Ginsenoside Rg3 restores hepatitis C virus–induced aberrant mitochondrial dynamics and inhibits virus propagation
Potential conflict of interest: Nothing to report.
Supported by the Korea Research Institute of Chemical Technology (KK1603-C00), the National Research Council of Science & Technology of the Korean government (CRC-16-01-KRICT), the Soonchunhyang University Research Fund (SCH Biopharm Human Resources Development Center Foundation), and the National Institutes of Health (AI085087 and DK077704, to A.S.).
Abstract
Hepatitis C virus (HCV) alters mitochondrial dynamics associated with persistent viral infection and suppression of innate immunity. Mitochondrial dysfunction is also a pathologic feature of direct-acting antiviral (DAA) treatment. Despite the high efficacy of DAAs, their use in treating patients with chronic hepatitis C in interferon-sparing regimens occasionally produces undesirable side effects such as fatigue, migraine, and other conditions, which may be linked to mitochondrial dysfunction. Here, we show that clinically prescribed DAAs, including sofosbuvir, affect mitochondrial dynamics. To counter these adverse effects, we examined HCV-induced and DAA-induced aberrant mitochondrial dynamics modulated by ginsenoside, which is known to support healthy mitochondrial physiology and the innate immune system. We screened several ginsenoside compounds showing antiviral activity using a robust HCV cell culture system. We investigated the role of ginsenosides in antiviral efficacy, alteration of mitochondrial transmembrane potential, abnormal mitochondrial fission, its upstream signaling, and mitophagic process caused by HCV infection or DAA treatment. Only one of the compounds, ginsenoside Rg3 (G-Rg3), exhibited notable and promising anti-HCV potential. Treatment of HCV-infected cells with G-Rg3 increased HCV core protein–mediated reduction in the expression level of cytosolic p21, required for increasing cyclin-dependent kinase 1 activity, which catalyzes Ser616 phosphorylation of dynamin-related protein 1. The HCV-induced mitophagy, which follows mitochondrial fission, was also rescued by G-Rg3 treatment. Conclusion: G-Rg3 inhibits HCV propagation. Its antiviral mechanism involves restoring the HCV-induced dynamin-related protein 1–mediated aberrant mitochondrial fission process, thereby resulting in suppression of persistent HCV infection. (Hepatology 2017;66:758–771)
Abbreviations
-
- CCCP
-
- carbonyl cyanide m-chlorophenylhydrazone
-
- CDK1
-
- cyclin-dependent kinase 1
-
- CHC
-
- chronic hepatitis C
-
- COX2
-
- cyclooxygenase 2
-
- ΔΨm
-
- mitochondrial membrane potential
-
- DAA
-
- direct-acting antiviral
-
- Drp1
-
- dynamin-related protein 1
-
- EGFP
-
- enhanced green fluorescent protein
-
- G-Rg3
-
- ginsenoside Rg3
-
- HCV
-
- hepatitis C virus
-
- HCVcc
-
- cell culture–derived HCV
-
- IFN
-
- interferon
-
- IgG
-
- immunoglobulin G
-
- Mfn2
-
- mitofusin 2
-
- mito-RFP-GFP
-
- signal peptide targeting mitochondria–red fluorescence protein–green fluorescence protein
-
- ND2
-
- mitochondrially encoded reduced nicotinamide adenine dinucleotide dehydrogenase subunit 2
-
- NS
-
- nonstructural protein
-
- p-
-
- phosphorylated
-
- TOM20
-
- translocase of outer mitochondrial membrane 20
-
- VDAC1
-
- voltage-dependent anion channel 1
Hepatitis C virus (HCV) infects 2-3% of the world population and is the leading cause of hepatocellular carcinoma and end-stage liver disease requiring liver transplantation. In contrast to most other viral infections, the hallmark of HCV infection is that a majority of patients develop chronic infection after viral exposure. Unfortunately, to date, an effective vaccine is not available for HCV.1
The standard treatment option for chronic hepatitis C (CHC) patients traditionally had been a combination of pegylated interferon (IFN) and ribavirin. However, this combinatorial treatment showed suboptimal efficacy in viral responses along with severe adverse reactions. The current standard therapy for CHC patients is direct-acting antivirals (DAAs), which have reached well-established efficacy. Telaprevir and boceprevir are the first wave of protease inhibitors that were introduced in 2011,1 and currently, many second-generation DAAs specifically targeting HCV nonstructural protein (NS) 3 protease and NS5A and NS5B polymerases are being used for the treatment of CHC patients.2, 3 The application of a pegylated IFN–free regimen with DAAs has shown very high sustained virologic response rates, up to nearly 100%, with insignificant side effects.3 Second-generation DAAs including sofosbuvir as a monotherapy or combined sofosbuvir and ledipasvir are currently prevalent in clinical practice.1, 3, 4
Sofosbuvir is an oral nucleotide analogue inhibitor that targets the HCV NS5B polymerase.1 Sofosbuvir-based IFN-free therapies for patients with HCV genotype 1 infection include sofosbuvir or ledipasvir with or without ribavirin for 12 weeks. Sofosbuvir plus ribavirin for 12 weeks (or 16 weeks for cirrhosis) is recommended as a treatment against HCV genotype 2 in the guidelines of both the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. However, information on the clinical outcomes of these new DAAs including adverse effects and other limitations has not been reported.1, 3, 4
