SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress
Potential conflict of interest: Nothing to report.
Supported by the National Natural Science Foundation of China–Canadian Institutes of Health Research (81261120565, to J.W.), the Program for Changjiang Scholars and Innovative Research Team in University (82000-18811100, to J.W.), the National Natural Science Foundation of China (81300705 and 81670782, to F.X.), the Pearl River S&T Nova Program of Guangzhou (201610010175, to F.X.), and the Fundamental Research Funds for the Central Universities (16ykpy12, to F.X.; 13ykpy31, to M.C.; and 15ykpy25, to W.X.).
Abstract
Recent studies have indicated that lipid-induced endoplasmic reticulum (ER) stress is a major contributor to the progression of hepatic steatosis. Exenatide (exendin-4), a glucagon-like peptide-1 receptor agonist, is known to improve hepatic steatosis, with accumulating evidence. In this study, we investigated whether exenatide could alleviate lipid-induced hepatic ER stress through mammal sirtuin 1 (SIRT1) and illustrated the detailed mechanisms. Male C57BL/6J mice challenged with a high-fat diet (HFD) were treated with exenatide or normal saline by intraperitoneal injection for 4 weeks. We observed that HFD feeding induced hepatic ER stress as indicated by increased expression of glucose-regulated protein 78, phosphorylated protein kinase-like ER kinase, and phosphorylated eukaryotic initiation factor 2α, while these increases were significantly inhibited by exenatide. Exenatide notably decreased the liver weight and hepatic steatosis induced by HFD challenge. Consistently, in human HepG2 cells and primary murine hepatocytes, exendin-4 also significantly alleviated the ER stress and lipid accumulation induced by palmitate. Importantly, further studies showed that exendin-4 enhanced the binding of heat shock factor 1 to the promoter of heat shock protein (HSP) genes through SIRT1-mediated deacetylation, which then increased the expression of molecular chaperones HSP70 and HSP40 to alleviate hepatic ER stress. Finally, inhibition of SIRT1 by genetic whole-body heterozygous knockout or by lentiviral short hairpin RNA knockdown greatly diminished the effect of exenatide on deacetylating heat shock factor 1, increasing HSP expression and alleviating ER stress and hepatic steatosis in HFD-fed mice. Conclusion: The SIRT1/heat shock factor 1/HSP pathway is essential for exenatide-alleviated, lipid-induced ER stress and hepatic steatosis, which provides evidence for a molecular mechanism to support exenatide and incretin mimetics as promising therapeutics for obesity-induced hepatic steatosis. (Hepatology 2017;66:809–824)
Abbreviations
-
- ChIP
-
- chromatin immunoprecipitation
-
- eIF2α
-
- eukaryotic initiation factor 2α
-
- ER
-
- endoplasmic reticulum
-
- GFP
-
- green fluorescence protein
-
- GLP-1
-
- glucagon-like peptide-1
-
- GRP78
-
- glucose-regulated protein 78
-
- H&E
-
- hematoxylin and eosin
-
- HFD
-
- high-fat diet
-
- HSF1
-
- heat shock factor 1
-
- HSP
-
- heat shock protein
-
- Lv
-
- lentivirus
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- p-
-
- phosphorylated
-
- PA
-
- palmitate
-
- PERK
-
- protein kinase–like ER kinase
-
- RNAi
-
- RNA interference
-
- SEM
-
- standard error of the mean
-
- shRNA
-
- short hairpin RNA
-
- SIRT1
-
- sirtuin1
-
- UPR
-
- unfolded protein response
-
- WT
-
- wild-type
Nonalcoholic fatty liver disease (NAFLD) is now recognized as the most common chronic liver disease worldwide.1, 2 It ranges from simple steatosis to more aggressive lesions including steatohepatitis, fibrosis, and cirrhosis.3 Recent studies have demonstrated that NAFLD is considered to be the hepatic component of metabolic syndrome, which is closely associated with obesity, type 2 diabetes, and dyslipidemia.4 Hepatic steatosis, characterized by excessive triglyceride accumulation in hepatocytes, is mainly associated with an overload of free fatty acids and serves as the key metabolic component of NAFLD.5 It has been shown that excess free fatty acids in the liver, especially saturated free fatty acids, could induce an endoplasmic reticulum (ER) stress response and contribute to the pathogenesis of NAFLD.6, 7 In human beings and rodent models with obesity and NAFLD, enhanced hepatic ER stress was observed, indicating that ER stress may represent a common pathophysiological mechanism involved in systemic metabolic disorders.8
ER stress, characterized by accumulation of unfolded proteins in the ER lumen due to extracellular stress signals, promotes the dissociation of chaperone glucose-regulated protein 78 (GRP78) and leads to the activation of an adaptive program called the unfolded protein response (UPR) to reestablish equilibrium in the ER. The UPR is mediated by three transducers that are transmembrane proteins of the ER: protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1α, and activating transcription factor 6. Each of these transducers activates specific pathways and collectively leads to decreased overall protein synthesis, enhanced ER folding capacity, and increased degradation of misfolded proteins, resulting in either recovery of ER homeostasis or cell death.9 During chronic and unresolved ER stress, which is characteristic of obesity and type 2 diabetes, prolonged activation of UPR plays an important role in regulating expression of hepatic lipogenic genes and contributes to the development of hepatic steatosis.10, 11 Importantly, studies have shown that activation of PERK and subsequent phosphorylation of eukaryotic initiation factor 2α (eIF2α) appear to promote lipogenesis and thus the development of hepatic steatosis.12, 13
Glucagon-like peptide-1 (GLP-1), an insulinotropic hormone released from the intestinal L cells in response to nutrient ingestion, has also been extensively shown to have extrapancreatic effects.14 Studies have shown that exendin-4 or exenatide (synthetic exendin-4), a GLP-1 receptor agonist, ameliorated hepatic steatosis both in vivo and in vitro,15, 16 and this effect was found to be dependent on sirtuin1 (SIRT1), a highly conservative protein deacetylase in mammals.17, 18 However, it has not been clearly defined whether exenatide alleviates lipid-induced hepatic ER stress and whether this alleviation is SIRT1-dependent. A study revealed that hepatic overexpression of SIRT1 attenuated ER stress and hepatic steatosis in diet-induced and genetically obese mice.19 Mechanistic studies also showed that the decreased SIRT1 expression could promote the greater acetylation of heat shock factor 1 (HSF1) and reduce its binding to the promoter of heat shock protein (HSP) genes, which then decreased the expression of molecular chaperone HSP and contributed to ER stress in neuronal cells and an experimental colitis model.20-22 Thus, these findings revealed a potential link between the SIRT1/HSF1/HSP pathway and ER stress.
