Autoreactive monoclonal antibodies from patients with primary biliary cholangitis recognize environmental xenobiotics
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health (R21AI115120 and DK067003).
Abstract
A major problem in autoimmunity has been identification of the earliest events that lead to breach of tolerance. Although there have been major advances in dissecting effector pathways and the multilineage immune responses to mitochondrial self-antigens in primary biliary cholangitis, the critical links between environmental factors and tolerance remain elusive. We hypothesized that environmental xenobiotic modification of the E2 subunit of the pyruvate dehydrogenase (PDC-E2) inner lipoyl domain can lead to loss of tolerance to genetically susceptible hosts. Previously we demonstrated that serum anti-PDC-E2 autoantibodies cross-react with the chemical xenobiotics 2-octynoic acid and 6,8-bis (acetylthio) octanoic acid and further that there is a high frequency of PDC-E2-specific peripheral plasmablasts. Herein we generated 104 recombinant monoclonal antibodies (mAbs) based on paired heavy-chain and light-chain variable regions of individual plasmablasts derived from primary biliary cholangitis patients. We identified 32 mAbs reactive with native PDC-E2, including 20 specific for PDC-E2 and 12 cross-reactive with both PDC-E2 and 2-octynoic acid and 6,8-bis (acetylthio) octanoic acid. A lower frequency of replacement somatic hypermutations, indicating a lower level of affinity maturation, was observed in the complementarity-determining regions of the cross-reactive mAbs in comparison to mAbs exclusively recognizing PDC-E2 or those for irrelevant antigens. In particular, when the highly mutated heavy-chain gene of a cross-reactive mAb was reverted to the germline sequence, the PDC-E2 reactivity was reduced dramatically, whereas the xenobiotic reactivity was retained. Importantly, cross-reactive mAbs also recognized lipoic acid, a mitochondrial fatty acid that is covalently bound to PDC-E2. Conclusion: Our data reflect that chemically modified lipoic acid or lipoic acid itself, through molecular mimicry, is the initial target that leads to the development of primary biliary cholangitis. (Hepatology 2017;66:885–895)
Abbreviations
-
- AA
-
- amino acid
-
- AMA
-
- antimitochondrial antibody
-
- BSA
-
- bovine serum albumin
-
- CD
-
- cluster of differentiation
-
- cDNA
-
- complementary DNA
-
- CDR
-
- complementarity-determining region
-
- Ig
-
- immunoglobulin
-
- IGHV
-
- Ig heavy-chain variable region
-
- IGKV
-
- Ig kappa-chain variable region
-
- IGLV
-
- Ig lambda-chain variable region
-
- LA
-
- lipoic acid
-
- mAb
-
- monoclonal antibody
-
- 2-OA
-
- 2-octynoic acid
-
- PBC
-
- primary biliary cholangitis
-
- PDC-E2
-
- E2 subunit of pyruvate dehydrogenase
-
- RSA
-
- rabbit serum albumin
-
- SAc
-
- 6,8-bis (acetylthio) octanoic acid
-
- SHM
-
- somatic hypermutation
Primary biliary cholangitis (PBC) is characterized by the presence of antimitochondrial antibodies (AMAs), a serological hallmark, in >95% of patients.1 Although extremely useful as a diagnostic marker, AMAs are not helpful for patient follow-up as their titer does not correlate with disease progression.2, 3 However AMAs can be detected long before the onset of symptoms. This unique feature suggests that data on the origin of AMA will help dissect the etiology of PBC.4-6 The major mitochondrial autoantigen in PBC is the E2 subunit of pyruvate dehydrogenase (PDC-E2).7, 8 Other autoantigens recognized by AMAs are the E2 subunits of enzyme complexes belonging to the 2-oxo-acid dehydrogenase family, including the branched-chain oxo-acid dehydrogenase, the oxo-acid dehydrogenase complexes, the E3 binding protein, and the E1 subunit of the pyruvate dehydrogenase complex (PDC-E1a).7-10 All the epitopes of these autoantigens are localized within their respective lipoyl domains.
