Statins in cirrhosis—Ready for prime time
Potential conflict of interest: Dr. Bosch consults, advises, and received grants from Conatus. He consults and received grants from Gilead. He advises, owns stock, and holds intellectual property rights with BLB. He consults for Exalenz.
See Article on Page 896
Abbreviations
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- RCT
-
- randomized clinical trial
Statins are inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase with lipid-lowering properties that are routinely prescribed for primary and secondary prevention of cardiovascular events.1 In recent years, experimental and observational studies have demonstrated that statins have pleiotropic effects over and above their antilipid mechanism of action and have been proposed as part of potential preventative strategies for decompensation in cirrhosis.1
There is a strong experimental rationale for such strategies. From a mechanistic perspective, simvastatin increases nitric oxide availability in the cirrhotic liver circulation by enhancing the expression and activity of endothelial nitric oxide synthase and, therefore, ameliorates portal hypertension but also prevents endothelial dysfunction during endotoxemia.2 An anti-inflammatory effect is achieved through a decreased production of inflammatory cytokines and leukocyte migration to the subendothelial space. Importantly, mainly mediated by up-regulation of the nuclear receptor KLF2, statins have antifibrotic effects due to inhibition of hepatic stellate cell activation by paracrine interaction with endothelial sinusoidal cells.3
In their article in Hepatology, Chang and coauthors used the Taiwan National Health Insurance database in a nested case–control study to estimate the effect of statins on the risk of decompensation, mortality, and hepatocellular carcinoma (HCC) in patients with cirrhosis. Index cases of cirrhosis were identified from a representative sample of 1,000,000 people who were followed from 2000 to 2013.4 The authors used propensity score matching and finally selected 675 patients with cirrhosis in each of the statin user and non–statin user group from a potential size of 1,172 statin users. Hepatitis C virus (HCV), hepatitis B virus, and alcohol were the included etiologies of cirrhosis. Statin users were defined as patients with cirrhosis with more than 28 cumulative defined daily doses of any statin. Statin users and nonusers were well matched in baseline characteristics including the Charlson Comorbidity Index. Use of statins was associated with a significantly lower risk of decompensation (hazard ratio [HR], 0.39), mortality (HR, 0.46), and HCC development (HR, 0.52) in a dose–response relationship in the overall population with cirrhosis, with users with a cumulative defined daily dose >365 days having the greatest benefit. When analyzed according to the etiology of cirrhosis, statin use was associated with a reduced risk of decompensation in hepatitis B virus and HCV and a trend for lower risk of decompensation in alcoholic liver disease but no change in the risk of mortality or HCC in independent etiologies (likely due to a lower number of patients/events in this subanalysis).
An advantage of this study compared to other population studies is that it derives from a well-validated general population database, thus significantly reducing the risk of selection bias. There are inevitable weaknesses as with other retrospective analyses, which include the lack of patients with nonalcoholic steatohepatitis cirrhosis, the absence of laboratory parameters (with ensuing inability to calculate and correct for the Model for End-Stage Liver Disease and Child-Pugh scores), a potential lead-time bias for the incident HCC cases and the low threshold (cumulative defined daily dose >28) to classify a patient as a statin user. Nevertheless, the propensity score matching was robust, and the presence of nonbleeding varices at baseline was added in the model to account for the presence of clinically significant portal hypertension. The dilution of the statin effect in individual etiologies of cirrhosis is most likely due to type II error rather than selective effectiveness in viral hepatitis over alcohol-induced cirrhosis.
This study adds further observational evidence to the potential beneficial effects of statins in patients with cirrhosis. In large cohort studies, statin use was protective against significant fibrosis in patients with nonalcoholic fatty liver disease5 and associated with an approximately 50% lower risk of progression to cirrhosis in patients with HCV6 and hepatitis B virus.7 In a cohort of 40,512 patients with compensated HCV cirrhosis, statin use was associated with a 40% lower risk of decompensation and death.8 Statins have also been associated with reduced risk for the development of HCC in various liver disease etiologies.1 Importantly, these studies suggest that such effects are class effects rather than related to a particular statin and apply to all noncholestatic etiologies of cirrhosis. However, though promising, these studies need confirmation by prospective randomized clinical trials (RCTs).
There have been two proof-of-concept prospective RCTs on the use of statins for cirrhosis so far. In an RCT of 59 patients, simvastatin given for 1 month significantly reduced portal pressure by an average of 8.3% and was associated with a marked improvement in liver indocyanine-green clearance, indicating a potential for improved liver function.9 Importantly, the effect on portal pressure was over and above that of nonselective beta-blockers. In a further RCT in 158 patients with decompensated cirrhosis due to previous variceal bleeding, addition of simvastatin to standard of care was independently associated with a survival benefit (HR, 0.55) in those patients with Child-Pugh A and B cirrhosis.10 The study is an important guidance to further study design; although the primary composite endpoint of reduction of rebleeding or death was not achieved, simvastatin had a significant effect on all-cause mortality, mostly due to less bleeding or infection-related deaths, further reinforcing the pleiotropic statin effect.10 This is in keeping with data from preclinical cirrhotic models showing that statins protect from liver failure secondary to sepsis and hypovolemic shock.2 In the BLEPS study, statins were not beneficial in patients with Child-Pugh C cirrhosis.10 Moreover, there were two cases of statin-induced rhabdomyolysis among Child-Pugh C patients compared to no cases in Child-Pugh A or B, thus raising efficacy and safety concerns in patients with more advanced liver disease. Figure 1 shows the potential beneficial effects of statins in the evolution of cirrhosis and combines the presented data.
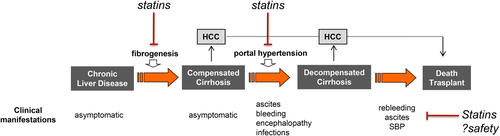
The above evidence justifies a phase 3 RCT of statins in patients with cirrhosis. Such a trial should only include compensated patients with Child-Pugh A (and potentially Child-Pugh B7), with the primary endpoint being a composite outcome of decompensation or death. Given the fact that most statins are inexpensive and generic drugs, public funding would be required. In an era of increasingly expensive medications with profound impact on health care budgets, exploring the repurposing of statins for cirrhosis is too good of an opportunity to be missed.
-
Emmanuel A. Tsochatzis, M.D., Ph.D.1
-
Jaime Bosch, M.D., Ph.D.2,3
-
1UCL Institute for Liver and Digestive Health and Sheila Sherlock Liver Unit, Royal Free Hospital and University College London, London, UK
-
2Hepatic Hemodynamic Laboratory, Hospital Clínic-IDIBAPS and CIBERehd, University of Barcelona, Barcelona, Spain
-
3Swiss Liver Center, Inselspital, Bern University, Bern, Switzerland




