Prevention and treatment of complications of selective internal radiation therapy: Expert guidance and systematic review
Potential conflict of interest: Dr. Flamen consults and received grants from Sirtex. He advises Novartis. He received grants from Bayer. Dr. Coldwell consults and is on the speakers' bureau for Sirtex. Dr. Kennedy advises and received grants from Sirtex. Dr. Bilbao consults and is on the speakers' bureau for Sirtex. Dr. Bester is on the speakers' bureau for Sirtex. Dr. Ricke consults, advises, and received grants from Sirtex. Dr. Sharma consults, is on the speakers' bureau, and received grants from Sirtex. Dr. Sangro advises and is on the speakers' bureau for Sirtex. He advises BTG.
Supported by the National Institute of Health Research UCL Biomedical Research Centre, the UCL Cancer Institute Research Trust, and Cancer Research UK (to R.A.S.).
Abstract
Selective internal radiation therapy (or radioembolization) by intra-arterial injection of radioactive yttrium-90-loaded microspheres is increasingly used for the treatment of patients with liver metastases or primary liver cancer. The high-dose beta-radiation penetrates an average of only 2.5 mm from the source, thus limiting its effects to the site of delivery. However, the off-target diversion of yttrium-90 microspheres to tissues other than the tumor may lead to complications. The most prominent of these complications include radiation gastritis and gastrointestinal ulcers, cholecystitis, radiation pneumonitis, and radioembolization-induced liver disease, which may occur despite careful pretreatment planning. Thus, selective internal radiation therapy demands an expert multidisciplinary team approach in order to provide comprehensive care for patients. This review provides recommendations to multidisciplinary teams on the optimal medical processes in order to ensure the safe delivery of selective internal radiation therapy. Based on the best available published evidence and expert opinion, we recommend the most appropriate strategies for the prevention, early diagnosis, and management of potential radiation injury to the liver and to other organs. (Hepatology 2017;66:969–982).
Abbreviations
-
- CRC
-
- colorectal cancer
-
- CT
-
- computed tomography
-
- FOLFOX
-
- folinic acid, fluorouracil, and oxaliplatin
-
- GI
-
- gastrointestinal
-
- HCC
-
- hepatocellular carcinoma
-
- LS
-
- lung shunt
-
- MAA
-
- macroaggregated albumin
-
- PPI
-
- proton pump inhibitor
-
- REILD
-
- radioembolization-induced liver disease
-
- SIRT
-
- selective internal radiation therapy
-
- SPECT
-
- single-photon emission computed tomography
-
- 90Y
-
- yttrium-90
Selective internal radiation therapy (SIRT, also known as radioembolization) involves the implantation of radiation sources into tumors using radioactive microspheres. Based on published evidence, this procedure is now widely available in specialist centers for the treatment of patients with liver metastases or primary liver cancer. There are two approved products available across continents using either glass or resin microspheres. Once administered into the hepatic artery that feeds liver tumors, the microspheres become permanently lodged in the terminal arterioles of the tumor where the emitted high-dose beta-radiation is delivered locally. The off-target diversion of yttrium-90 (90Y) microspheres to tissues other than the tumor may lead to complications. The most prominent complications are gastrointestinal (GI) ulcers, radiation pneumonitis, and radioembolization-induced liver disease (REILD). The aim of this review is to provide recommendations to multidisciplinary teams on the most appropriate strategies for the prevention, early diagnosis, and management of potential radiation injury, based on the best available published evidence and expert opinion.
Methods
Recommendations were developed by a panel of experts with extensive experience with SIRT, representing the relevant medical disciplines: medical oncology, interventional radiology, hepatology, radiation oncology, and nuclear medicine. The methodology used for the systematic search of the literature and panel discussion of findings and recommendations is available in the Supporting Information. In brief, the literature published between January 1990 and June 2016 was systematically searched. A directed appraisal identified 196 papers that were reviewed in detail. An in-depth analysis was performed by complication, and all authors had an opportunity to comment on each recommendation. A number of recommendations involving technical aspects of the interventional radiological procedures are beyond the scope of this review and will be discussed elsewhere.