We have hypothesized that environmental xenobiotic modification of the PDC-E2 inner lipoyl domain can lead to loss of tolerance to PDC-E2 and have demonstrated the presence of anti-PDC-E2 autoantibodies that are cross-reactive to chemical xenobiotics including 2-octynoic acid (2-OA), a material commonly found in perfumes and cosmetics, and 6,8-bis (acetylthio) octanoic acid (SAc), a metabolite of acetaminophen that is structurally similar to lipoic acid (LA).11 More recently, we demonstrated a high frequency of PDC-E2-specific plasmablasts in the periphery of PBC, suggesting ongoing activation of PDC-E2-specific B cells during the disease course of PBC.12 Herein, we have investigated the origin and evolution of anti-PDC-E2 antibodies by analyzing the immunoglobulin (Ig) gene sequences of circulating plasmablasts and the reactivity of recombinant monoclonal antibodies (mAbs) generated from Ig gene sequences.
Patients and Methods
STUDY SUBJECTS AND BLOOD SAMPLES
In this labor-intensive analysis, 3 AMA-positive PBC patients, all stage I or II and diagnosed using established criteria, were enrolled. All participants provided written informed consent, and the study protocol was approved by the institutional review board at the University of California, Davis, prior to initiation of study. Blood samples were collected using acid-citrate-dextrose as the anticoagulant. B cells were isolated by negative selection with RosetteSep Human B cell Enrichment cocktail (Stemcell Technologies, Vancouver, BC, Canada); purity was 70%-80%.
ISOLATION OF SINGLE PLASMABLASTS BY FLUORESCENCE-ACTIVATED CELL SORTING
Freshly isolated B cells were treated with human Fc receptor binding inhibitor (eBioscience, San Diego, CA) to block the Fc receptor prior to staining. Cells were then stained with fluorescein isothiocyanate–conjugated anti–cluster of differentiation 27 (anti-CD27), phycoerythrin-conjugated anti-CD19, phycoerythrin-Cy7-conjugated anti-CD20, BV421-conjugated anti-CD38, and allophycocyanin-conjugated anti-CD3 (Biolegend, San Diego, CA). The LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies, Carlsbad, CA) was used to determine the viability of cells. Single plasmablasts (CD3–CD19+CD20lo/– CD27hiCD38hi) were sorted into 96-well plates with 4 μL of cell lysis buffer containing 10 mM dithiothreitol (Invitrogen, Carlsbad, CA) and ribonuclease inhibitor (Eppendorf) in each well.13 Cell sorting was performed using a BD Influx cell sorter.
SINGLE-CELL RT-PCR AND Ig GENE AMPLIFICATION
Complementary DNA (cDNA) was synthesized as described.13 Briefly, the iScript cDNA Synthesis Kit (Bio-Rad) was used to synthesize cDNA in a total volume of 20 µL/well in the original 96-well sorting plates. Variable region gene transcripts of IgH, Igκ, and Igλ were then amplified independently by nested PCR.13 The primer sequences for PCR amplification and cycling conditions have been described.13, 14
Ig GENE SEQUENCE ANALYSIS
Purified PCR products were sequenced at Quintara Biosciences. The resultant transcript sequences were analyzed by the National Center for Biotechnology Information's IgBLAST comparison with GenBank to determine the functional sequences and to identify the germline V(D)J gene segments with the highest identity. The IgG, IgM, or IgA isotype of each sorted plasmablast was determined by isotype gene-specific PCR during library preparation for sequencing.
CONSTRUCTION OF Ig EXPRESSION VECTORS
PCR products were digested with respective restriction enzymes to isolate the gene segments encoding the Ig heavy-chain or light-chain variable regions, which were then cloned into corresponding vectors containing a murine Ig signal peptide sequence (GenBank accession no. DQ407610) and a multiple cloning site upstream of the human Igγ1, Igκ, or Igλ constant regions, respectively, as described.14
PRODUCTION OF RECOMBINANT mAbs
Ig expression vectors were transfected into HEK293 cells using the X-tremeGENE 9 DNA transfection reagent (Roche, Mannheim, Germany) or JetPRIME transfection reagent (Polyplus Transfection, NY). Cells were seeded in six-well plates (2 × 106 cells/well) or 75-cm2 flasks (6 × 106 cells/flask) in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, then incubated overnight at 37oC with 5% CO2 to allow attachment. Unattached cells were removed, and culture medium was replaced with serum-free Dulbecco's modified Eagle's medium. Transfection reagent was mixed with IgH or IgL (either Igκ or Igλ) expression vectors and incubated for 20 minutes at room temperature. The mixture was then added to cultured cells and incubated for 24 hours. The transfection culture medium was then changed to high-glucose Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. The supernatant with secreted recombinant mAbs was harvested at days 6 and 12 posttransfection, and the mAbs were concentrated with Amicon Ultra-4 Centrifugal Filters or purified with HiTrap Protein G HP (GE Healthcare Life Sciences, NJ). The concentration of each mAb was measured with human IgG enzyme-linked immunosorbent assay quantitation sets (Bethyl Laboratories, Inc., TX).