Mitochondrial dynamics is crucial for the regulation of cell homeostasis.5 Mitochondria are readily damaged by various physiological changes induced by HCV infection, resulting in disruption of mitochondrial membrane potential (ΔΨm) and subsequent mitochondrial dysfunction followed by apoptosis.6-9 We have recently shown that HCV perturbs mitochondrial dynamics by promoting dynamin-related protein 1 (Drp1)–mediated mitochondrial fission followed by Parkin-mediated mitophagy, which is associated with attenuation of HCV-induced apoptosis and innate immune response.8, 9
Ginseng, as a traditional herbal medicine, has been widely used in Asian medicine.10, 11 The ginsenoside compounds in ginseng are known to exert a wide range of pharmacologic and immunologic effects.10, 11 The National Center for Complementary and Integrative Health, one of the centers of the US National Institutes of Health, has been supporting research aimed at obtaining a better understanding of the potential of Asian ginseng to treat various diseases, including its interactions with other herbs and drugs.12 In this study, we show that ginsenoside Rg3 (G-Rg3), one of the ginsenosides, exhibits a strong antiviral activity against HCV infection. G-Rg3 inhibits HCV-induced abnormal mitochondrial fission and mitophagy, which supports persistent viral infection. In doing so, it reverses the damage caused by viral infection when used in combination with anti-HCV DAAs. These results together reinforce the homeostatic effects of Rg3 in HCV treatment regimens.
Materials and Methods
CELL CULTURE AND VIRUS
Human hepatoma Huh7 and Huh7.5.1 cells were grown in high-glucose Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% minimal essential medium nonessential amino acids (Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco). The R-1 HCV subgenomic replicon cells were maintained in Dulbecco's modified Eagle's medium with 0.5 mg/mL G418.13 Cell culture–derived HCV Japanese fulminant hepatitis 1 genotype 2a (HCVcc) was propagated and prepared as described.14 HCV infection was performed at a multiplicity of infection of 3.
IMMUNOFLUORESCENCE
HCV-infected cells and those posttreated with G-Rg3 (100 μM) were grown on coverslips and used in immunofluorescence assays as described.8, 9, 15 For monitoring mitophagic process, Huh7 cells transfected with phosphorylated signal peptide targeting mitochondria–red fluorescence protein–enhanced green fluorescence protein (p-mito-mRFP-EGFP) reporter were infected with HCVcc for 1 day and then treated with G-Rg3. At 2 days posttreatment, cells were immunostained with HCV core antibody. Confocal images were visualized under a 100 × oil objective using an Olympus FluoView 1000 confocal microscope or Zeiss LSM700 laser scanning confocal microscope. Analyses of colocalization of proteins and mitochondrial lengths were quantified by ImageJ software and FV10-ASW 3.0 viewer software (Olympus), respectively.
PLASMIDS, ANTIBODIES, AND REAGENTS
The p-mito-mRFP-RFP and pFLAG-CMV-HCV core DNA plasmids used in this study have been described.8, 15, 16 The primary antibodies used in this study include the following: rabbit monoclonal anti-Drp1 (Cell Signaling), rabbit monoclonal anti-phospho-Drp1 (Ser616; Cell Signaling), mouse monoclonal anti-HCV core (Thermo Scientific), rabbit polyclonal anti-β-actin (Cell Signaling), mouse monoclonal anti–translocase of outer mitochondrial membrane 20 (TOM20; BD Biosciences), human monoclonal anti-HCV E2,17 mouse monoclonal anti-HCV NS3 (Abcam), mouse monoclonal anti-mitofusin 2 (Mfn2; Abcam), and rabbit polyclonal anti–voltage-dependent anion channel 1 (VDAC1; Cell Signaling). The secondary antibodies used for western blot analysis were horseradish peroxidase–conjugated antimouse immunoglobulin G (IgG) and horseradish peroxidase–conjugated antirabbit IgG (both from Promega). The secondary antibodies for immunofluorescence were Alexa Fluor 350, 488, 594, or 647 donkey antimouse, rabbit, or goat IgG and Alexa Fluor 555 goat antihuman IgG (all from Molecular Probes). The chemical reagents used in this study were carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Sigma), sofosbuvir (Selleckchem), and ginsenoside compounds (Sigma).
WESTERN BLOT ANALYSIS
For western blot analysis, whole-cell lysates were extracted from cells, homogenized, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane (Thermo Scientific). Western blot analysis with antibodies against the indicated proteins was then performed as described.8, 9, 15 Densitometric graphs were generated using Image J (National Institutes of Health).18
REAL-TIME QUANTITATIVE RT-PCR
Real-time quantitative RT-PCR analysis of HCV RNA, mitochondrially encoded reduced nicotinamide adenine dinucleotide dehydrogenase subunit 2 (ND2) and cyclooxygenase 2 (COX2) levels was performed as described.9
CELL VIABILITY ASSAY
A previously described cell viability assay9 was used to assess the cytotoxic effects of ginsenoside compounds during HCV infection.