Radiation Pneumonitis
THE MEDICAL PROBLEM
Radiation pneumonitis is a very rare but worrisome complication that may occur 1-6 months after SIRT and is characterized by the appearance of restrictive ventilatory dysfunction and bilateral lung infiltrates1, 2 usually with exertional dyspnea and dry cough. Only six cases2, 3 have ever been reported in detail, none of which were from clinical trials; and of these, three were fatal events that occurred at a single center.3
Microspheres can circumvent the liver sinusoidal network through the tumor or through arteriovenous channels present in the cirrhotic liver and ultimately reach the lung filter. During the pretreatment workup, technetium-99m macroaggregated albumin (MAA) is injected into the hepatic arteries. The lung shunt (LS) is estimated based on the fraction of MAA which becomes deposited in the pulmonary vasculature. The risk of pneumonitis arises when LS exceeds 10%3 and is particularly high when LS exceeds 20%. Treatment-limiting LS fractions >20% are atypical but more common in hepatocellular carcinoma (HCC) than other liver tumors (14% versus 3%).4
The relevance of subclinical lung damage as a determinant of future cancer treatment is unknown. Further radiation exposure to the lungs is not recommended within 6 months of SIRT. Anecdotal evidence suggests that in cases where a second SIRT procedure is administered within 6-12 months of the first, the maximum tolerated dose in the lung should be <50% of the dose delivered to the lungs during the first procedure.5
PREVENTION OF PNEUMONITIS
Dose reductions of 20% and 40% are recommended by the manufacturer of resin microspheres if LS exceeds 10% or 15%, respectively; and SIRT is contraindicated if LS exceeds 20%. Strict adherence to these limits largely prevents this complication, with no reported cases in recent multicenter retrospective analyses of 606 patients with metastatic colorectal cancer (CRC),6 325 patients with HCC,7 and 112 patients with various tumor types.8 For glass and resin microspheres, an upper limit of 30 Gy to the lung has been empirically established and again shown to largely prevent this complication. Yet, this dosimetric approach has several pitfalls. There are no reliable methods for assessing the real dose of radiation delivered to the lung, a normal tissue complication probability analysis for lung damage is lacking for SIRT, and dosimetric models do not consider functional lung volume. Indeed, pneumonitis may rarely occur in patients with LS <20% even if the dose threshold of 30 Gy is not reached, as illustrated in Fig. 1. No drug has shown a positive effect in preventing lung damage produced by external beam radiation therapy. Therefore, prevention of SIRT-induced pneumonitis by medical therapy is not available.
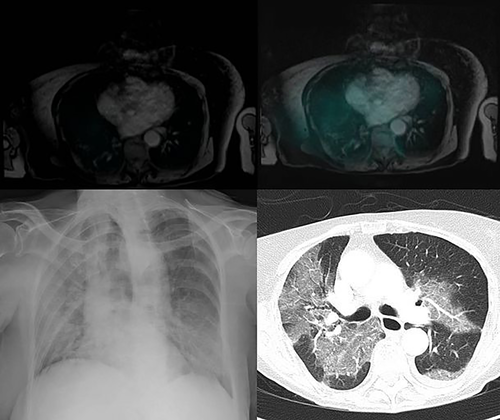
LS is also important because a lower delivered tumor dose will also diminish efficacy. When LS is ≥15%, the SIRT team should strongly consider an alternative treatment approach rather than reducing the activity of 90Y delivered or alternatively refer the patient to, or discuss the case with, a center with high-volume experience.
DIAGNOSTIC WORKUP FOR SUSPECTED PNEUMONITIS
If lung uptake is observed in imaging procedures following SIRT, we suggest checking the oxygen saturation by pulse oximetry or performing a 6-minute walk test on a weekly or biweekly basis, with a chest computed tomographic (CT) scan in case of oxygen desaturation or dyspnea. Similarly, any patient with LS >10% that presents with dyspnea within the first 3 months after SIRT should have a chest CT. If the characteristic bilateral symmetric ill-defined patchy opacities and ground-glass nodularity with relative peripheral/hilar sparing3 are observed and an infectious or cardiac cause can be ruled out, it is worth starting therapy without delay. Functional tests show a restrictive pattern with an altered diffusing capacity of the lung for carbon monoxide. The decision to perform a bronchoalveolar lavage and/or a transbronchial lung biopsy should be based on the likelihood of an alternative diagnosis that may be identified with these procedures.