This study was aimed to investigate whether exenatide (exendin-4) alleviated lipid-induced hepatic ER stress through the SIRT1/HSF1/HSP pathway. We demonstrate that exenatide (exendin-4) increases HSP expression to alleviate lipid-induced ER stress and hepatic steatosis through SIRT1-mediated HSF1 deacetylation, which provides a novel mechanism to support the notion that exenatide and incretin mimetics act as promising therapeutics for obesity-induced hepatic steatosis.
Materials and Methods
ANIMAL MODELS
Seven-week-old male C57BL/6J mice were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After a 1-week acclimatization period, mice were randomly distributed into two initial groups fed with a chow diet (4% fat [wt/wt]; Guangdong Medical Laboratory Animal Center, Guangzhou, China) or a high-fat diet (HFD) (35.8% fat [wt/wt], D12331; Research Diets, New Brunswick, NJ). After 12-week diet intervention, each group of mice was then randomly subdivided to receive either exenatide (24 nmol/kg/d; Eli Lilly and Company, Indianapolis, IN) or normal saline as control by intraperitoneal injection during the light cycle with the respective diet feeding for 4 weeks. Body weight and daily food intake were monitored every 2 weeks. Glucose tolerance tests and insulin tolerance tests were carried out at the end of intervention as described in the Supporting Information.
The whole-body Sirt1 heterozygous knockout (Sirt1+/–) mice in a C57BL/6J genetic background were from J. Ye's laboratory at the Pennington Biomedical Research Center (Louisiana State University, Baton Rouge, LA)23 and bred in the animal experimental center of our hospital. Eight-week-old male Sirt1+/– mice and their wild-type (WT) littermates were both challenged with an HFD for 12 weeks and then randomly subjected to either exenatide or normal saline for 8 weeks in the same way as described above.
A Sirt1 knockdown mouse model was constructed in C57BL/6J mice by tail vein injection of lentivirus (Lv) expressing short hairpin RNA (shRNA) targeting Sirt1, which was designed and chemically synthesized by Shanghai GeneChem Co. Ltd. (Shanghai, China). The Lv vector expressing green fluorescence protein (GFP) only was used as the RNA inference (RNAi) control. Eight-week-old male C57BL/6J mice were randomly divided into two groups fed a chow diet or an HFD for 8 weeks. The HFD-fed mice were further divided into four groups: a normal saline–treated group (HFD), an exenatide-treated group (HFD+Exe), an Lv-GFP with exenatide–treated group (HFD+Exe+GFP), and an Lv-Sirt1 RNAi with exenatide–treated group (HFD+Exe+Sirt1 RNAi). One week after the injection of Lv (5 × 107 transfection units) or control solvent, mice were subjected to either exenatide or normal saline for 2 weeks as described above.
All animals received humane care, and all study procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University. Serum sampling and tissue collection, liver histology and immunohistochemistry, assays of serum and liver lipids, as well as serum adiponectin are detailed in the Supporting Information.
CELL CULTURE AND TREATMENT
Human HepG2 hepatocytes were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells at 70%-80% confluence were incubated in serum-free medium for 4 hours before treatment. Primary murine hepatocytes were isolated from 8-week-old male C57BL/6J mice using a two-step collagenase perfusion technique as described24 and cultured on type I collagen–coated plates in Williams E medium supplemented with 2 mM L-glutamine, 100 nM dexamethasone, and antibiotics described above.
Thapsigargin and palmitate (PA) (Sigma-Aldrich, St. Louis, MO) were used as ER stress inducers. To examine the effect of exendin-4 (Sigma-Aldrich) on hepatic ER stress in vitro, hepatocytes were incubated in medium containing ER stress inducers with or without 100 nM exendin-4 for 24 hours. A SIRT1 inhibitor, EX-527 (Sigma-Aldrich), as well as two SIRT1 agonists, resveratrol (Sigma-Aldrich) and SRT1720 (Selleck, Houston, TX), were also included to investigate the role of SIRT1 in the effect of exendin-4 on PA-induced ER stress. For Sirt1 knockdown in vitro, HepG2 cells were transfected with Lv Sirt1 shRNA or scrambled shRNA at a multiplicity of infection (=10/20) according to the manufacturer's instructions. Transfected cells were then incubated in medium containing PA with or without exendin-4 for 24 hours.
IMMUNOPRECIPITATION
Total protein (500 μg) was incubated with 10 μL specific antibody against HSF1 (Millipore, Billerica, MA) overnight at 4 °C with continuous mixing. Then Protein A/G Mix Magnetic Beads (Millipore) were added, followed by incubation for 2 hours at 4 °C. After washing three times, the immunocomplexes were resuspended with denaturing elution buffer, heated at 100 °C for 5 minutes, and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by immunoblotting with primary antibody against HSF1. For acetylation western blotting, 50 mM trishydroxymethylaminomethane (pH 7.5) with 10% (vol/vol) Tween-20 and 1% peptone was used for blocking, and 50 mM trishydroxymethylaminomethane (pH 7.5) with 0.1% peptone was used to prepare primary antibody against acetylated-lysine (Cell Signaling Technology, Danvers, MA) and secondary antibody.25
CHROMATIN IMMUNOPRECIPITATION ASSAY
Chromatin immunoprecipitation (ChIP) reactions were performed with an EZ-Magna ChIP HiSens kit (Millipore) according to the manufacturer's instructions. Briefly, chromatin samples isolated from hepatocytes were sonicated, followed by incubation with 5 μL anti-HSF1 antibody (Millipore) or with control immunoglobulin G and ChIP A/G magnetic beads overnight at 4 °C with continuous mixing. Immunoprecipitated DNA and input genomic DNA were amplified with primers targeting the Hsp70.1 promoter region containing heat shock element by real-time PCR as described above. Primers used for the Hsp70.1 promoter were (forward) 5′-TGTCCCCTCCAGTGAATCCCA-3′ and (reverse) 5′-TATTCCAGGGTTTTCGCCTCC-3′. Results were normalized to reactions performed with 100% input samples.