ΔΨm MEASUREMENT
Huh7 cells infected with HCVcc or treated with sofosbuvir, telaprevir, boceprevir, and CCCP at the indicated concentrations were stained with JC-1 reagent (Invitrogen) for 1 hour and immediately used for fluorescence-activated cell sorting analysis.
HUMAN LIVER BIOPSY SPECIMENS
Frozen human liver biopsy specimens (n = 6) were collected in the Soonchunhyang University Hospital Seoul from anti-HCV-negative patients (n = 3) and anti-HCV-positive patients (n = 3). All three anti-HCV-negative patients were nearly at the complete healing stage of drug-induced hepatitis, while all three anti-HCV-positive patients had active CHC. Liver biopsy samples were taken after obtaining written informed consent.
STATISTICAL ANALYSIS
Statistical analyses using the unpaired Student t test were performed using Sigma Plot software (Systat Software, San Jose, CA).
Results
SOFOSBUVIR CAUSES ABERRANT MITOCHONDRIAL FISSION
DAAs, particularly sofosbuvir, are key components in the current IFN-sparing treatment regimens for CHC patients. Despite the high efficacy of sofosbuvir, its role in altering mitochondrial dynamics and physiology has not been studied, although mitochondrial toxicity caused by DAAs has been reported.19 Because sofosbuvir is an effective and one of the most widely used DAAs, we first investigated if sofosbuvir perturbs mitochondrial dynamics associated with modulation of mitochondrial structure and function. We examined the changes in ΔΨm in the presence of sofosbuvir. Treatment of human hepatoma Huh7 cells with sofosbuvir at the half-maximal effective concentration induced a decrease in the ΔΨm (Fig. 1A). Similar results were obtained in cells treated with the mitochondrial uncoupler CCCP, which causes mitochondrial depolarization (Fig. 1A; Supporting Fig. S1A).20 We also observed that telaprevir and boceprevir, which are HCV NS3 protease inhibitors, cause ΔΨm loss (Supporting Fig. S1A). An increase in depolarized mitochondria triggers the mitochondrial recruitment of phosphorylated Drp1 (Ser616), which induces mitochondrial fission.21 Sofosbuvir induced distinct mitochondrial fission in human hepatoma cells, whereas dimethyl sulfoxide–treated cells showed the typical tubular network of mitochondria (red color in the figures indicates TOM20-positive mitochondrial morphology) (Fig. 1B; Supporting Fig. S1B,C). Telaprevir and boceprevir also induced mitochondrial fragmentation (white color in Supporting Fig. S1B indicates MitoTracker-positive mitochondria morphology). Further, confocal microscopy revealed that the expression level of p-Drp1 (Ser616) was significantly higher in sofosbuvir-treated cells than in dimethyl sulfoxide–treated control cells as indicated by the p-Drp1 (Ser616) intensity immunostained green in Fig. 1B. More importantly, sofosbuvir promoted mitochondrial recruitment of p-Drp1 (Ser616), as evidenced by colocalization of p-Drp1 (Ser616) and TOM20 (yellow spots in Fig. 1B), which supported the increase in Drp1-mediated mitochondrial fission seen in sofosbuvir-treated cells. Telaprevir and boceprevir also induced mitochondrial translocation of p-Drp1 (Supporting Fig. S2). The sofosbuvir-induced increase in the expression level of p-Drp1 was further analyzed by western blot assays using whole-cell lysates extracted from hepatoma cells treated with sofosbuvir (accompanying graph in Fig. 1C). Together, these results indicate that HCV inhibitors such as sofosbuvir, telaprevir, and boceprevir induce loss of ΔΨm followed by mitochondrial translocation of p-Drp1 (Ser616), which promotes Drp1-mediated mitochondrial fission.
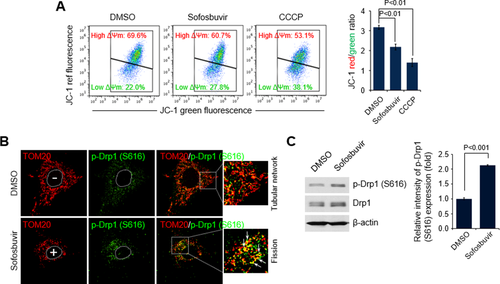
Sofosbuvir induces mitochondrial damage and Drp1-mediated mitochondrial fission. (A) Fluorescence-activated cell sorting analysis showing decrease in ΔΨm in the presence of sofosbuvir. At 24 hours after treatment with sofosbuvir (100 nM), Huh7 cells were stained with JC-1 dye and then analyzed on a flow cytometer. The mitochondrial uncoupler CCCP (5 μM) was used as a control. The accompanying graph indicates that sofosbuvir induced decreases in the level of ΔΨm, as indicated by the JC-1 red/green ratio (right panel). (B) Representative confocal microscopic image showing p-Drp1 translocation to mitochondria and mitochondrial fission in the presence of sofosbuvir. At 24 hours after treatment with sofosbuvir (100 nM), Huh7 cells were immunostained with antibodies against TOM20 (red) and p-Drp1 (S616; green). Nuclei are demarcated with white dotted circles. Treated (+) and untreated (–) cells are marked. In the zoomed images, the arrows indicate the colocalization of TOM20 and p-Drp1 (S616) in sofosbuvir-treated cells (yellow spots). (C) Western blot analysis of p-Drp1 (S616) and Drp1 expression in sofosbuvir-treated cells. Whole-cell lysates extracted from Huh7 cells treated with sofosbuvir (100 nM) for 24 hours were analyzed by immunoblotting with antibodies specific to p-Drp1 (S616) and Drp1 proteins. β-actin was used as an internal loading control. The accompanying graph indicates that sofosbuvir stimulates Drp1 phosphorylation (right panel). Abbreviation: DMSO, dimethyl sulfoxide.
