Transintestinal cholesterol excretion: A secondary, nonbiliary pathway contributing to reverse cholesterol transport
Potential conflict of interest: Nothing to report.
Cholesterol is an important component of cellular membranes. However, when excess cholesterol is accumulated within the cell, it becomes cytotoxic. Because almost all cells in the major tissues need a continuous supply of cholesterol, a complex series of biosynthetic, transport, and regulatory mechanisms have evolved in the body. Cholesterol can be obtained from the intestinal absorption derived from diet and bile as well as the de novo biosynthesis from acetyl coenzyme A within the body. Because human cells do not possess enzymes to degrade the ring structure of cholesterol, this sterol cannot be metabolized to CO2 and water. Thus, excess cholesterol must be excreted to prevent a potentially hazardous accumulation in the body. To achieve this task, cholesterol is usually excreted from the body either as an unaltered molecule or after conversion to other sterol products such as bile acids and steroid hormones. Consequently, there is little net accumulation of cholesterol in the body, and yet sufficient cholesterol is always available to meet the metabolic needs of the various tissues.
Classically, the reverse cholesterol transport (RCT) encompasses the transporting of excess cholesterol accumulating within peripheral tissues back to the liver for biliary excretion into the feces (Fig. 1). In the late 1950s, a novel nonbiliary transport route for RCT was proposed,1 challenging this classic view of RCT by demonstrating that the intestine also significantly contributes to mass fecal neutral sterol (FNS) excretion, independently of biliary cholesterol excretion. However, these studies were performed under conditions of severe hepatobiliary disease and inappropriate experimental approaches. Using different mouse models with new experimental methods, many findings have shown that direct transintestinal excretion of plasma-derived cholesterol may contribute to RCT in mice,2, 3 which may account for ∼30% of total FNS excretion under basal conditions and could be modulated by liver X receptor, peroxisome proliferator–activated receptor-delta, and farnesoid X receptor.4 This pathway might represent a novel therapeutic target to increase RCT and thereby confer protection against cardiovascular disease.5 Although in vitro evidence for the activity of this pathway has been reported in explants from human small intestine mounted in Ussing chambers,6 the existence and importance of transintestinal cholesterol excretion (TICE) in humans remains elusive.
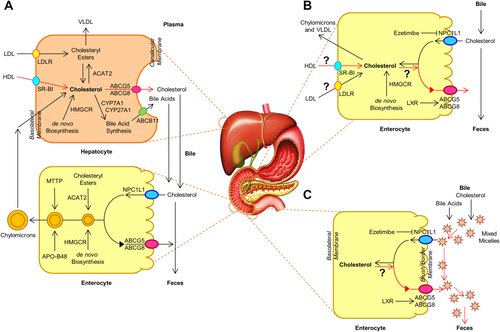
A recent publication in Cell Metabolism investigated the contribution of TICE to total FNS excretion in humans.7 Using a cholesterol balance approach with combined stable isotopes that label cholesterol and bile acid molecules, the authors quantified the body cholesterol fluxes in 15 men with mild hypercholesterolemia. Subsequently, 10 of these volunteers on a regular diet repeated the protocol after ezetimibe (10 mg/day) administration for 4 weeks (i.e., selectively blocking the intestinal Niemann-Pick C1-Like 1 [NPC1L1] cholesterol influx transporter). They found that under basal conditions approximately 65% of daily FNS excretion was derived from biliary secretion, with the remainder (∼35%) being contributed likely by TICE. Moreover, ezetimibe treatment led to a 4-fold increase in total FNS excretion, possibly through TICE. Studying chow-fed adenosine triphosphate–binding cassette transporter G8 (Abcg8) knockout versus wild-type mice treated with ezetimibe at 0 or 8 mg/kg/day for 2 weeks, they observed that most of the ezetimibe-induced TICE flux is determined likely by the intestinal sterol efflux transporters Abcg5/Abcg8. These results implied that TICE does exist in humans and that most of ezetimibe-induced TICE flux may be regulated by Abcg5/Abcg8. Therefore, TICE may be a novel target to promote the removal of excess cholesterol from the body in patients at risk for cardiovascular disease.