STATISTICAL ANALYSIS
Data are presented as mean ± standard error of the mean (SEM). The unpaired two-tailed Student t test was used to determine significant differences between groups. P < 0.05 was considered statistically significant.
Additional materials and methods are described in the Supporting Information.
Results
EXENATIDE REDUCES BODY WEIGHT AND RESTORES SYSTEMIC AND HEPATIC GLUCOSE HOMEOSTASIS IN HFD-FED MICE
From the fourth week of intervention, the mice in the HFD group began to show a significant increase in body weight compared to the chow diet group, which lasted until the end of intervention (Fig. 1A). Interestingly, the HFD-induced mice subjected to 4-week exenatide treatment exhibited significant weight loss compared to the HFD group, especially in the first 2 weeks, which was accompanied by a decrease in daily food intake (Fig. 1A,B). Meanwhile, exenatide treatment showed beneficial effects on glucose tolerance and insulin sensitivity in HFD-challenged mice as indicated by glucose tolerance tests and insulin tolerance tests (Fig. 1C,D). To investigate the physiological mechanisms responsible for exenatide-restored systemic glucose homeostasis, the expression of hepatic glycometabolic genes was measured. As a result, the mRNA levels of gluconeogenic genes including Pepck and Pck1 were increased in the liver by HFD challenge, and these changes were significantly reduced by exenatide. In contrast, the expression of a glycolytic gene, Gck, which was obviously reduced by HFD feeding, was restored by exenatide treatment (Fig. 1E). These findings demonstrate that exenatide reduces body weight and restores systemic and hepatic glucose homeostasis in HFD-fed mice.
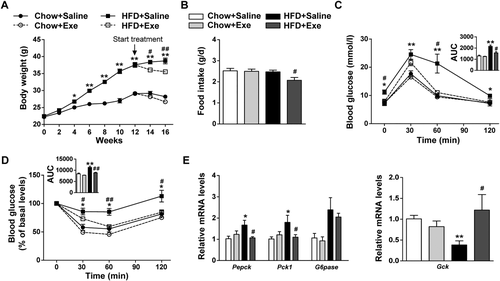
Exenatide reduces body weight and restores systemic and hepatic glucose homeostasis in HFD-fed mice. C57BL/6J mice fed a chow diet or an HFD for 12 weeks were randomly assigned to either normal saline or exenatide treatment (24 nmol/kg) by intraperitoneal injection for 4 weeks with the diet intervention maintained (n = 5-6/group). (A) Body weight detected every 2 weeks. (B) Daily food intake during exenatide treatment. (C) Glucose tolerance tests after exenatide treatment and the area under curve. (D) Insulin tolerance tests after exenatide treatment and the area under curve. (E) Relative mRNA levels of gluconeogenic genes (Pepck, G6pase, and Pck1) and a glycolytic gene (Gck) by quantitative real-time PCR. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with the chow diet group; #P < 0.05, ##P < 0.01 compared with the HFD group. Abbreviations: AUC, area under curve; Exe, exenatide.
EXENATIDE ALLEVIATES ER STRESS AND HEPATIC STEATOSIS IN HFD-FED MICE
To investigate whether exenatide alleviates lipid-induced hepatic ER stress in an HFD-fed mouse model, the protein levels of the ER stress marker GRP78 and the PERK/eIF2α arm of the UPR pathway were measured in the liver tissues. We found that HFD challenge strongly induced hepatic ER stress, as indicated by increased protein levels of GRP78, phosphorylated PERK (p-PERK) and p-eIF2α in the livers of the HFD group compared with the chow diet group. However, these increases were significantly inhibited by exenatide treatment (Fig. 2A). Similarly, both the liver weight and the ratio of liver to body weights in the HFD group were significantly decreased by exenatide (Fig. 2B). Consistently, histological analysis including hematoxylin and eosin (H&E) staining and oil red O staining revealed that exenatide greatly alleviated the lipid accumulation in the livers of HFD-fed mice, which was also indicated by the reduced liver triglyceride and cholesterol levels (Fig. 2C,D). The same trends were observed in the serum levels of triglyceride and cholesterol (Fig. 2E). Furthermore, the serum levels of adiponectin, an adipose-derived cytokine, were decreased by HFD challenge but reversed after exenatide treatment (Fig. 2E). The gene expression of two adiponectin receptors, AdipoR1 and AdipoR2, was reduced in the livers of the HFD group. However, only the expression of AdipoR2 was significantly increased by exenatide (Fig. 2F). Taken together, these results indicate that exenatide alleviates lipid-induced ER stress and hepatic steatosis in HFD-fed mice.
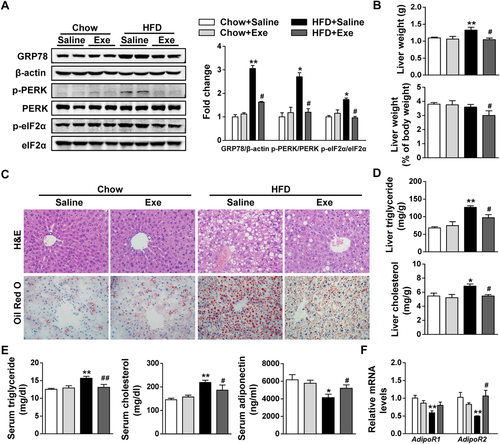
Exenatide alleviates ER stress and hepatic steatosis in HFD-fed mice. C57BL/6J mice fed a chow diet or an HFD for 12 weeks were randomly treated with either normal saline or exenatide (24 nmol/kg/day) by intraperitoneal injection for 4 weeks with the diet intervention maintained (n = 5-6/group). (A) Western blot analysis of ER stress markers (GRP78, p-PERK, PERK, p-eIF2α, and eIF2α) in liver tissues and β-actin used as a loading control. (B) Liver weight and the ratio of liver weight to body weight. (C) Representative images of the liver, H&E staining and oil red O staining with ×400 magnification. (D) Liver triglyceride and cholesterol levels. (E) Serum triglyceride, cholesterol, and adiponectin levels. (F) Relative mRNA levels of AdipoR1 and AdipoR2 by quantitative real-time PCR. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with the chow diet group; #P < 0.05, ##P < 0.01 compared with the HFD group. Abbreviation: Exe, exenatide.