G-Rg3 INHIBITS HCV PROPAGATION
Cytoprotective effects of ginsenosides have been implicated in the treatment for many diseases including bacterial and viral infections.11 Furthermore, its effects on mitochondrial dynamics have also been reported.22, 23 We reasoned that these cytoprotective roles of ginsenosides may counter the adverse effects of antiviral DAAs seen in patients under treatment.
To investigate the antiviral effect of ginsenosides in HCV-infected cells, we first screened several representative ginsenoside compounds isolated from the root of Korean red ginseng (Panax ginseng C.A. Meyer).10 To conduct in vitro screening with ginsenoside compounds, we established an in vitro HCV infection system using HCVcc24 and Huh7.5.1, a highly permissive cell line for HCV infection.25 The infectivity of HCVcc in Huh7.5.1 cells was confirmed using confocal microscopy and immunostaining with an antibody specific to HCV core protein (Fig. 2A).
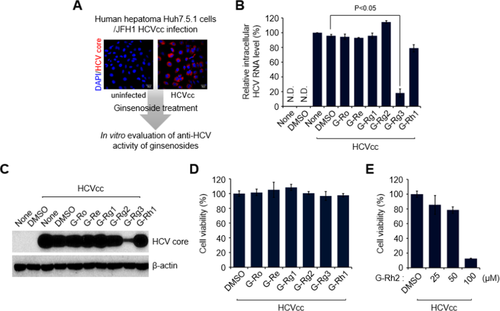
G-Rg3 inhibits HCV propagation. (A) A strategy for screening ginsenosides that shows antiviral effects during HCV propagation. Huh7.5.1 cells infected with Japanese fulminant hepatitis 1 HCVcc for 1 day at a multiplicity of infection of 5 were treated with various ginsenosides at 100 μM. At 2 days posttreatment, cells were harvested and used for analyses of intracellular HCV RNA (B) and protein expression (C). Confocal-microscopic images show HCV core protein expression (red) in uninfected (left) and infected (right) cells. Nuclei are immunostained with 4′,6-diamidino-2-phenylindole (blue). (B) Intracellular HCV RNA levels were analyzed by real-time quantitative RT-PCR as described in the Materials and Methods. Glyceraldehyde 3-phosphate dehydrogenase was used as the control for determining the normalized changes in HCV RNA expression. (C) Western blot analysis showing the reduction in HCV core protein expression induced by G-Rg3 treatment. Whole-cell lysates extracted from HCV-infected cells were analyzed by immunoblotting with an antibody specific to HCV core protein. (D) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay data showing the viability of HCV-infected cells treated with ginsenosides for 2 days. Cell viability was measured as described in Materials and Methods. (E) Viability of HCV-infected cells treated with G-Rh2. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; JFH1, Japanese fulminant hepatitis 1.
These analyses revealed that G-Rg3 remarkably suppresses the level of HCV RNA, as determined by real-time quantitative RT-PCR with primers specific to the HCV 5′-untranslated region (Fig. 2B). Also, western blot and cell viability assays showed that G-Rg3 reduces the expression level of HCV core protein in HCV-infected cells without cellular cytotoxicity (Fig. 2C,D). However, treatment of HCV-infected cells with G-Rh2, which is a protopanaxadiol type of ginsenoside like G-Rg3, induced very high cytotoxicity (Fig. 2E). These results suggest that G-Rg3 effectively inhibited HCV propagation.
G-Rg3 RESTORES HCV-INDUCED ABERRANT MITOCHONDRIAL FISSION
We have recently shown that HCV induces Drp1-mediated mitochondrial fission, which promotes robust HCV propagation.8 To examine an inhibitory mechanism of G-Rg3 in robust HCV infections, we analyzed the role of G-Rg3 in ΔΨm loss caused by HCV infection6, 26 because the HCV-induced loss of ΔΨm leads to mitochondrial fission.6, 8, 26 It is known that G-Rg3 inhibits the opening of mitochondrial permeability transition pores by free radical scavenging action.27 Consistent with our previous study,6 HCV infection decreased ΔΨm compared to uninfected cells (Fig. 3A).28, 29 Further, the HCV-induced loss of ΔΨm was remarkably restored by G-Rg3 treatment (third panel and accompanying graph in Fig. 3A). We also observed that G-Rg3 restored the ΔΨm loss caused by DAA treatment (Supporting Fig. S1A).