IMMUNOBLOTTING
Immunoblotting was performed with a sodium dodecyl sulfate-polyacrylamide gel electrophoresis system. Briefly, 10 μg of each protein to be tested, including recombinant PDC-E2, bovine serum albumin (BSA), rabbit serum albumin (RSA), 2OA-BSA, SAc-BSA, LA-BSA, and LA-RSA, was electrophoresed on 4%-12% NuPAGE Novex Bis-Tris gels with NuPAGE 3-(N-morpholino)propane sulfonic acid sodium dodecyl sulfate running buffer, then transferred to a nitrocellulose membrane. Membranes were blocked in phosphate-buffered saline plus Tween 20 containing 3% of skim milk for 2 hours at 4°C, then incubated with either concentrated or purified recombinant mAbs for 1 hour. A pool of plasma from PBC patients, at various dilutions (1:200,000 for PDC-E2 and 1:3,000 for 2OA-BSA or SAc-BSA), was used as a positive control. The membranes were then incubated with horseradish peroxidase–labeled antihuman IgG (H+L; 1:3,000) and developed with SuperSignal Chemiluminescent Substrates (ThermoFisher Scientific, Rockford, IL).
STATISTICAL ANALYSIS
Data were analyzed using one-way analysis of variance. P ≤ 0.05 was considered statistically significant.
Results
Ig GENE USAGE AND ISOTYPE DISTRIBUTION OF CIRCULATING PLASMABLASTS IN PBC PATIENTS
The Ig genes of 1,680 sorted single plasmablasts from 3 PBC subjects (S1, S2, and S3) were individually sequenced and analyzed for their antibody variable regions (Supporting Fig. S1). After excluding nonfunctional transcripts including those with out-of-frame sequences or stop codons in the reading frame, a total of 922 variable regions of Ig heavy-chain (IGHV) and 790 Ig kappa-chain (IGKV) or lambda-chain (IGLV) transcripts were recovered, resulting in 568 variable heavy:light pairs derived from the same cell. Out of these 568 pairs, IGHV3 family members, in particular IGHV3-23, IGHV3-30, and IGHV3-7, were the most frequent IGHV genes, followed by IGHV4-39 and IGHV1-18 (Fig. 1A). In Vκ gene usage, IGKV3-20 and IGKV3-15 were the most frequent IGKV3s, followed by IGKV4-1 and IGKV1-39. In Vλ gene usage, the IGLV2 family, in particular IGLV2-14, was the most represented, followed by IGLV1 (Fig. 1B). Twenty heavy-chain and light-chain gene combinations were used by 3 subjects (data not known), suggesting a diverse Ig repertoire of plasmablasts in PBC patients. Regarding the isotype distribution of the circulating plasmablast repertoire, IgA isotype (55.6 ± 5.6%) predominated in all 3 subjects, followed by IgM (20.6 ± 8.5%) and IgG (22.8 ± 13.9%) (Fig. 1C).