However, for ethical reasons, daily biliary cholesterol and bile acid secretion rates were not determined in humans; and for technical reasons, only 30-minute hepatic bile samples were collected through a cannulation in the gallbladder to measure daily biliary cholesterol and bile acid secretion rates in mice. Such indirect measurements and calculations may not lead to a precise analysis of daily total FNS excretion by the cholesterol balance method. Because ezetimibe is a potent cholesterol absorption inhibitor, it dramatically deters intestinal absorption of both dietary and biliary cholesterol by suppressing NPC1L1 expression, greatly increasing the total mass of FNS excretion in humans and mice. The efficiency of cholesterol absorption could be determined by the net effect between influx and efflux of intraluminal cholesterol molecules crossing the brush border membrane of enterocytes (Fig. 1).8 Obviously, some of the absorbed cholesterol from the diet and bile and the cholesterol taken up from plasma through TICE are exported by ABCG5/G8 from the inside of enterocytes to the intestinal lumen for fecal excretion. However, the results reported by Jakulj et al.7 cannot discriminate between the absorbed cholesterol molecules and those taken up from plasma. Thus, no direct evidence was provided to qualify the contribution of ABCG5/G8 to the fraction of total FNS excretion through TICE. Nevertheless, their results support the potential existence of TICE in humans. However, TICE's importance relative to the biliary pathway for RCT is awaiting further investigation with new experimental techniques.
TICE is now recognized as a significant alternative route to the biliary pathway of RCT; however, the molecular and cellular mechanisms underlying the role of TICE in the regulation of RCT remain unclear. It has not been established how the cholesterol molecules are transported from extrahepatic tissues to the intestine for TICE and how the cholesterol is transferred within the enterocytes from the basolateral membrane to the apical membrane for its efflux to the intestinal lumen mediated by ABCG5/G8. Notably, the physical forms of cholesterol molecules can greatly influence intestinal absorption efficiency. The contribution of de novo cholesterol biosynthesis in the small intestine to TICE should be assessed because the small intestine is the second largest organ for cholesterol biosynthesis in the body. It is likely that part of the newly synthesized cholesterol is used for the assembly of very low-density lipoprotein during the period between meals and that other parts may be exported to the intestinal lumen by ABCG5/G8 through TICE. The physical forms of cholesterol in the intestinal lumen during the postprandial period and under fasting conditions are still the focus of particular interest. In the intestinal lumen, it remains unclear what is the acceptor to hold these cholesterol molecules exported from the inside of enterocytes by ABCG5/G8 because cholesterol is highly insoluble in water. It is likely that the intestinal bile acids may be a potential candidate for the acceptor and carrier (Fig. 1). First, bile acids form mixed micelles that function as a concentrated reservoir and transport vehicle for cholesterol molecules across the unstirred water layer and the surface mucous coat toward the brush border of enterocytes to facilitate uptake of monomeric cholesterol by the enterocytes.8 These mixed micelles may accept the cholesterol molecules exported by ABCG5/G8 and move them back from the brush border of enterocytes to the bulk phase of the intestinal contents because bile acids are less absorbed in the proximate small intestine. Although the biliary elimination of cholesterol is the single most important determinant of how the body regulates and disposes of excess cholesterol, biliary cholesterol is often reabsorbed efficiently by NPC1L1 and subsequently exported by ABCG5/G8. It is a technical challenge to distinguish the cholesterol molecules taken up from plasma and those absorbed from the bile and diet. Thus, to precisely quantify the body cholesterol fluxes derived from TICE strongly requires the development of new methods to discriminate between them. We sincerely look forward to reading new reports from the Groen lab and other research groups, elucidating the importance of each of the biliary and nonbiliary (TICE) pathways in the molecular regulation of RCT and in the prevention and treatment of cardiovascular disease.
-
David Q.-H. Wang, M.D., Ph.D.1
-
Piero Portincasa, M.D., Ph.D.2
-
Patrick Tso, Ph.D.3
-
1Department of Medicine Division of Gastroenterology and Liver Diseases
-
Marion Bessin Liver Research Center
-
Albert Einstein College of Medicine
-
Bronx, NY
-
2Department of Biomedical Sciences and Human Oncology
-
Clinica Medica “A. Murri”
-
University of Bari “Aldo Moro” Medical School
-
Bari, Italy
-
3Department of Pathology and Laboratory Medicine
-
University of Cincinnati College of Medicine
-
Cincinnati, OH
REFERENCES
Author names in bold designate shared co-first authorship.