TREATMENT OF PNEUMONITIS
Steroids form the mainstay of treatment (Table 1), although there is little evidence to support their use. The patient may receive steroids for a minimum period of 2 weeks, followed by slow tapering.9 Oxygen supply may be needed, and treatment may be augmented with pentoxifylline, again on a totally empiric basis.3
| Prevention | Workup | Treatment |
|---|---|---|
| Reductions of 20%, 40%, and 100% in injected activity if LS exceeds 10%, 15%, or 20%, respectively (for resin microspheres). | Chest CT scan if hypoxemia, cough, or dyspnea within the first 2 months post-SIRT, particularly if LS on MAA scan was >10%. | Steroids on a very empiric basis (methylprednisolone 500 mg intravenously twice daily or prednisolone 60 mg per os and slow tapering). |
| Dose of radiation to lung tissue under 30 Gy based on MAA scan (for resin or glass microspheres). | Functional tests to confirm restrictive pattern and altered carbon monoxide diffusion level. | Oxygen supply as needed. |
| If LS is ≥15%, strongly consider an alternative treatment approach (balance between safety and efficacy). | The decision to perform a bronchoalveolar lavage and/or a transbronchial lung biopsy should be based on the likelihood of alternative diagnosis. |
Gastrointestinal Ulcerations
THE MEDICAL PROBLEM
Radiation-induced GI ulcerations are an uncommon complication of SIRT that presents usually 2-6 weeks after SIRT with symptoms of acute epigastric pain, nausea, vomiting, dyspepsia, and sometimes anorexia.10 Ulcers are usually multiple (0.5-2 cm in size) but can also be “diffuse” and unmeasurable11 and often associated with diffuse mucositis.12 The ulcers typically have a chronic, insidious course with symptoms persisting for weeks despite appropriate therapy. In the largest clinical trial on SIRT, the incidence of GI ulcers was 2.4% among patients with metastatic CRC.13 Several retrospective analyses of large cohorts reported a similar incidence of 1.9%-3.2%.6, 7, 11, 14-16 The natural history has been described in detail.12 Symptoms are usually mild to moderate and last for 4-10 months despite treatment, but full symptomatic and endoscopic recovery normally occurs, as shown in Fig. 2. Complications include pyloric stricture, hemorrhage, severe anemia with transfusion needs, bilioenteric fistula, and death.7, 11, 12, 17
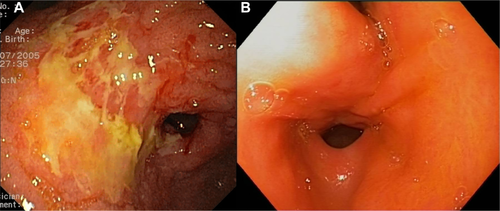
90Y microspheres can be found in gastric biopsies obtained months to years after SIRT in asymptomatic patients. The administration of agents with a potential to produce gastric injury to these patients with subclinical GI damage may pose some theoretical concern, particularly antiangiogenics. Among patients with metastatic CRC treated with SIRT plus folinic acid, fluorouracil, and oxaliplatin (FOLFOX) in a large trial, the rate of bleeding or GI ulcers was reassuringly similar in those who received bevacizumab and those who had not.13
PREVENTION OF GI ULCERS
Prevention of GI complications in SIRT largely depends on the identification of the vascular anatomy that allows microspheres injected into the hepatic arteries to gain access to the stomach or duodenum. A comprehensive evaluation of the liver arterial vasculature, careful evaluation of any MAA uptake that may be located outside the liver, the judicious use of coil embolization of collateral vessels to manage parasitization/collateralization, and the prevention of stasis and reflux during infusion should minimize the risk of GI ulcers. Yet, ulcers can still occur due to unnoticed or recanalized collateral vessels or changes in flow dynamics during treatment.
Although commonly used, there is no scientific evidence to support the use of proton pump inhibitors (PPIs) in the prevention of radiation-induced damage to the GI tract. Routine PPI therapy should therefore not be considered mandatory. If PPIs are prescribed, they should be maintained for at least 8 weeks, when SIRT-related GI ulcers usually become symptomatic. Other prophylactic approaches, including use of the prostaglandin E2 inhibitor misoprostol or the identification and eradication of Helicobacter pylori prior to SIRT, may be considered but equally lack scientific evidence supporting their use.
The detection of extrahepatic uptake of radioactivity in post-SIRT imaging procedures that is consistent with GI exposure to radiation deserves special consideration. The limitations in space resolution of these imaging techniques, including the effect of respiratory movements, may produce false-positive observations. The use of a prophylactic PPI is generally recommended, but gastroscopy does not need to be performed in the absence of significant symptoms. The use of innocuous radioprotective agents such as amifostine might be considered in some cases.18
DIAGNOSTIC WORKUP FOR SUSPECTED GI ULCERATIONS
An upper endoscopy should be performed in every patient who develops persistent upper abdominal pain 4-8 weeks after SIRT, particularly if pain is associated with nausea, loss of appetite, or anemia. Early gastroscopy is also encouraged for those patients in whom anemia develops or worsens 4-6 weeks after SIRT. Failure to retrospectively detect extrahepatic uptake of radioactivity in the post-SIRT scans in these patients should not lead to delayed evaluation. The presence of multiple mucosal erosions and ulcerations or a single large ulcer, usually associated with diffuse mucositis, and the distribution of the lesions, usually involving the distal gastric body, antrum, pylorus, and the duodenal bulb, allow the presumptive diagnosis.10, 12, 19-23 The poor healing of the radiated site often discourages regular biopsies that will unlikely change patient management.