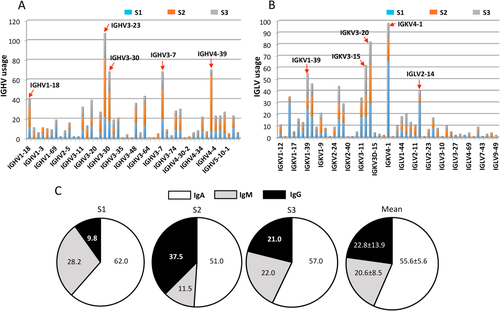
Single-cell analysis of antibody repertoire and isotype distribution. Antibody repertoire of plasmablasts. (A) There were 992 functional IGHV transcripts identified, with IGHV3-23 transcripts being the most frequent. Red arrows indicate the frequently used IGHV families. (B) There were 790 functional IGLV transcripts identified, with the IGKV3-20 and IGKV4-1 transcripts being the most frequent. Red arrows indicate the frequently used IGLV families. (C) Antibody isotype distribution. The 568 functional and paired variable heavy:light antibody repertoire was analyzed. The variation in the frequency of IgA, IgM, and IgG among the 3 PBC patients is presented in pie charts.
IDENTIFICATION OF PDC-E2-REACTIVE AND XENOBIOTIC-REACTIVE mAbs
We randomly selected 104 paired variable heavy:light cDNAs derived from single plasmablasts to construct human Igγ1, Igκ, or Igλ expression vectors for expression of recombinant mAbs. The reactivity of each mAb against PDC-E2 and xenobiotics was examined by immunoblotting. Among these 104 single mAbs, 32 (30.8%) reacted to PDC-E2, including 20 that reacted to PDC-E2 only and 12 that cross-reacted with PDC-E2 and xenobiotics (Fig. 2A). No mAbs that recognize xenobiotics but not PDC-E2 were identified. Indirect immunofluorescence staining of HEp-2 cells using a representative PDC-E2 reactive mAb showed a cytoplasmic staining pattern as expected for AMAs (Supporting Fig. S2). For the cross-reactive mAbs, we further confirm that their binding target was xenobiotics per se rather than the BSA conjugates, using unconjugated BSA or RSA and BSA conjugated with a biologically and structurally irrelevant molecule, methacrylic acid, as the testing antigen (Supporting Fig. S3). Finally, we analyzed the original isotype distribution of the expressed plasmablast variable heavy:light pairs in each reactivity group. Among the total of 104 plasmablasts, 63 were IgA, 22 were IgM, and 19 were IgG plasmablasts. Out of the 20 PDC-E2-specific mAbs, 15 (75%) were derived from IgA plasmablasts, which was higher than the IgA frequency in the PDC-E2/xenobiotic–cross-reactive groups (7 of 12, 58.3%) and in the PDC-E2-negative group (41 of 72, 56.9%). In contrast, IgG appears to be underrepresented in the PDC-E2-specific and cross-reactive groups as only one IgG mAb was identified in each of these groups (Fig. 2B). The frequency of IgM in PDC-E2-specific group (4 of 20, 20%) mAbs is similar to the frequency of IgM plasmablasts in the PDC-E2-negative group (14 of 72, 19.4%) (Fig. 2B).
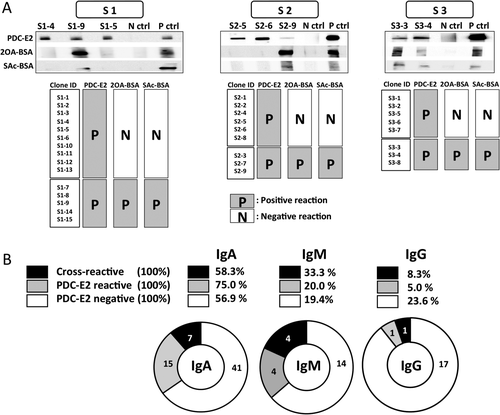
Antigen specificity and cross-reactivity of mAbs against PDC-E2, 2OA-BSA, and SAc-BSA. (A) Immunoblot assay was used to identify antigen specificity and cross-reactivity. mAbs cross-reacting with PDC-E2, 2OA-BSA, and SAc-BSA were detected in all 3 subjects (S1, S2 and S3). Culture media only were served as negative controls; PBC patient sera diluted from 1:1,000 to 1:200,000 served as positive controls. (B) The frequencies of PDC-E2-negative, PDC-E2-reactive, and PDC-E2/xenobiotic–cross-reactive clones. Antibody isotype distribution was examined from 104 randomly selected clones and screened for antigen specificity and cross-reactivity against PDC-E2, 2OA-BSA, and SAc-BSA (IgA, n = 63; IgM, n = 22; IgG, n = 19). Numbers in pie charts reflect the number of PDC-E2-negative, PDC-E2-reactive, and PDC-E2/xenobiotic–cross-reactive clones, respectively. Abbreviations: N ctrl, negative control; P ctrl, positive control.