EXENDIN-4 ALLEVIATES PA-INDUCED ER STRESS AND LIPID ACCUMULATION IN HEPATOCYTES
To confirm whether exendin-4 alleviates lipid-induced hepatic ER stress in vitro, human HepG2 cells and primary murine hepatocytes were introduced in our study. Thapsigargin, modifying Ca2+ concentration in the ER lumen by inhibiting Ca2+ adenosine triphosphatase, was served as a positive ER stress inducer. Here, thapsigargin exposure was observed to increase the protein levels of GRP78, p-PERK, and p-eIF2α in parallel with the lipid accumulation in HepG2 cells (Fig. 3B; Supporting Fig. S1), suggesting that ER stress could directly lead to lipid accumulation in hepatocytes. Both immunofluorescence staining and western blotting revealed that PA exposure also induced the protein levels of GRP78, p-PERK, and p-eIF2α in HepG2 cells, to a lesser extent than thapsigargin (Fig. 3A,B). Notably, the increased expression of these ER stress markers induced by thapsigargin and PA was significantly inhibited by exendin-4 treatment (Fig. 3A,B). Meanwhile, exendin-4 significantly alleviated the lipid deposition induced by thapsigargin and PA in HepG2 cells (Fig. 3C; Supporting Fig. S1). We also confirmed that exendin-4 reduced PA-induced lipid accumulation in primary murine hepatocytes (Fig. 3D). Immunofluorescence staining revealed that the enhanced positive staining of GRP78 located in the cytoplasm of primary murine hepatocytes induced by PA was substantially reduced by exendin-4 treatment (Fig. 3E). Likewise, the protein levels of GRP78 and p-eIF2α were increased by PA exposure alone and significantly decreased by exendin-4 (Fig. 3F). Collectively, these results suggest that exendin-4 alleviates PA-induced ER stress, which may contribute to its amelioration of lipid accumulation in hepatocytes.
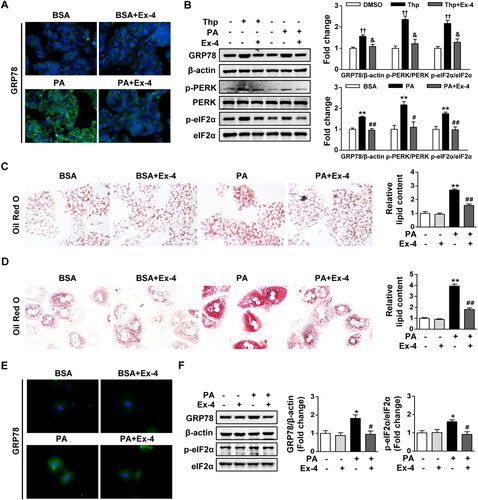
Exendin-4 alleviates PA-induced ER stress and lipid accumulation in hepatocytes. HepG2 cells and primary murine hepatocytes exposed to thapsigargin (500 nM) or PA (300 μM) were treated with or without Ex-4 (100 nM) for 24 hours. (A) Representative images of immunofluorescence staining for GRP78 protein (green fluorescence) in HepG2 cells with × 400 magnification. (B) Western blot analysis of ER stress markers (GRP78, p-PERK, PERK, p-eIF2α, and eIF2α) in lysates from HepG2 cells, with β-actin as a loading control. (C,D) Representative images of oil red O staining were captured in HepG2 cells (C) and primary murine hepatocytes (D) with × 400 magnification. Absorbance of the oil red O content was determined at 510 nm with a spectrophotometer. (E) Representative images of immunofluorescence staining for GRP78 protein (green fluorescence) in primary murine hepatocytes with ×400 magnifications. (F) Western blot analysis of ER stress markers (GRP78, p-eIF2α, and eIF2α) in lysates from primary murine hepatocytes, with β-actin as a loading control. Data are expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the BSA group; #P < 0.05, ##P < 0.01 compared with the PA group; †P < 0.05, ††P < 0.01 compared with the DMSO group; &P < 0.05, &&P < 0.01 compared with the Thp group. Abbreviations: BSA, bovine serum albumin; DMSO, dimethylsulfoxide; Ex-4, exendin-4; PA, palmitate; Thp, thapsigargin.
EXENDIN-4 ALLEVIATES PA-INDUCED HEPATIC ER STRESS IN A SIRT1-DEPENDENT MANNER
In HepG2 cells transfected with scrambled shRNA, PA exposure was observed to increase the protein levels of GRP78, p-PERK, and p-eIF2α in parallel with the reduced SIRT1 expression; and these changes were significantly reversed by exendin-4 treatment. By contrast, no obvious change in the expression of GRP78, p-PERK, and p-eIF2α was observed after exendin-4 treatment when Sirt1 was knocked down by Sirt1 shRNA (Fig. 4A). We noted that PA exposure induced a higher expression of GRP78 and p-eIF2α in HepG2 cells transfected with Sirt1 shRNA than that with scrambled shRNA (Fig. 4A). We also observed that exendin-4 significantly decreased the mRNA expression of Grp78 in PA-exposed HepG2 cells, but this was greatly blocked by pretreatment with a SIRT1 inhibitor, EX-527 (Fig. 4B). In addition, the ability of exendin-4 to restore the reduced SIRT1 expression and the increased expression of ER stress markers induced by PA exposure was close to two SIRT1 activators, resveratrol and SRT1720 (Fig. 4C,D). These data demonstrate that exendin-4 alleviates PA-induced hepatic ER stress in a SIRT1-dependent manner.