TREATMENT OF GI ULCERATIONS
Radiation-induced GI ulceration is poorly responsive to therapy, but the most relevant recommendations are summarized in Table 2. Mild to moderate symptoms can be treated by dietary modification and starting or increasing to maximal the dose of PPI, the gastroprotective agent sucralfate, antiemetics, analgesics, and gastric promotility agents such as domperidone or cinitapride.12 Nonsteroidal anti-inflammatory drugs and any other medication potentially harmful for the GI mucosa must be withdrawn. In more severe cases, pain and nausea relief can only be achieved by total parenteral nutrition. When the situation persists for weeks, jejunostomy may provide long-term symptom control until ulcers heal and proper nourishment can be secured.23 Recurrent GI hemorrhage can be managed by direct endoscopic therapy or selective bland targeted arterial embolization. Rarely (<10% of cases), perforation or bleeding may require surgical excision of the involved GI tract segment with bypass.24, 25
| Prevention | Workup | Treatment |
|---|---|---|
|
Conventional or CT angiography to identify hepaticoenteric vessels and avoid them by coil embolization, a more distal injection, or flow redistribution. SIRT simulation using MAA test with • Perchlorate to avoid false-positive gastric uptake. • MAA prepared within 30 minutes before injection. • Injection from the same site as treatment. • SPECT imaging performed within 1 hour after injection. If a false-positive result is suspected, repeat MAA test before contraindicating SIRT. Use isotonic dextrose 5% as the administrative agent for resin microspheres. Intra-arterial lidocaine or nitroglycerin may ameliorate vasospasm during SIRT. |
Upper endoscopy in every patient who develops upper abdominal pain 4-8 weeks after SIRT, particularly if associated with nausea, loss of appetite, or anemia (even if no extrahepatic uptake of radioactivity in the MAA SPECT/CT or 90Y positron emission tomographic scans). Presumptive diagnosis based on gross morphology (multiple erosions and ulcerations or a single large ulcer, with diffuse mucositis, usually involving the distal gastric body, antrum, pylorus, and duodenal bulb). Biopsies not mandatory unless an alternative cause is considered. |
High-dose PPIs (omeprazole 40 mg daily or equivalent doses of pantoprazole, lansoprazole, etc.), sucralfate, antiemetics, analgesics, and gastric promotility agents (domperidone or cinitapride). Avoid nonsteroidal anti-inflammatory drugs and any other medications that may produce gastritis or ulcers. In more severe cases consider total parenteral nutrition or jejunostomy. |
Liver Complications
THE MEDICAL PROBLEM
Identifying liver toxicity in patients with liver cancer treated with liver-directed therapies is not an easy task. The liver that harbors a cancer is by definition nonhealthy. Patients with primary liver cancer usually have a chronic liver disease frequently in the cirrhotic stage. Those with liver metastases from distant cancers quite commonly have steatosis or fibrosis either at diagnosis or after exposure to several chemotherapeutic agents, including oxaliplatin, irinotecan, and fluoroderivatives.26 Tumor growth in the liver may per se ultimately impair liver functions. On the other hand, the liver is a multifunctional organ, and different variables may reveal liver dysfunction, from laboratory values to organ rigidity to distant signs of portal hypertension.
Radioembolization-Induced Liver Disease
REILD is a well-defined syndrome characterized by the appearance of jaundice and ascites 4-8 weeks after SIRT in the absence of tumor progression or bile duct occlusion.27 REILD is always associated with elevated bilirubin (>3 mg/dL), with variable increases in alkaline phosphatase and gamma-glutamyltransferase and virtually no changes in transaminases (aspartate aminotransferase and alanine aminotransferase). This syndrome is clinically distinct from the anicteric ascites caused by external beam radiation therapy.28 REILD resembles other forms of sinusoidal obstruction syndrome. Indeed, in the most severe cases liver biopsy shows veno-occlusive disease, the histological hallmark of sinusoidal obstruction syndrome.27, 29 Importantly, sinusoidal obstruction syndrome may also occur in CRC patients who receive oxaliplatin-based or irinotecan-based chemotherapy regimes,30 which is the clinical setting for many SIRT-treated patients.