SOMATIC HYPERMUTATION LOAD IN THE COMPLEMENTARITY-DETERMINING REGION WAS SIGNIFICANTLY LOWER IN CROSS-REACTIVE ANTIBODIES
Introduction of somatic hypermutation (SHM) shapes the Ig repertoire diversity and increases the affinity of antibodies for their cognate antigens during antibody responses. We compared the SHM loads in the framework and complementarity-determining region (CDR) of IGHV (Fig. 3A) and IGLV (Fig. 3B) genes among PDC-E2/xenobiotic–cross-reactive, PDC-E2-specific, and PDC-E2-negative antibodies. The average number of all mutations in the entire variable region of heavy chain and light chain was significantly smaller in PDC-E2/xenobiotic–cross-reactive antibodies compared to PDC-E2-negative antibodies (Fig. 3A). Importantly, a significantly lower rate of replacement mutation was observed in the entire variable region of heavy chain and the CDR of both heavy and light chains used by PDC-E2/xenobiotic–cross-reactive antibodies compared to PDC-E2-specific or PDC-E2-negative antibodies, whereas such a difference was not observed for the silent mutations (Fig. 3A,B). Taken together, these results indicate that on average the mAbs recognizing PDC-E2 exclusively or those recognizing irrelevant antigens have adopted more amino acid (AA) changes from their germline sequences in comparison to the cross-reactive mAbs.
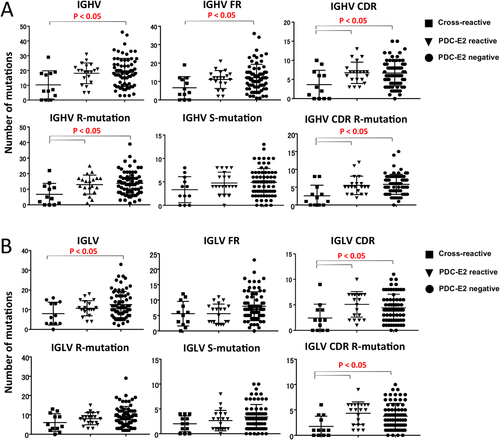
Comparison of number of mutations in IGHV regions and IGLV regions in PDC-E2/xenobiotic–cross-reactive, PDC-E2-reactive, and PDC-E2-negative mAbs. (A) Comparison of the number of mutations in IGHV, framework, and CDR regions of IGHV sequences (upper panels). Comparison of the number of replacement and silent mutations in IGHV and replacement mutation in CDR region of IGHV sequences (lower panels). (B) Comparison of the number of mutations in IGLV, framework, and CDR regions of IGLV sequences (upper panels). Comparison of the number of replacement and silent mutation in IGLV and replacement mutations in the CDR region of IGLV sequences (lower panels). Abbreviations: R, replacement; S, silent.
CDR1 SEQUENCE OF THE HEAVY CHAIN IN THE IGHV4-61:IGLV2-14 FAMILY IS CRITICAL FOR PDC-E2 RECOGNITION
We explored the relationship between PDC-E2 reactivity and AA sequence of the mAbs by examining six evolutionarily closely related mAbs using the same IGHV4-61:IGLV2-14 pair (Fig. 4A). The mAbs S1-3, S1-10, S1-11, and S1-12 were PDC-E2-reactive, whereas S1-was PDC-E2-negative Three of the PDC-E2 reactive mAbs (S1-3, S1-10, and S1-11) had a G→S replacement in CDR1, whereas the other two PDC-E2-reactive mAbs (S1-12 and S1-13) did not have the G→S replacement but had a nearby I→V replacement in CDR1, indicating that the AA differences at the site of the two AA replacements (G→S and I→V) in CDR1 did not affect the reactivity to PDC-E2. The PDC-E2-negative mAb S1-16 had the same I→V replacement in CDR1 as the PDC-E2-positive mAbs S1-12 and S1-13 but an additional G→D replacement next to the I→V replacement. Based on three-dimensional structure modeling of these mAbs, this G→D replacement in the PDC-E2-negative S1-16 occurred on the surface of the heavy chain, resulting in alteration of surface structure in comparison to the other five mAbs (Fig. 4B and data not shown). To evaluate the impact of AA variability in the light chain on the mAb reactivity to PDC-E2, we generated an artificial mAb S1-13HV: S1-16LV with the heavy chain of S1-3 (PDC-E2-reactive) and the light chain of S1-16 (PDC-E2-negative) (Fig. 4C). This artificial mAb retained the reactivity to PDC-E2 (Fig. 4D). These results suggested that within this group of mAbs using the IGHV4-61:IGLV2-14 pair, the conformational structure in the CDR of the heavy chain was critical for reactivity against the autoantigen PDC-E2.