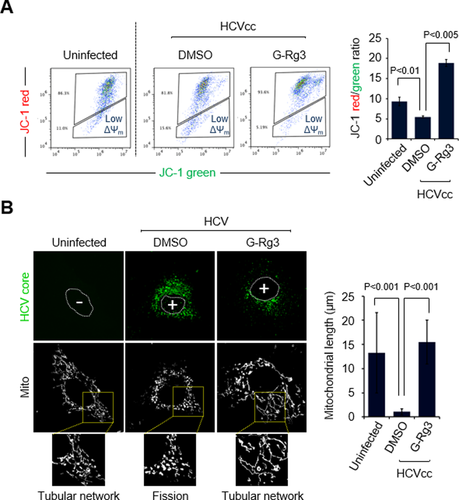
G-Rg3 inhibits HCV-induced mitochondrial fission. (A) Representative fluorescence-activated cell sorting analysis showing restoration of HCV-induced reduction of ΔΨm in the presence of G-Rg3. Cells infected with HCVcc for 1 day were treated with G-Rg3 (100 μM). At 1 day posttreatment, HCV-infected Huh7 cells were stained with JC-1 dye and then analyzed on a flow cytometer. The accompanying graph indicates that G-Rg3 restores the HCV-induced decrease in the level of ΔΨm, as indicated by the JC-1 red/green ratio (right panel). Data are averages of two independent experiments. (B) Representative confocal images showing the mitochondrial tubular network in HCV-infected cells treated with G-Rg3. Huh7 cells infected with HCVcc at a multiplicity of infection of 1 were treated with G-Rg3 (100 μM). At 1 day posttreatment, cells prestained with MitoTracker (white) were immunostained with HCV core antibody (green). Nuclei are demarcated with white dots. Infected (+) and uninfected (–) cells are marked. In the zoomed images, uninfected (left panel) and HCV-infected/G-Rg3 (right panel) cells show the typical tubular mitochondrial network, whereas HCV-infected cells (middle panel) display a fragmented mitochondrial structure. The accompanying graph is a quantitative analysis of mitochondrial length in left confocal images (mean ± standard error of the mean; n = 10 mitochondria, two independent experiments). Abbreviations: DMSO, dimethyl sulfoxide; Mito, MitoTracker.
We next investigated if G-Rg3 affects HCV-induced aberrant mitochondrial fission. Consistent with our previous results,8 HCV infection induced mitochondrial fission (second panel in Fig. 3B), whereas confocal microscopic analysis of uninfected cells using immunostaining with MitoTracker displayed the typical tubular network of mitochondria (first panel in Fig. 3B). Interestingly, G-Rg3 treatment inhibited HCV-induced mitochondrial fission formation and displayed a normal tubular mitochondrial network (third panel in Fig. 3B). This effect is similar to silencing the mitochondrial fission factor or Drp1 and results in mitochondrial fusion status.8 The accompanying graph in Fig. 3B indicates the restoring effect of G-Rg3 using quantification data of the mitochondrial length during HCV infection. The present data demonstrate that G-Rg3 prevented the HCV-induced loss of ΔΨm and inhibited HCV-induced aberrant mitochondrial fission.
G-Rg3 SUPPRESSES HCV PROPAGATION BY DOWN-REGULATION OF Drp1
We have shown that HCV induces mitochondrial translocation of p-Drp1, leading to mitochondrial fission.8 Therefore, we used confocal microscopy to further examine whether G-Rg3 inhibits mitochondrial translocation of p-Drp1. As indicated by the yellow spots in the zoomed image of the third panel in Fig. 4A, HCV-infected cells showed activation and translocation of p-Drp1 onto fragmented mitochondria. In contrast, HCV-infected cells treated with G-Rg3 displayed no translocation of p-Drp1 on the tubular mitochondrial network (red mitochondrial morphology in the zoomed image of the fourth panel in Fig. 4A). The accompanying graph in Fig. 4A shows the quantitative colocalization of p-Drp1 and mitochondrial marker TOM20.