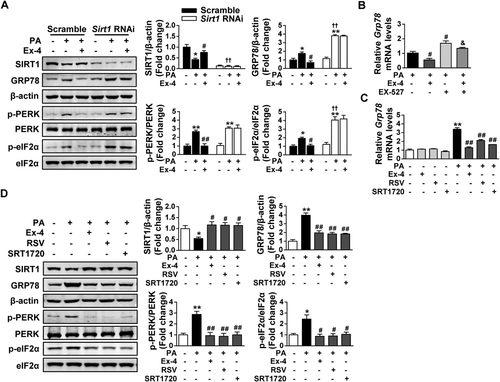
Exendin-4 alleviates PA-induced hepatic ER stress in an SIRT1-dependent manner. (A) HepG2 cells were transfected with lentiviral Sirt1 shRNA or scrambled shRNA and then incubated in medium containing PA (300 μM) with or without Ex-4 (100 nM) for 24 hours. Western blot analysis of SIRT1 and ER stress markers (GRP78, p-PERK, PERK, p-eIF2α, and eIF2α) was performed in the lysates, and β-actin was used as a loading control. Data are expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the corresponding BSA group; #P < 0.05, ##P < 0.01 compared with the corresponding PA group; †P < 0.05, ††P < 0.01 compared with the PA group transfected with scrambled shRNA. (B) HepG2 cells were pretreated with the SIRT1 inhibitor EX-527 (10 μM) for 1 hour and then incubated in medium containing PA (300 μM) with or without Ex-4 (100 nM) for 24 hours. Relative mRNA levels of Grp78 were analyzed by quantitative real-time PCR. (C,D) HepG2 cells with or without PA (300 μM) were treated with Ex-4 (100 nM) or two SIRT1 agonists, RSV (50 μM) and SRT1720 (5 μM), for 24 hours. (C) Relative mRNA levels of Grp78 by quantitative real-time PCR. (D) Western blot analysis of SIRT1 and ER stress markers (GRP78, p-PERK, PERK, p-eIF2α, and eIF2α) in lysates, with β-actin as a loading control. (B-D) Data are expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the BSA group; #P < 0.05, ##P < 0.01 compared with the PA group; &P < 0.05, &&P < 0.01 compared with the PA with Ex-4–treated group. Abbreviations: BSA, bovine serum albumin; Ex-4, exendin-4; RSV, resveratrol.
EXENDIN-4-AMELIORATED PA-INDUCED HEPATIC ER STRESS IS ASSOCIATED WITH INCREASED HSP EXPRESSION THROUGH SIRT1-MEDIATED HSF1 DEACETYLATION
HSP, a large family of molecular chaperones that regulate protein homeostasis, prevent protein aggregation and participate in refolding or elimination of misfolded proteins in their capacity.26, 27 To investigate whether HSP were involved in exendin-4-ameliorated, PA-induced hepatic ER stress, we screened the expression of the HSP family in this system. As a result, exendin-4 treatment significantly increased the protein levels of HSP70 and HSP40 in PA-induced HepG2 cells. However, this effect disappeared when Sirt1 expression was inhibited by Sirt1 shRNA (Fig. 5A). The same trend was confirmed in the mRNA levels by quantitative real-time PCR (Fig. 5B; Supporting Fig. S2). Moreover, the effect of exendin-4 on gene expression induction of HSP was greatly blocked by the pretreatment with EX-527 (Fig. 5C). By contrast, exendin-4 was observed to up-regulate the gene expression of HSP70 and HSP40 comparable to resveratrol and SRT1720 (Fig. 5C). Taken together, these findings show that exendin-4-ameliorated, PA-induced hepatic ER stress is associated with increased HSP expression in a SIRT1-dependent manner.
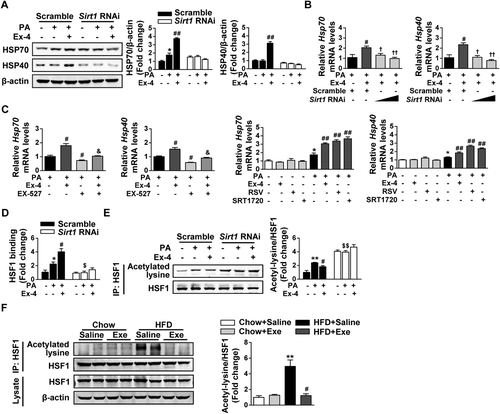
Exendin-4-ameliorated, PA-induced hepatic ER stress is associated with increased HSP expression through SIRT1-mediated HSF1 deacetylation. (A,B,D,E) HepG2 cells were transfected with lentiviral Sirt1 shRNA or scrambled shRNA and then incubated in medium containing PA (300 μM) with or without Ex-4 (100 nM) for 24 hours. (A) Western blot analysis of HSP (HSP70 and HSP40) in lysates, with β-actin as a loading control. (B) Relative mRNA levels of Hsp70 and Hsp40 by quantitative real-time PCR. (D) Relative HSF1 binding to the Hsp70 promoter by ChIP and quantitative real-time PCR. (E) Acetylated lysine and total HSF1 expression by immunoprecipitation and western blot analysis. (A,B,D,E) Data are expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the corresponding BSA group; #P < 0.05, ##P < 0.01 compared with the corresponding PA group; †P < 0.05, ††P < 0.01 compared with the PA with Ex-4–treated group transfected with scrambled shRNA; $P < 0.05, $$P < 0.01 compared with the PA group transfected with scrambled shRNA. (C) HepG2 cells were pretreated with a SIRT1 inhibitor EX-527 (10 μM) for 1 hour and then incubated in medium containing PA (300 μM) with or without Ex-4 (100 nM) for 24 hours. In addition, HepG2 cells with or without PA (300 μM) were treated with Ex-4 (100 nM) or two SIRT1 agonists, RSV (50 μM) and SRT1720 (5 μM), for 24 hours. Relative mRNA levels of Hsp70 and Hsp40 were analyzed by quantitative real-time PCR. Data are expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 compared with the BSA group; #P < 0.05, ##P < 0.01 compared with the PA group; &P < 0.05, &&P < 0.01 compared with the PA with Ex-4–treated group. (F) Acetylated lysine and total HSF1 expression in the livers of C57BL/6J mice described in Fig. 1 by immunoprecipitation and western blot analysis. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with the chow diet group; #P < 0.05, ##P < 0.01 compared with the HFD group. Abbreviations: BSA, bovine serum albumin; Ex-4, exendin-4; Exe, exenatide.