REILD is an uncommon event. Incidence rates of 5.4%31 and 4%32 have been reported on large series with a mix of tumor types. REILD appears mainly, if not exclusively, in two groups: patients who do not have cirrhosis but who are exposed to systemic chemotherapy prior to SIRT and treated in a whole-liver fashion because of extensive tumor load (e.g., CRC or breast cancer patients treated in a salvage setting) and patients with cirrhosis and reduced liver functional reserve, even if treated in a more selective fashion (e.g., patients with HCC).31 The risk of REILD is increased if patients are exposed to chemotherapy in the 2-month period following SIRT,31 in the presence of a small liver (total volume <1.5 L)31 or increased baseline bilirubin and aspartate aminotransferase,31 after an intense treatment,16, 31 and with repeated whole-liver SIRT.16 In many patients, the disease may be controlled by therapy, but severe complications including overt liver failure may rapidly ensue.33 Although there are no reports on prospective long-term follow-up of patients with REILD, median survival as short as 95 days from SIRT has been reported.32 For any SIRT center, the outcomes of a regular review and/or an audit process are essential to identify whether complication rates are within the expected limits reported in the recent literature.6, 31 There is no clear indication that the occurrence of REILD is different for resin or glass microspheres, although the highest incidence of liver decompensation (36.5% at 6 months) has been reported after SIRT using glass microspheres in intermediate or advanced HCC patients.34
SIRT may produce subclinical liver injury. A significant although clinically irrelevant increase in total bilirubin has been reported in CRC,35 breast cancer,36 and HCC37 patients. The implications of this subclinical liver damage on the tolerability of subsequent treatments are not known and require further investigation in well-designed, prospective studies.
Portal Hypertension
Noncirrhotic portal hypertension may very rarely develop months to years after SIRT. Early asymptomatic increases in splenic volume with or without low platelet count are frequently observed, even after lobar SIRT.32, 36, 38 Similar findings may be seen in patients treated with adjuvant FOLFOX for stage II-III CRC due to sinusoidal injury.39 Gastroesophageal varices have been anecdotally identified years after SIRT.40 Histological findings observed in these patients indicate nodular regenerative hyperplasia (unpublished data) with40 or without29 bridging fibrosis. Encephalopathy and variceal bleeding have not been reported in patients who do not have cirrhosis.
Biliary Tree Damage
Biliary tree injury post-SIRT has been reported infrequently, including bile duct necrosis or strictures. Among 569 SIRT treatments in 327 patients, 3 patients (1%) developed large bilomas requiring drainage,41 while asymptomatic biliary necrosis and strictures were observed in 3.9% and 2.4%, respectively. In this large series, biliary complications were more common in metastatic disease with multiple disseminated tumors than in primary HCC. Bile duct compression by tumors cannot be ruled out in many cases. The consensus, based on expert opinion, is that SIRT is unlikely to be the primary cause of isolated biliary strictures. This is partially supported by recent evidence of minimal or no significant biliary complications with stereotactic body radiation therapy or high-dose hypofractionated radiation therapy for tumors located within the perihilar region.42-44 Ischemic cholangitis leading to diffuse biliary strictures has only once been reported after SIRT.45
PREVENTION OF REILD
Patients with poor liver functional reserve, such as those who present with total bilirubin >2 mg/dL or have nontumoral ascites, should not be considered candidates for SIRT. Individual yet conservative decisions should be made in patients with bilirubin values slightly below this threshold in whom a rapid increase is observed within the weeks previous to SIRT evaluation.
Adaptation of the calculated activity is recommended in all cases where patients may have a low functional liver reserve because of steatosis, steatohepatitis, hepatitis, or cirrhosis, where the liver is small (<1.5 L), and in patients who have received multiple lines of prior chemotherapy. Both the type and duration of prior systemic treatment are important, with anecdotal evidence, for example, that the more hepatotoxic therapies used for breast cancer may increase the risk of REILD.
The body surface area formula is the recommended method for calculating the prescribed activity for SIRT using resin microspheres, while for glass microspheres activity is calculated from the volume of liver that is targeted. There is general agreement that the body surface area formula may overestimate the activity and that liver toxicity is lower when the prescribed activity is reduced.6 A tailored treatment protocol has recommended a dual strategy of either more selective lobar or segmental treatment (enabling a greater spared liver volume) or a reduction in the prescribed activity for patients receiving whole-liver SIRT.31 Tables that display a modified body surface area formula according to tumor load and LS have been used in large clinical trials in CRC patients.13
For both resin and glass microspheres, the development of reliable dosimetric methods for activity prescription represent the holy grail.5, 46, 47 Dosimetric analysis indicates that better tumor responses are associated with higher mean absorbed doses. From the safety perspective, however, the use of dosimetric methods faces several problems including heterogeneous distribution of particles in the nontumoral liver, potential changes in particle distribution between MAA and 90Y microsphere injections due to catheter position and local hemodynamic conditions,48 and suboptimal measurement of the tumor to nontumor ratio for multinodular tumors. Such methods may, however, be reliable for prescribing the activity to patients receiving selective treatment if the tumor to nontumor uptake ratios of 90Y microspheres can be calculated accurately.31, 49 But these methods have not been validated in prospective trials and are not appropriate for whole-liver treatment or for tumors without significant uptake of MAA.