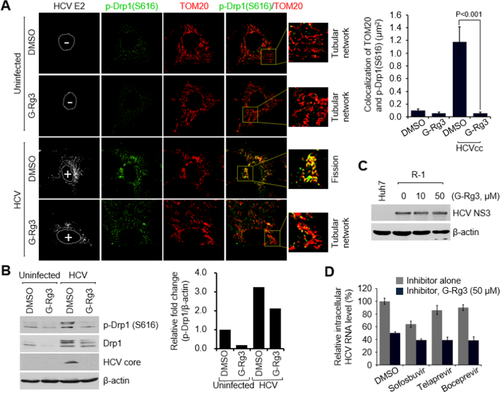
G-Rg3 inhibits HCV-induced mitochondrial recruitment of Drp1. (A) Confocal microscopic images showing inhibition of Drp1 translocation to mitochondria in HCV-infected cells in the presence of G-Rg3. At 2 days after treatment with G-Rg3 (100 μM), Huh7 cells infected with HCVcc were immunostained with antibodies against TOM20 (red), p-Drp1 (S616; green), and HCV E2 protein (white). Nuclei are demarcated with white dotted circles. Infected (+) and uninfected (–) cells are marked. In the zoomed images, the arrows indicate the colocalization of TOM20 and p-Drp1 (S616) in HCV-infected cells (yellow spots). The accompanying graph is a quantitative analysis of the colocalization of TOM20 and p-Drp1 (S616) in left panel (mean ± standard error of the mean; n = 10 mitochondria, two independent experiments). (B) Western blot analysis of p-Drp1 (S616) and Drp1 expression in HCV-infected cells treated with G-Rg3. Whole-cell lysates extracted from HCV-infected cells treated with G-Rg3 (100 μM) for 2 days were analyzed by immunoblotting with antibodies specific to p-Drp1 (S616), Drp1, and HCV core protein. β-Actin was used as an internal loading control. The accompanying graph is quantitation of western blot data in left panel. (C) Western blot analysis showing the antiviral effect of G-Rg3 in R-1 HCV subgenomic replicon cells. Whole-cell lysates of R-1 cells treated with G-Rg3 were analyzed by immunoblotting with antibodies specific to HCV NS3 protein. β-Actin was used as an internal loading control. (D) HCV-infected Huh7 cells were treated with various HCV inhibitors at 10 nM (representing an approximate 10-fold dilution of half-maximal effective concentration) or in combination with G-Rg3 (50 μM). At 1 day posttreatment, cells were harvested and used for analyses of intracellular HCV RNA by real-time quantitative RT-PCR as described in Materials and Methods. Glyceraldehyde 3-phosphate dehydrogenase was used as the control for determining the normalized changes in HCV RNA expression. Abbreviation: DMSO, dimethyl sulfoxide.
Drp1 translocation to mitochondria requires that Drp1 is phosphorylated at the Ser616 position. We analyzed the expression level of Drp1 (S616) using whole-cell lysates prepared from HCV-infected cells treated with G-Rg3. Analysis of these lysates shows that HCV-infected cells treated with G-Rg3 display decreased levels of Drp1 phosphorylation (Fig. 4B). There was also a modest reduction in the expression levels of Drp1 protein in these lysates. Together, these data suggest that G-Rg3 regulates Drp1 phosphorylation and subsequent mitochondrial translocation, thereby preventing HCV-induced abnormal mitochondrial fission.
Because HCV-induced mitochondrial fission is associated with virus secretion,8 we investigated if G-Rg3 affects HCV secretion. We first determined whether G-Rg3 inhibits viral replication in R-1 cells harboring HCV subgenomic replicon, supporting only HCV replication.13 G-Rg3 did not inhibit HCV replication of HCV subgenomic replicon (Fig. 4C). Thus, it is likely that the inhibitory effect of G-Rg3 is associated with viral secretion but not with replication. We further examined if G-Rg3 has any synergistic anti-HCV effect in combination with known HCV antivirals which inhibit HCV. We chose a 50 μM concentration of Rg-3, which does not affect viral replication, to evaluate its combinatorial synergic effect. Sofosbuvir and other nucleotide analogues used in this study inhibit viral replication. Rg3 treatment produced a synergic anti-HCV effect with sofosbuvir, telaprevir, and boceprevir and suppressed viral propagation (Fig. 4D).
G-Rg3 INHIBITS HCV-INDUCED DEGRADATION OF CYTOPLASMIC p21
HCV stimulates the activity of cyclin-dependent kinase 1 (CDK1), which promotes Drp1 phosphorylation at Ser616 in the cytoplasm.8 The subsequent mitochondrial translocation of p-Drp1 mediates mitochondrial fission during HCV infection.8, 21, 30 CDK1 activity is central to Drp1 phosphorylation at Ser616 in the cytosol during HCV infection.30 To examine how HCV promotes CDK1 activity, we analyzed the expression level of the cytoplasmic p21 protein, which directly inhibits CDK1 activity.31 The expression level of cytosolic p21 protein was strikingly lower in liver biopsy materials than in the control materials (see the light brown color in the cytoplasmic area in Fig. 5A). Cytoplasmic p21-positive cells are quantified in Fig. 5B, which clearly shows that HCV down-regulates cytoplasmic p21 expression to promote CDK1 activity.
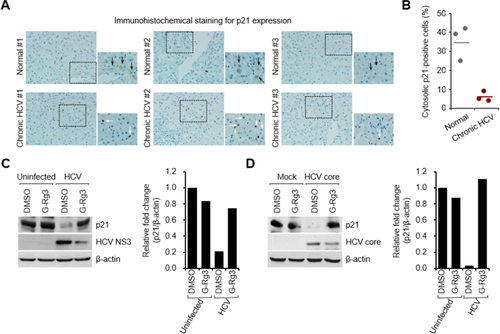
G-Rg3 restores the HCV-induced decrease in p21 expression. (A) Immunohistochemical analysis showing the reduction of cytosolic p21 expression in liver biopsy materials from patients with CHC. The immunohistochemical assay was performed with a specific antibody for p21 protein (dark brown). In the zoomed images, black arrows (dark brown) indicate the cytosolic expression of p21 protein, whereas white arrows indicate p21-positive staining in nuclei in HCV-infected tissue. (B) Quantitative analyses of p21-positive signals targeting the cytoplasm in (A). (C,D) Western blot analyses showing rescue of the decreased cytosolic p21 expression level in G-Rg3-treated, HCV-infected cells (C) and HCV core protein–transfected cells (D). Crude cytosolic fractions isolated from G-Rg3-treated, HCV-infected cells (C) and HCV core protein–transfected cells (D) were analyzed by immunoblotting with antibodies specific to p21 and HCV core protein. β-Actin was used as an internal loading control. Accompanying graphs show quantitation of western blot data in left panel. Abbreviation: DMSO, dimethyl sulfoxide.