Because HSF1 is a major transcription factor regulating the expression of HSP genes,28 we performed a ChIP assay to investigate whether exendin-4 influenced the binding of HSF1 to the promoter of HSP genes in a SIRT1-dependent manner. As a result, exendin-4 significantly increased the binding of HSF1 to the Hsp70 promoter in PA-induced HepG2 cells. However, no change was observed after exendin-4 treatment in Sirt1 shRNA-transfected cells (Fig. 5D). Because SIRT1 functions as a protein deacetylase, the acetylation status of HSF1 was determined. In scrambled shRNA-transfected HepG2 cells, PA exposure increased the acetylation of HSF1, and this was significantly reversed by exendin-4 treatment. In Sirt1 shRNA-transfected HepG2 cells, the acetylation of HSF1 was significantly higher and had no change regardless of exendin-4 treatment (Fig. 5E). We also confirmed that the acetylation of HSF1 was increased in the liver after HFD challenge, while this was significantly inhibited by exenatide (Fig. 5F). Altogether, these data indicate that exendin-4-ameliorated, PA-induced hepatic ER stress is associated with increased HSP expression through SIRT1-mediated HSF1 deacetylation.
EXENATIDE-INCREASED HSP EXPRESSION TO ATTENUATE ER STRESS AND HEPATIC STEATOSIS IS DIMINISHED IN HFD-FED Sirt1+/– MICE
To validate in vitro findings in animal studies, an HFD-fed Sirt1+/– mouse model was introduced in our study. We observed decreased SIRT1 expression in combination with an exaggerated ER stress response indicated by increased protein levels of GRP78 and p-PERK in the livers of Sirt1+/– mice compared to WT controls under HFD feeding (Fig. 6A). Not surprisingly, increased SIRT1 expression in parallel with decreased protein levels of GRP78, p-PERK, and p-eIF2α were observed after exenatide treatment in HFD-fed WT mice. However, no significant change was found in HFD-fed Sirt1+/– mice regardless of exenatide treatment (Fig. 6A). In addition, exenatide notably increased the protein levels of HSP70 and HSP40 in HFD-fed WT mice, while this effect was greatly diminished in HFD-fed Sirt1+/– mice (Fig. 6B). Histological analysis including H&E staining and oil red O staining showed more severe lipid accumulation in the livers of Sirt1+/– mice compared to WT controls under HFD feeding. The hepatic lipid accumulation induced by HFD challenge was dramatically improved by exenatide in WT controls. However, this effect was greatly weakened in Sirt1+/– mice (Fig. 6C). Consistently, the same tendency was observed in the triglyceride and cholesterol contents in both the liver and serum (Fig. 6D,E). These results suggest that exenatide-increased HSP expression to attenuate ER stress and hepatic steatosis is diminished in HFD-fed Sirt1+/– mice.
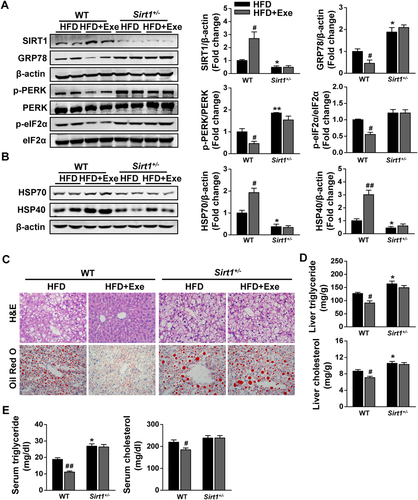
Exenatide-increased HSP expression to attenuate ER stress and hepatic steatosis is diminished in HFD-fed Sirt1+/– mice. Sirt1+/– mice and their WT controls fed with an HFD for 12 weeks were randomly treated with either normal saline or exenatide (24 nmol/kg/day) by intraperitoneal injection for 8 weeks with the diet intervention maintained (n = 5-6/group). (A) Western blot analysis of SIRT1 and ER stress markers (GRP78, p-PERK, PERK, p-eIF2α, and eIF2α) in liver tissues, with β-actin as a loading control. (B) Western blot analysis of HSP (HSP70 and HSP40) in liver tissues, with β-actin as a loading control. (C) Representative images of liver H&E staining and oil red O staining with ×400 magnification. (D) Liver triglyceride and cholesterol levels. (E) Serum triglyceride and cholesterol levels. Data are expressed as mean ± SEM. #P < 0.05, ##P < 0.01 compared with the corresponding HFD group; *P < 0.05, **P < 0.01 compared with the HFD group in WT mice. Abbreviation: Exe, exenatide.
Lv Sirt1 KNOCKDOWN ABOLISHES THE EFFECT OF EXENATIDE ON HSF1 DEACETYLATION, HSP INDUCTION, AND ALLEVIATION OF ER STRESS AND HEPATIC STEATOSIS
A Sirt1 knockdown mouse model was also used to further confirm our conclusion. As a result, neither SIRT1 and p-eIF2α nor HSP70 and HSP40 displayed any change in protein expression after Lv-GFP injection compared to the HFD with the exenatide-treated group, while Lv-Sirt1 RNAi injection dramatically decreased SIRT1 expression and abolished the effect of exenatide on increasing HSP expression and reducing p-eIF2α expression in the livers of HFD-fed mice (Fig. 7A,B). Consistent with the in vitro finding, we observed that exenatide-inhibited acetylation of HSF1 was greatly blocked by Lv-Sirt1 RNAi injection (Fig. 7C). As shown by H&E staining and oil red O staining, 2-week exenatide treatment ameliorated the hepatic steatosis induced by 8-week HFD challenge, which was consistent with the results described above. Unlike Lv-GFP injection, Lv-Sirt1 RNAi injection greatly diminished exenatide-ameliorated hepatic steatosis (Fig. 7D). Triglyceride and cholesterol quantification in the liver and serum displayed a similar tendency with the histological analysis of liver (Fig. 7E,F). By contrast, the increased serum adiponectin levels and AdipoR2 gene expression in the liver induced by exenatide treatment were not changed after Lv-Sirt1 RNAi injection (Fig. 7F; Supporting Fig. S3A). In addition, exenatide-restored hepatic glucose homeostasis in HFD-fed mice was significantly weakened by Lv-Sirt1 RNAi injection (Supporting Fig. S3B). Taken together, these findings verify again that Sirt1 knockdown abolishes the effect of exenatide on HSF1 deacetylation, HSP induction, and alleviation of ER stress and hepatic steatosis.