Sequential lobar treatment (i.e., deferring treatment of the contralateral liver lobe for 6 weeks) may improve liver tolerance to SIRT for several reasons. Deferring subclinical liver injury in the contralateral lobe may lower the likelihood of clinical decompensation. Increasing contralateral lobe volume may reduce the relative intensity of treatment in that lobe. This measure provides clinicians with an opportunity to evaluate the tolerability of SIRT and modify activity prescribed to the contralateral lobe. Finally, the induction of hepatocyte growth factor and other proregenerative factors after lobar SIRT37 might make the contralateral lobe more tolerant to SIRT.50 As a major counterpart, sequential lobar treatment increases treatment-derived costs of an already costly therapy. Small case series have suggested that this approach resulted in fewer grade 3/4 toxicities and liver damage,51 but has not been proven to improve strong outcomes including overall survival. General recommendation should therefore await prospective validation and cost-economic analysis.
Prophylactic treatments such as pentoxifylline, ursodeoxycholic acid, and low–molecular weight heparin have been evaluated to reduce the liver damage caused by conditioning total-body irradiation regimens prior to bone marrow transplantation with equivocal results.52-54 Pentoxifylline prevents activation of stellate and endothelial cells by interfering with transforming growth factor-beta signaling; low–molecular weight heparin may prevent thrombosis; and ursodeoxycholic acid protects endothelial cells from injury55 and hepatocytes from cytokine-induced damage,56 while it is known that SIRT is followed by a systemic inflammatory response.37
Building on this concept, the combination of ursodeoxycholic acid (300 mg twice daily) and methylprednisolone (4-8 mg once a day) was incorporated into a tailored treatment protocol that also included changes in the algorithm for activity calculation. Prophylactic drug therapy was not independently predictive of the reduced incidence of liver decompensation. In a recent small prospective study, the incidence of focal radiation-induced liver damage at 6 weeks was significantly lowered when patients were treated with pentoxifylline, ursodeoxycholic acid, and low-dose low–molecular weight heparin after image-guided interstitial brachytherapy for low metastatic CRC.57 Although ursodeoxycholic acid and low-dose steroids are generally innocuous, currently there is insufficient evidence to recommend prophylactic medication to reduce the incidence of REILD after SIRT.
DIAGNOSTIC WORKUP FOR SUSPECTED REILD
REILD has to be considered in any patient, with or without cirrhosis, who develops jaundice and ascites within the first 3 months after SIRT. Patients may be instructed to provide the result of blood tests including total bilirubin, alkaline phosphatase, and transaminases 4-6 weeks post-SIRT, as well as to report on the presence of edema or abdominal swelling. Upon the discovery of bilirubin >3 mg/dL, the physician should request an abdominal ultrasound to rule out bile duct obstruction and to confirm the presence of ascites and the patency of the portal and hepatic veins, as well as additional blood tests to measure liver function including albumin and coagulation factors (at least prothrombin activity). Other laboratory tests such as viral hepatitis markers should be ordered in case of markedly elevated aspartate aminotransferase/alanine aminotransferase (>1,000 IU/mL) or if clinically advised. Contrast-enhanced CT or (better) magnetic resonance imaging scanning is recommended to accurately evaluate intrahepatic and extrahepatic tumor progression. If the diagnosis is not obvious based on the result of tests and imaging procedures and the course of the disease is stable or worsening, an early liver biopsy is strongly recommended because it may guide treatment. Biopsy is not recommended for patients with cirrhosis because the histological findings may be equivocal27 and there is increased risk of bleeding. Once the diagnosis of REILD is established, liver function tests should be repeated (at least weekly) to identify those patients who may be in transit to liver insufficiency, with declining coagulation and increasing bilirubin.