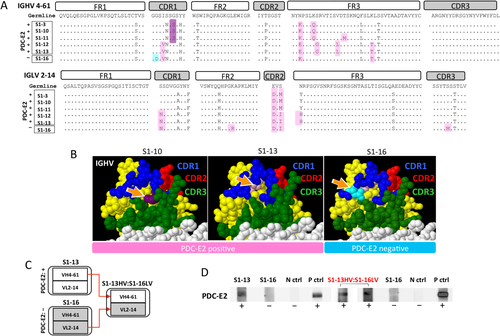
CDR1 AA sequence in the IGHV 4-61:IGLV 2-14 family is critical for PDC-E2 recognition. (A) Evolutionary AA replacements were analyzed in six evolutionarily closely related clones, which used IGHV4-61 and IGLV2-14. (B) Three-dimensional structures of PDC-E2-reactive clones (S1-3, S1-10, S1-11, S1-12, and S1-13) and a PDC-E2-negative clone (S1-16). Orange arrows indicate the sites of CDR mutations. Note that two AA replacements (G→S and I→V) in CDR1 did not affect the reactions to PDC-E2, whereas one AA replacement (G→D) located on the surface in the CDR1 region resulted in loss of PDC-E2 recognition. (C) Illustration of artificial recombinant mAb construction. The IGHV segment was cloned from PDC-E2-positive antibody S1-13, and the IGLV segment was cloned from PDC-E2-negative S1-16. (D) The AA replacements in IGHV were more critical for antigen reactivity than that in IGLV. Immunoblotting results demonstrated that PDC-E2 reactivity was comparable between the original clone S1-13 and the designer clone “S1-13 IGHV: S1-16 IGLV” (S1-13 IGHV was constructed by pairing IGLV from the PDC-E2-negative clone S1-16). Abbreviations: N ctrl, negative control; P ctrl, positive control.
GERMLINE-ENCODED AUTOANTIBODY REACTS STRONGLY TO XENOBIOTICS BUT WEAKLY TO PDC-E2
Mutation analysis demonstrated that cross-reactive mAbs have fewer mutated CDRs in their heavy and light chains in comparison to the mAbs reactive to PDC-E2 exclusively (Fig. 3). Two cross-reactive mAbs, S1-8 and S1-9, were selected for further evaluation of the effects of SHM load on the antibody cross-reactivity. These two mAbs used the same IGHV3-30 gene (Fig. 5A). S1-9 had a germline sequence–encoded heavy chain, whereas S1-8 had five replacement mutations in the heavy chain including one located in CDR3. In contrast, S1-8 used a germline sequence–encoded light chain Vκ4-1, whereas S1-9 used a Vλ 1−44 gene with one mutation. We swapped the light chains of S1-8 and S1-9 to generate two artificial mAbs, S1-8′ and S1-9′, so that both the heavy chain and the light chain of S1-9′ were encoded by germline sequences, whereas both the heavy chain and the light chain of S1-8′ were mutated (Fig. 5B). Next we compared the reactivity of these two native and two artificial mAbs to PDC-E2 and 2OA-BSA (Fig. 5C). The artificial clone S1-9′, with its heavily mutated heavy-chain gene from the native S1-9 reverted to germline sequence, exhibited a sharply reduced reactivity to PDC-E2 while retaining its reactivity to 2OA-BSA (Fig. 5C). In contrast, a comparably strong reactivity to 2OA-BSA was detected in S1-9 and S1-8′, which shared the lightly mutated Vλ 1−44 gene. Taken together, these results suggest that while both the heavy chain and the light chain are important for antigen recognition, a heavily mutated IGHV3-30 heavy chain is associated with increased antibody reactivity to the autoantigen PDC-E2, whereas the germline-encoded or lightly mutated light chains are associated with cross-reactivity to the xenobiotics.