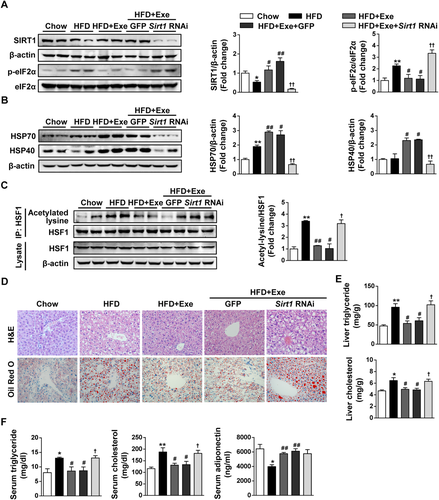
Lentiviral Sirt1 knockdown abolishes the effect of exenatide on HSF1 deacetylation, HSP induction, and alleviation of ER stress and hepatic steatosis. Male C57BL/6J mice were randomly divided into two groups fed a chow diet or an HFD for 8 weeks, and the HFD-fed mice were further divided into four groups: normal saline–treated group (HFD), exenatide-treated group (HFD+Exe), Lv-GFP with exenatide–treated group (HFD+Exe+GFP), and Lv-Sirt1 RNAi with exenatide–treated group (HFD+Exe+Sirt1 RNAi). One week after the injection of lentivirus or control solvent, mice were subjected to either normal saline or exenatide (24 nmol/kg/day) for 2 weeks (n = 5-6/group). (A) Western blot analysis of SIRT1 and ER stress markers (GRP78, p-eIF2α, and eIF2α) in liver tissues, with β-actin as a loading control. (B) Western blot analysis of HSP (HSP70 and HSP40) in liver tissues, with β-actin as a loading control. (C) Acetylated lysine and total HSF1 expression in the liver by immunoprecipitation and western blot analysis. (D) Representative images of liver H&E staining and oil red O staining with ×400 magnification. (E) Liver triglyceride and cholesterol levels. (F) Serum triglyceride, cholesterol, and adiponectin levels. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 compared with the chow diet group; #P < 0.05, ##P < 0.01 compared with the HFD group; †P < 0.05, ††P < 0.01 compared with the Lv-GFP injection group. Abbreviation: Exe, exenatide.
Discussion
In the current study, we identify a mechanism that exenatide (exendin-4) increases HSP expression to alleviate obesity-induced ER stress and hepatic steatosis by up-regulating HSF1 deacetylation through SIRT1. It further supports the notion that exenatide and incretin mimetics act as promising therapeutics for obesity-induced hepatic steatosis.
GLP-1 receptor agonists, like exenatide and liraglutide, have been clinically licensed as antidiabetic agents. An early animal study by Ding et al. revealed the beneficial effect of exendin-4 on hepatic steatosis.15 In addition, exenatide was observed to reverse fatty liver better than intensive insulin therapy in patients with obesity, NAFLD with elevated liver enzymes, and type 2 diabetes.29 New clinical studies also demonstrated that liraglutide, which was safe and well tolerated, led to histological resolution of nonalcoholic steatohepatitis.30, 31 However, the mechanism involved in the beneficial effect of GLP-1 receptor agonists on hepatic steatosis has not been clearly defined. Accumulating evidence has indicated that the chronic and unresolved ER stress could contribute to the progression of hepatic steatosis through increased lipogenesis.10, 11 Genetic ablation studies revealed that the PERK/eIF2α arm of UPR appeared to promote the lipogenesis and development of hepatic steatosis.12, 13 In this study, we identified that exenatide (exendin-4) ameliorated hepatic lipid accumulation in a diet-induced obese mouse model and in PA-induced hepatocytes due to the alleviation of ER stress, which was indicated by significantly decreased expression of ER stress markers including GRP78, p-PERK, and p-eIF2α. This is the first time to prove that exenatide ameliorates lipid-induced hepatic ER stress to improve lipid deposition in hepatocytes both in vivo and in vitro. In support of our findings, a previous study showed that liraglutide protected against ER stress in the livers of HFD-induced insulin-resistant rats.32 These findings collectively reveal the ability of GLP-1 receptor agonists to protect against ER stress and hepatic steatosis. Moreover, our study validated that exenatide (exendin-4) alleviated hepatic ER stress in a SIRT1-dependent manner by using genetic or pharmacological SIRT1 inhibition both in vivo and in vitro. This finding was supported by a study demonstrating that hepatic overexpression of SIRT1 attenuated ER stress and hepatic steatosis in obese mice.19 Another study also revealed that SIRT1 activation could alleviate PA-induced ER stress in hepatocytes.33 However, it has not been clearly defined how SIRT1 regulates hepatic ER stress.