TREATMENT OF REILD
Table 3 summarizes the most relevant recommendations. Initial symptomatic therapy should include diuretics (low-dose spironolactone and/or furosemide). Defibrotide, a single-stranded polydeoxy-ribonucleotide with antithrombotic, thrombolytic, anti-inflammatory, and anti-ischemic properties, has been successfully used in veno-occlusive disease post–stem cell transplantation at doses ranging from 10 to 40 mg/kg over a median of 18 days.58, 59 Based on this experience, treatment with defibrotide may be considered in patients with REILD and rapidly increasing bilirubin (>6 mg/dL) or altered coagulation (decline in prothrombin activity with or without low platelet count). If medical treatment is ineffective, prompt transjugular intrahepatic portosystemic stent-shunt placement is recommended as a potentially life-saving procedure for patients with ongoing decline in liver function following SIRT. This recommendation is based on largely anecdotal experience with REILD27 and the collective published literature in life-threatening clinical syndromes resulting from sinusoidal congestion.60, 61
| Prevention | Workup | Treatment |
|---|---|---|
|
Contraindicate if total bilirubin > 2 mg/dL or nontumoral ascites. Consider individually late changes in bilirubin. Consider reducing the recommended activity for patients with chronic liver disease (including steatohepatitis or cirrhosis), where the liver is <1.5 L, and in patients who have received multiple prior chemotherapy regimes. Spare as many liver segments as appropriate and technically feasible. |
Suspect REILD in any patient who develops jaundice and ascites within the first 3 months after SIRT. Request Doppler ultrasound to check for bile duct obstruction, ascites, and portal/hepatic vein patency, plus blood test to measure liver damage and function (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, albumin, international normalized ratio). If bile duct obstruction is discarded, request contrast-enhanced CT or (better) magnetic resonance imaging to rule out tumor progression. Repeat liver function tests at least weekly. If the problem persists or worsens within 2 weeks, consider liver biopsy (only for patients who do not have cirrhosis) and consultation with hepatologist. |
Treat initially with diuretics (spironolactone 100 mg and/or furosemide 40 mg daily) and adjust the dose based on weight loss and renal function. If liver function starts to decline (e.g., total bilirubin ≥6 mg/dL and prothrombin activity ≤60% or international normalized ratio ≥1.4), consider defibrotide intravenously at a starting dose of 10 mg/kg. If liver failure develops despite medical treatment, consider transjugular intrahepatic portosystemic stent-shunt placement. |
Radiation Cholecystitis
THE MEDICAL PROBLEM
Acute cholecystitis is a rare complication of SIRT. Symptoms are characterized by upper right quadrant abdominal pain, nausea or vomiting, malaise, and occasional fever. On the other hand, a thickened gallbladder wall is occasionally observed in patients who remain asymptomatic. In a detailed analysis of CT changes in the gallbladder following SIRT, thickening and hyperenhancement of the gallbladder wall were observed in 10 out of 42 patients (90% had metastases adjacent to the gallbladder). These features were most prominent on the first follow-up scan at 20-30 days after treatment.62 The incidence and natural history of gallbladder toxicity are largely unknown, but fewer than 10 cases of acute cholecystitis have been reported in the literature and only five in detail.63, 64 Surprisingly, in 4 out of these 10 cases, review of MAA and 90Y single-photon emission computed tomography (SPECT) images did not reveal increased radioisotope uptake in the gallbladder.64 Cholecystectomy was performed in 7 of these patients without any serious complication.41, 63-65
PREVENTION OF CHOLECYSTITIS
Gallbladder imaging changes are largely asymptomatic, but the deposition of 90Y-microspheres in the gallbladder should also be avoided, to maximize the delivery of microspheres to the target tumor tissue. As a general rule, placement of the catheter distal to the cystic artery is recommended; and only if this is not feasible may temporary occlusion of the cystic artery be performed, preferably immediately before SIRT, injecting Gelfoam particles66 or inducing a vasospasm of the cystic artery using a microwire.67 Coil embolization of the cystic artery (which may induce acute ischemic cholecystitis68) and preventive cholecystectomy are not recommended.
RELEVANCE OF SUBCLINICAL DAMAGE TO THE GALLBLADDER
On imaging, some shrinkage of the gallbladder may be evident 6 months post-SIRT, but this is not thought to be clinically significant.
Empiric preventative measures using steroids and antibiotics in patients judged to be at risk of cholecystitis (based on scans during the pretreatment workup)67 are not recommended.
DIAGNOSTIC WORKUP FOR SUSPECTED RADIATION CHOLECYSTITIS
Radiation cholecystitis should be suspected in any patient who develops persistent right upper quadrant tenderness and fever 4-6 weeks following SIRT. The presence of a thickened (≥3.5 mm) wall with pericholecystic fluid, intramural gas, or hydrops on ultrasound, magnetic resonance imaging, or CT helps confirm the diagnosis. A thickened, hyperenhanced gallbladder wall alone should not prompt a diagnosis of cholecystitis in the absence of consistent signs on physical examination, including Murphy's sign. If reevaluation of the MAA scan, Brehmsstralung SPECT scan, or 90Y positron emission tomography reveals no evidence of intense gallbladder uptake, cholecystitis is highly unlikely. In such cases, an upper endoscopy may be needed to differentiate cholecystitis from gastric and duodenal ulcerations.