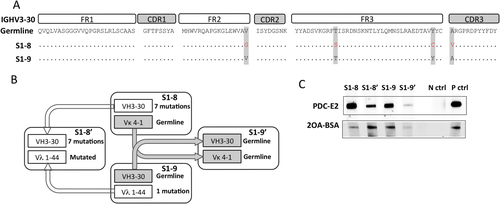
Germline-encoded clone strongly reacted to xenobiotics. (A) AA sequences of cross-reactive clones S1-8 and S1-9 aligned with IGHV 3-30 germline sequence. (B) Schematic representation of conversion of germline IGHV3-30-reverted antibody generation. (C) A side-by-side comparison of antigen reactivities against PDC-E2 and 2OA-BSA using immunoblots. Equal concentrations of mAbs were used to probe PDC-E2 and 2OA-BSA. Abbreviations: N ctrl, negative control; P ctrl, positive control.
CROSS-REACTIVE mAbs RECOGNIZE LA
Given the similarity between the structure of xenobiotic SAc and natural LA and the importance of LA in both enzymatic function and antigenicity of the PDC-E2 autoantigen, we examined the reactivity of cross-reactive mAbs to LA-conjugated RSA (LA-RSA) and unconjugated RSA. Four mAbs that were encoded by germline or lightly mutated (fewer than three total AA replacements) sequences were tested. All these mAbs recognized LA-RSA but not RSA (Fig. 6), suggesting that LA is the dominant epitope of the cross-reactive mAbs.
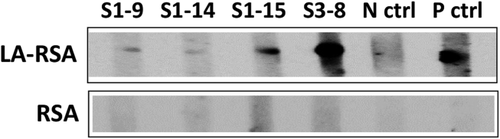
Cross-reactive mAbs recognize LA. Reactivity of PDC-E2/xenobiotic–cross-reactive clones to LA. Immunoblotting results demonstrate that PDC-E2/xenobiotic–cross-reactive mAbs react to LA-RSA but not RSA. Abbreviations: N ctrl, negative control; P ctrl, positive control.
Discussion
Epidemiological studies, clinical research, and animal model studies have pointed to a role of environmental agents in triggering autoimmunity.15-17 However, the precise contribution of these agents to disease development is unknown. Our quantitative structure–activity relationship studies have demonstrated that AMA-positive PBC sera react to a number of xenobiotic modified PDC-E2 structures, in particular those modified by SAc or 2OA.11, 18 To address this issue at the level of individual B-cell clones, we have investigated the circulating plasmablasts, precursors of mature plasma cells, which enter circulation 5-7 days after B-cell activation before they migrate into inflamed tissues and bone marrow, the major site of antibody production.12, 19-21
To date, very few studies have been conducted on the immunological repertoire in PBC.22, 23 In the current study, we focused on the circulating plasmablasts, which are derived from recently activated naive or memory B cells. We reasoned that analyses of the Ig gene sequence and antibody reactivity of B cells that were recently activated in PBC patients could reveal the footsteps of B-cell evolution at the initial stage of autoimmunity. We successfully generated 104 recombinant mAbs that were derived from single plasmablasts isolated from 3 patients with PBC. A total of 32 (30.8%) mAbs recognized PDC-E2. These included 20 (19.2%) mAbs that were PDC-E2-specific and 12 (11.5%) mAbs that were cross-reactive to PDC-E2 and xenobiotics 2OA-BSA and SAc-BSA. Analyses of the Ig gene sequences demonstrated that the signature autoantibodies of PBC, AMAs, were encoded by a diversified Ig repertoire. Of note, the cross-reactive mAbs had fewer replacement mutations in their heavy-chain and light-chain genes in comparison to the mAbs that recognize PDC-E2 but not xenobiotics, suggesting that the high-affinity autoreactive B cells evolved from low-affinity cross-reactive B cells through adoption of SHMs. Further evidence was provided by the artificial mAbs we generated, which demonstrated that reversion of highly mutated Ig gene to the germline sequence resulted in drastically reduced PDC-E2 reactivity with retained xenobiotic reactivity. In addition, we demonstrated that LA is the primary antigenic target of the cross-reactive mAbs. Taken together, our findings suggest that B-cell autoimmunity in PBC is initiated with exposure to environmental xenobiotics, which activate xenobiotic-specific naive B cells that cross-react with PDC-E2, and perpetuated with the mutation of Ig genes that increase the antibody affinity to the autoantigen. The affinity maturation of these antibodies results in highly reactive AMA, the serological hallmark of PBC.