To substantiate the role of G-Rg3 in down-regulated cytoplasmic p21 during HCV infection, we prepared a cytosolic fraction from HCV-infected cells and analyzed by western blot assays using an antibody specific to p21 protein. Figure 5C shows that HCV infection caused a decrease in the expression level of cytoplasmic p21. Surprisingly, this reduction was rescued by G-Rg3 treatment.
HCV core protein is a strong inhibitor of p21 expression.32 To further investigate the role of G-Rg3 in HCV core protein–dependent regulation of cytoplasmic p21 expression during HCV infection, we isolated the cytosolic fraction from cells transiently expressing HCV core protein and then analyzed the expression level of cytoplasmic p21 protein. Similar to the HCV-induced decrease in cytoplasmic p21 expression shown in Fig. 5C, HCV core protein dramatically reduced the expression level of cytosolic p21 (Fig. 5D). This reduction was again rescued by G-Rg3 treatment. These data collectively suggest that restoration of cytosolic p21 by G-Rg3 during HCV infection (Fig. 5C) was not due to inhibition of HCV propagation by G-Rg3 but that G-Rg3 reversed the reduction in HCV core protein–dependent cytoplasmic p21 expression during HCV infection. In summary, G-Rg3 increased p21 and decreased CDK1 activity, which has a direct bearing on Drp1 phosphorylation and mitochondrial fission.
G-Rg3 INHIBITS HCV-INDUCED ABNORMAL MITOPHAGY
Mitochondrial fission usually triggers mitophagy orchestrated by activation of PINK1 and Parkin recruitment to the mitochondria.33 To investigate the effect of G-Rg3 on HCV-induced mitophagy, we used a novel vector containing mRFP-enhanced GFP (EGFP) dual luciferase gene fused in frame with a mitochondrial targeting sequence (mito-mRFP-EGFP), as described (Fig. 6A).8, 15, 34 The hybrid protein displays a yellow image when expressed in mitochondria. However, if mitochondria fuse with lysosomes to complete the process of mitophagy, only RFP (red) is expressed as EGFP (green) is unstable in the acidic environment of lysosomes and quenched off.35, 36 In cells undergoing complete mitophagy, red puncta are visible, indicating expression of this hybrid protein in the lysosomes.35, 36 Consistent with our previous observation,8, 15 HCV infection induced mitochondrial fission and mitophagy (Fig. 6B, middle panel, and C). However, in the presence of G-Rg3, mitophagy was inhibited (Fig. 6B, lower panel). During HCV infection, Parkin, the key mediator of mitophagy, recruited to the mitochondria ubiquitinates and degrades outer mitochondrial substrates like VDAC1 and Mfn2.9 To determine the function of G-Rg3 in mitochondrial Parkin activity during HCV infection, we performed western blot analysis of cellular lysates using specific VDAC1 and Mfn2 antibodies. G-Rg3 prevented ubiquitin degradation of VDAC1 and Mfn2 proteins (Fig. 6D and accompanying graph). We further show that G-Rg3 inhibited the HCV-induced decreases in the gene expression of mitochondrial DNA, ND2, and COX2 proteins (Fig. 6E).
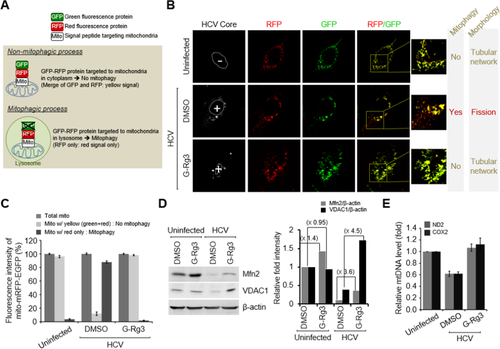
G-Rg3 inhibits HCV-induced mitophagy. (A) A system for monitoring the mitophagosomal maturation process by which mitophagosomes are delivered to lysosomes (mitophagy) using a dual fluorescence reporter/sensor, p-mito-mRFP-EGFP. Lysosomal delivery of the tandem fusion protein mito-mRFP-EGFP targeting entire mitochondria results in differential quenching and degradation of the two individual fluorochromes, thereby allowing for visual analysis of mitophagic flux. (B) Confocal microscopic images showing G-Rg3-mediated inhibition of HCV-induced mitophagy. HCV-infected cells transiently expressing mito-RFP-GFP were treated with G-Rg3 (100 μM) for 48 hours and then immunostained with anti-HCV core antibody (white). Nuclei are demarcated with white dotted circles. Infected (+) and uninfected (–) cells are marked. The fluorescence signals in the zoomed images indicate the expression of mito-RFP-GFP targeting mitochondria: yellow color, no mitophagy; red color, mitophagy. (C) Quantitative analyses of the fluorescence signals targeting mitochondria in (A). (D) Western blot analyses of Mfn2 and VDAC1 expression in HCV-infected cells treated with G-Rg3. Whole-cell lysates extracted from HCV-infected cells treated with G-Rg3 (100 μM) for 2 days were analyzed by immunoblotting with antibodies specific to Mfn2 and VDAC1 protein. β-Actin was used as an internal loading control. (E) Real-time quantitative RT-PCR analysis of mitochondrial DNA level in HCV-infected cells treated with G-Rg3. Mitochondrial ND2 and COX2 DNAs isolated from HCV-infected cells treated with G-Rg3 (100 μM) for 2 days were analyzed by real-time quantitative PCR with primers specific to ND2 and COX2 genes. β-actin was used for normalization. Abbreviation: DMSO, dimethyl sulfoxide; mtDNA, mitochondrial DNA.
