2 that is associated with loss of BSEP, and could be an invaluable model to study pathogenesis and test novel therapeutics. It is important to note that targeted inactivation of BSEP in mice by itself did not yield a progressive cholestatic phenotype, though mice showed modest but stable CLD.32 Only upon additional deletion of Mdr1a and Mdr1b in the BSEP null background was there evidence of progressive cholestasis and enhanced mortality and morbidity (albeit at around 6 months) and with distinct differences from PFIC clinically.33 Dual loss of β- and γ-catenin in the liver, on the other hand, was sufficient to lead to sufficient deregulation in levels of biliary transporters such as BSEP as well as additional junctional components, which led to a more profound disease reminiscent of PFIC. Although our model is an exciting one in the study of disease pathogenesis, further studies are necessary to evaluate whether loss of AJ catenins may itself be an important cause for subsets of CLD such as PFIC.
The barrier function in tissues is regulated primarily by TJ.34 In the liver, TJ are also thought to be the chief regulators of the blood–biliary barrier, although very few studies have directly demonstrated such function.35, 36 These junctions prevent blood and bile from mixing. Unlike TJ, AJ are viewed primarily as facilitators of cell–cell adhesion.37 While studies have suggested AJ can influence TJ organization, the molecular basis of AJ and TJ crosstalk remains incompletely understood.38 Studies have suggested important roles of E-cadherin in the development and organization of TJ through proper localization of proteins or through regulation of claudins.39, 40
We show that β-catenin, downstream of Wnt, regulates expression of claudin-2. We have previously reported claudin-2 to be a target of β-catenin in the liver.41 Although it is a pore-forming claudin whose loss would not make TJ more permeable, it has been shown to be pertinent in proper biliary canaliculi formation and bile flow.42, 43 In fact, a basal decrease in bile flow rate in β-catenin was attributed to loss of claudin-2, whereas no structural defect was observed in TJ.41 Thus, one major function of β-catenin in the liver that contributes to appropriate biliary homeostasis is regulating the expression of claudin-2 in hepatocytes, which is not compensated by γ-catenin in β-catenin knockout.6, 7
To unequivocally address the mechanism of how dual loss of catenins in hepatocytes yielded progressive intrahepatic cholestasis, we carefully elucidated the sequence of molecular perturbations that were evident as β- and γ-catenin were ablated. As expected, claudin-2 expression dissipated almost around the time of loss of β-catenin (and γ-catenin). The next major deregulation was that observed in E-cadherin protein levels, which was posttranscriptional and also preceded any losses in protein levels of occludin and plaque-forming claudin-3 and -5. At AJ, β-catenin—and in its absence, γ-catenin—appears to be critical for the stability of E-cadherin. Lack of this association in both DKO mice, DKO2 mice, and in vitro led to proteasomal degradation of E-cadherin. Indeed, β-catenin, through its physical association, is known to mask the PEST sequence motif on E-cadherin to prevent its recognition and degradation by ubiquitination.44 γ-Catenin appears to be efficiently performing this function when β-catenin was absent, as seen in single β-catenin knockout,7, 8 hence contributing to E-cadherin stability and maintaining AJ. We also show that catenins and E-cadherin complex with TJ proteins like occludin. Loss of catenins or E-cadherin promoted degradation of occludin and claudin-3, which could be prevented in vitro by a proteasome inhibitor. Proteasome-dependent degradation of occludin in various cell types has also been reported, although no prior connection to AJ proteins has been shown.45, 46 Thus, we divulge a complex interactome composed of key AJ and TJ proteins that help stabilize one another to eventually maintain junctional integrity. It appears that β-catenin—and, in its absence, γ-catenin—is especially critical in its role, perhaps because of its dual function at junctions and as a Wnt pathway component, where it additionally regulates claudin-2 mRNA expression.
Thus, loss of β-catenin alone or loss of Wnt/β-catenin signaling through combined deletion of Wnt coreceptors LRP5-6 is well-tolerated despite lack of claudin-2, since these animals lack any overt CLD.5, 6, 21, 41, 47 This is because of the continued presence of occludin and plaque-forming claudins, the major tetraspanins at the TJ,48 due to γ-catenin, which binds and stabilizes E-cadherin. However, when two major cornerstones of TJ structural integrity, claudins and occludin, are lost simultaneously, the hepatic tissue barrier gives way, and CLD ensues.
In conclusion, our study suggests an evolutionarily conserved and higher order function of β-catenin at AJ than as a component of the Wnt signaling pathway. This can be concluded because γ-catenin is adapted to fulfill a more vital function at AJ upon β-catenin loss and this redundancy is indispensable to survival, since DKO mice show severe morbidity and mortality. γ-Catenin increase that is evident in β-catenin KO is not compensating for rescue of Wnt signaling seen by continued lack of its transcriptional targets. Our study also highlights function of AJ as master regulators of TJ structure and eventually barrier function. Anomalies in the expression of key junctional proteins may thus be an important mechanism of cholemia and CLD.
REFERENCES
Author names in bold designate shared co-first authorship.