Posttranslational modifications lead to generation of neoepitopes capable of eliciting both innate and adaptive immune responses, which may be the foundation of autoantibody recognition in autoimmune diseases.24 Upon continuous exposure to neoantigen, B-cell epitope spreading expands the immune response from modified epitopes to nonmodified epitopes and eventually to other proteins complexed with the initial antigen during the course of an autoimmune response.17, 25 A limited number of proteins are known to be lipoylated in humans, including the PBC signature mitochondrial antigen PDC-E2.26 2-OA is widely used in detergents, perfumes, and cosmetics; and SAc is a metabolite of acetaminophen that is structurally very similar to LA. 2-OA and SAc have the ability to modify the lipoyl domain of PDC-E2, resulting in the formation of neoantigens. SAc is a modified form of LA in which both sulfur atoms of the disulfide bond of the lipoyl ring are modified by acetyl groups, thereby maintaining PDC-E2 in a reduced state by preventing disulfide bond formation.18, 27-29 Our study demonstrates that a subpopulation of anti-PDC-E2 autoantibodies, encoded with fewer mutated variable region transcripts, exhibits cross-reactivity to chemical xenobiotics including 2-OA and SAc. Of importance is the finding that, in fact, these cross-reactive mAbs also recognize lipoylated PDC-E2 and LA. These data strongly support the significance of epitope spreading and molecular mimicry as triggers in autoimmunity.
IgA anti-PDC-E2 antibodies have been detected not only in serum but also in bile, saliva, and urine; and the presence of IgA anti-PDC-E2 in serum and in saliva has been associated with histological progression of PBC.30-32 Biliary epithelial cells have the capacity to export dimeric IgA to the bile through polymeric Ig receptor–mediated transcytosis.33, 34 Our data demonstrate that the majority of PDC-E2-specific and PDC-E2/xenobiotic–cross-reactive plasmablasts (75% and 58%, respectively) are IgA. If IgA AMA binds to mitochondrial proteins during transcytosis through the biliary epithelium, mitochondrial function may be consequently disrupted, which further leads to epithelial apoptosis in PBC. Our finding of the predominant presence of IgA AMA+ plasmablasts sheds light on IgA-mediated tissue damage in PBC. Moreover, our data reveal that one third of the PDC-E2/xenobiotic–cross-reactive group are IgM plasmablasts. The presence of peripheral plasmablasts indicates recent activation of naive B cells or memory B cells.20, 35 IgM plasmablasts may derive not only from newly activated naive B cells36, 37 but also from IgM memory B cells primed by PDC-E2 and xenobiotics. However, we were unable to distinguish cross-reactive plasmablasts derived from naive B cells or from IgM memory B cells because the specific markers for naive-derived plasmablasts remain unclear. IgM+ plasmablasts bear fewer mutation loads, which might derive from newly activated naive B cells. In support of our thesis, we note that a recent Ig repertoire analysis using two complementary approaches, including single cell–based next-generation sequencing and serum proteomics, revealed that naive B cell–derived circulating plasmablasts produce unmutated or low mutated disease-specific autoantibodies in patients with systemic lupus erythematosus.38 Taken together, our data obtained through single-cell analyses of activated B cells in PBC suggest that the autoreactive antibody response in PBC is initiated with exposure to environmental xenobiotics that mimic the native lipoic acid moiety on the autoantigen PDC-E2. Subsequent affinity maturation of the cross-reactive Ig genes results in a diversified high-affinity antibody repertoire, which collectively forms the signature AMA in PBC.
REFERENCES
Author names in bold designate shared co-first authorship.