TREATMENT OF RADIATION CHOLECYSTITIS
As summarized in Table 4, conservative therapy includes intravenous hydration and analgesics, while steroids are not recommended. Patients with fever, intense pain, or signs of wall necrosis or rupture on imaging should be considered for cholecystostomy (drainage of the gallbladder) and/or cholecystectomy. Cholecystectomy is the mainstay of the treatment of acalculous cholecystitis. but patients who are critically ill or at high risk for surgical complications are better managed by percutaneous cholecystostomy and eventually delayed cholecystectomy. In cancer patients, incorporation of the surgical risk and the patient-specific survival and the impact of antineoplastic therapy in the decision-making process may be beneficial.69
| Prevention | Workup | Treatment |
|---|---|---|
|
When a significant amount of activity is likely to be diverted to the gallbladder and to maximize the delivery of microspheres to the tumor, place the catheter distal to the cystic artery. If this is not feasible, perform temporary occlusion of the cystic artery using microwire-induced vasospasm or Gelfoam particles immediately before SIRT. |
Suspect radiation cholecystitis in any patient with persistent right upper quadrant tenderness 4-6 weeks following SIRT. The presence of a thickened (≥3.5 mm) wall with pericholecystic fluid, intramural gas, or hydrops on imaging may confirm the diagnosis if there are consistent signs on physical examination (Murphy). Reevaluation of Brehmsstralung SPECT scan or 90Y positron emission tomography to confirm gallbladder uptake of radiation is helpful. |
Provide intravenous hydration and analgesics on demand. Consider cholecystostomy (preferred) or cholecystectomy in patients with fever, intense pain, or signs of wall necrosis or rupture on imaging. Weigh surgical risk, patient-specific survival, and the impact of antineoplastic therapy before making a surgical decision. |
Other Potential Complications
In a minority of patients, SIRT can be followed by acute symptoms during or shortly after infusion that resemble the postembolization syndrome observed after transarterial chemoembolization and are probably due to the embolizing nature of the radioactive beads. In large series of HCC and CRC patients, reported rates are 13%-39% for abdominal pain, 2%-12% for fever, and 17%-32% for nausea and/or vomiting.7, 70-72 These symptoms are usually mild, last for a few hours, and are easily managed by medical treatment including non-narcotic analgesics (paracetamol 1 g with or without codeine phosphate 30-60 mg orally or intravenously) and ondansetron (8 mg orally or intravenously).
There is no strong published evidence that patients with a “violated ampulla” due to stenting, papillotomy, or surgical biliary bypass are at increased risk of infectious complications. Nevertheless, these patients are usually considered as having a relative contraindication to SIRT and are therefore underrepresented in large cohorts. The expert consensus is that biliary tree instrumentation should not be considered an absolute contraindication for SIRT but that treatment should not be conducted in patients with a history of previous sepsis or biliary drainage without prophylactic antibiotics.
Initially, there was a concern that SIRT might increase the morbidity of subsequent liver resection or transplantation. A specific study addressing this question in which 100 patients were analyzed (71 liver resections and 29 transplantations) showed that the reported rates of complications do not differ much from what can be expected if the patients were operated without prior SIRT.73
Conclusions
Selective internal radiation therapy is used to treat patients with primary and secondary liver cancer. The vast majority of patients have no or mild procedure-related symptoms. If a significant amount of radioactive particles reach nontargeted organs such as the lung, the GI tract, or the gallbladder, the radiation may produce tissue damage. This will occur rarely if the procedure is performed to adequate quality assurance standards. However, a certain amount of radiation is always delivered to the nontumoral liver tissue irrigated by the artery in which the microspheres are administered. Again, this will only rarely lead to complications if the recommendations of the manufacturers are followed to calculate the amount of radioactivity that should be injected. It should be noted that some patients have livers that are more sensitive to radiation or that have a reduced functional reserve, and these patients are at greater risk of clinically significant tissue damage. The impact of radiation-induced tissue damage on a patient's health ultimately depends on the damage induced by the treatment and, quite importantly, the relevance of the organ involved. Pneumonitis is a potentially fatal but exceptionally rare complication. REILD is uncommon but also potentially life-threatening. GI ulcers and cholecystitis may impact quality of life but generally have less severe implications.
Local practices used to prevent and treat such complications vary between centers. In this article, we have extensively analyzed the literature to identify the incidence, natural course, and risk factors for all of these main four complications of SIRT as well as the relevance of subclinical damage to the corresponding tissues. Based on the available evidence, we have provided recommendations and proposed new medical approaches. Specific recommendations have been made for the diagnostic workup and treatment of complications induced by SIRT. We believe that this expert guidance may help multidisciplinary teams and individual physicians to make sound decisions with the aim of improving care for patients with primary and secondary malignancies of the liver.