SIRT1, the closest mammalian homolog of yeast silent information regulator 2, is now also recognized as a stress-response protein deacetylase.34 HSF1, a stress-inducible transcription factor, translocates to the nucleus and binds to the promoter of HSP genes to up-regulate their transcription under a variety of stresses. Then the increased HSP serve as molecular chaperones to dampen proteotoxic stresses and maintain protein homeostasis through refolding of misfolded peptides and restraining protein aggregation.28 A recent study demonstrates that SIRT1 deacetylates HSF1 and potentiates its binding to the promoter of HSP genes in vitro.20 Thus, we infer that the SIRT1/HSF1/HSP pathway may serve as a mechanism involved in ER stress and metabolic disorders that are closely related with protein homeostasis. As a result, we found that exenatide (exendin-4) increased the deacetylation of HSF1 and HSP expression to alleviate lipid-induced ER stress and hepatic steatosis in a SIRT1-dependent manner. We identify an effect of regulation of the SIRT1/HSF1/HSP pathway on ER stress in hepatocytes, which is a new mechanism different from adenosine monophosphate–activated protein kinase activation that might account for exenatide-ameliorated hepatic steatosis.17, 18
In addition, we observed that exenatide (exendin-4) up-regulated the expression of molecular chaperones including HSP70 and HSP40 to alleviate lipid-induced ER stress and hepatic steatosis both in vivo and in vitro, which emphasized the link between molecular chaperones and ER stress–related metabolic disorders. In support of our findings, studies have shown that activation of the inducible isoform of HSP70 protected against obesity-induced insulin resistance.35, 36 Hepatic overexpression of GRP78, an endogenous ER chaperone protein belonging to the HSP70 family, inhibited ER stress and sterol regulatory element binding protein 1c activation to reduce hepatic steatosis in mice.37 Furthermore, chemical chaperones were found to reduce hepatic ER stress in a mouse model of type 2 diabetes and in PA-induced hepatocytes.38, 39 Taken together, these findings indicate that strategies targeting molecular chaperones may be novel therapeutics for the treatment of metabolic disorders involved with ER stress. A previous report showed that exendin-4 enhanced GRP78 expression but decreased ER stress–related cell death in fat-loaded hepatocytes.40 By contrast, decreased GRP78 expression and increased HSP expression after exendin-4 treatment were observed simultaneously in our study. This inconsistency in GRP78 expression might reflect the different extent of ER stress existing in fat-loaded hepatocytes after exendin-4 treatment, which varied due to the different dosage and time course that both fatty acid and exendin-4 used. Because GRP78 is an ER chaperone protein belonging to the HSP70 family, we speculate that it is a direct transcriptional target of both UPR signaling and HSF1 signaling based on previous studies.9, 28 Therefore, the decreased GRP78 expression after exendin-4 treatment in our study might be mainly attributed to the down-regulated UPR signaling rather than the increased HSF1 deacetylation.
Recent publications have indicated a protective effect of adiponectin on hepatic steatosis and fibrosis.41, 42 AdipoR1 and AdipoR2 are two receptors of adiponectin, which are both expressed in the liver. AdipoR1 is primarily expressed in activated hepatic stellate cells, while AdipoR2 is primarily expressed in other liver cells such as hepatocytes. Adiponectin binds to its receptors and stimulates adenosine monophosphate–activated protein kinase in the liver, a crucial factor in glucose and lipid metabolism.42, 43 Thus, we also detected an effect of exenatide on adipose-derived adiponectin and its hepatic downstream signaling in this study. It showed that exenatide restored the reduced serum adiponectin levels and significantly up-regulated the mRNA expression of AdipoR2 in the livers of HFD-induced obese mice, which indicated that the beneficial effect of exenatide-increased adiponectin was mainly mediated by AdipoR2 in the liver. Besides, our previous research verified that exenatide could up-regulate the adenosine monophosphate–activated protein kinase activity in the liver.17 Taken together, our findings are consistent with a report indicating that exenatide increased serum adiponectin levels in obese mice,15 which also provides another possible mechanism to explain the effect of exenatide on improving hepatic steatosis.
In our study, tail vein injection of viral shRNA was used for in vivo gene knockdown as described.44, 45 Due to the liver first-pass effect of virus, we infer that Lv-Sirt1 shRNA by tail vein injection in our mouse model might primarily target to the liver. To confirm this conjecture, we examined SIRT1 expression in different organs with western blot analysis. As expected, liver was the organ that SIRT1 was knocked down the most (∼90% reduction). There were no significant changes of SIRT1 expression in other insulin-sensitive organs including muscle, white adipose tissue (epididymal fat), and brown adipose tissue after Lv-Sirt1 RNAi injection compared with Lv-GFP control (Supporting Fig. S4A). Meanwhile, immunohistochemical analysis of liver sections also revealed a marked decrease in SIRT1 expression after Lv-Sirt1 RNAi injection (Supporting Fig. S4B). Consistently, the acetylation of p53 at Lys382, a specific deacetylation site of SIRT1,19 showed a 2-fold to 3-fold increase in the liver after Lv-Sirt1 RNAi injection (Supporting Fig. S4C). Studies have shown that SIRT1 regulates the production of adiponectin in adipocytes through forkhead box O1.46, 47 Importantly, we found no change in the serum adiponectin levels between Lv-Sirt1 RNAi injection and Lv-GFP control. Thus, our result provided another credible proof that tail vein injection of Lv-Sirt1 RNAi did not affect the white adipose tissue because adiponectin was primarily produced and secreted by white adipose tissue. Taken together, this Lv-Sirt1 knockdown mouse model in our study could be considered as a proper model for studying the causal role of SIRT1 in mediating exenatide's effect on the liver. However, it should be noted that this mouse model has limitations due to the potential for Lv-Sirt1 shRNA to target other non-insulin-sensitive organs and that the liver-specific Sirt1 knockout mouse is the ideal mouse model for this study. Therefore, by combination with the two mouse models of SIRT1 inhibition and in vitro models, our results provide strong biochemical evidence that the SIRT1/HSF1/HSP pathway mediates the beneficial effect of exenatide on lipid-induced ER stress and hepatic steatosis associated with obesity.
In summary, our study demonstrates that exenatide (exendin-4) increases HSP expression to alleviate lipid-induced ER stress and hepatic steatosis through SIRT1-mediated HSF1 deacetylation. This indicates that the SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced ER stress and hepatic steatosis, which provides a novel mechanism supporting exenatide and incretin mimetics as promising therapeutics for obesity-induced hepatic steatosis.
REFERENCES
Author names in bold designate shared co-first authorship.