The effect of G-Rg3 on innate immune responses during HCV infection was also examined (Supporting Fig. S3). G-Rg3-treated cells showed comparable antiviral responses with nontreated control during HCV infection. Therefore, it is highly likely that G-Rg3 suppresses HCV propagation not by immune responses but by performing a protective role against HCV-induced mitophagy. These results collectively suggest that G-Rg3 rescues HCV-infected cells from mitophagy.
Discussion
G-Rg3 is an effective potential candidate drug for treating hepatitis C infection along with other DAAs. G-Rg3 predominantly displayed antiviral activities in HCV-infected cells. G-Rg3 restored HCV-induced aberrant mitochondrial fission and had a synergic antiviral effect in combination with sofosbuvir, telaprevir, and boceprevir, respectively.
Nucleotide inhibitors that were incorporated by the mitochondrial RNA polymerase inhibited mitochondrial protein synthesis and produced a corresponding decrease in mitochondrial oxygen consumption in cells. In contrast, nucleotide inhibitors containing multiple ribose modifications, including the active forms of mericitabine and sofosbuvir, were poor substrates for mitochondrial RNA polymerase and did not show mitochondrial toxicity in cells.37 However, we observed unequivocal reductions in ΔΨm when sofosbuvir, telaprevir, or boceprevir was present at half-maximal effective concentration. An increase in depolarized mitochondria promotes mitochondrial recruitment of cytosolic p-Drp1, thereby facilitating mitochondrial fission. More importantly, sofosbuvir promoted the mitochondrial recruitment of p-Drp1, as evidenced by the colocalization of p-Drp1 and TOM20, inducing increases in Drp1-mediated mitochondrial fission in sofosbuvir-treated cells. Together, these mechanisms result in sofosbuvir-induced loss of ΔΨm and Drp1-mediated mitochondrial fission. Recognized adverse effects of nucleotide inhibitors include fatigue, headache, muscle ache, nausea, and insomnia; and these may be related to mitochondrial toxicity.
Mitochondrial fission is associated with robust HCV propagation, with our recent data showing that the inhibition of mitochondrial fission by silencing Drp1 and mitochondrial fission factor suppressed HCV secretion.8 Along the same line as the inhibition of mitochondrial fission, we showed that G-Rg3 inhibits Drp1-mediated mitochondrial fission, thereby suppressing HCV secretion. A preventive effect of G-Rg3 on abnormal mitochondrial fission caused by HCV infection or on HCV inhibitors could also provide improved therapies for CHC. Thus, using G-Rg3 in combination with sofosbuvir is likely to reduce the treatment costs if a regimen involving sofosbuvir at a low concentration and G-Rg3 can be established.
In the era of DAAs, meeting the demand for effective therapies for harder-to-treat populations is a huge concern for policymakers. HCV management is required in harder-to-treat populations with DAAs, which include elderly patients and patients with decompensated liver cirrhosis, renal impairment, and the emergence of resistance-associated variants. Moreover, those who do not respond to pegylated IFN plus ribavirin treatment are also difficult to treat. The high cost of DAAs might reduce accessibility to patients, thus restricting social benefits. It may be necessary to stratify and prioritize patients based on cost-effectiveness, stage of disease, and potential gain from treatment. However, the cost of treatment might decrease as more curative drugs become licensed, so drugs exhibiting comparable efficacy and lower cost compared to DAAs are needed.
G-Rg3 was a strong inhibitor of HCV propagation, restoring HCV-induced abnormal mitochondrial fission followed by mitophagy by inhibiting activation of the CDK1–Drp1 pathway. CDK1 activity was modulated by cytosolic p21, and G-Rg3 restored the p21 expression level that had been suppressed by HCV core protein (Fig. 7). These observations indicate that G-Rg3 is a strong and safe suppressor of HCV infection. Its antiviral mechanism involves modulating HCV-induced aberrant mitochondrial dynamics, thereby suppressing persistent HCV infection. Also, G-Rg3 acts as a synergistic and complementary agent with nucleotide inhibitors, especially sofosbuvir. Our data suggest that G-Rg3 is a valuable new candidate for treating HCV patients either as a monotherapy or in combination with sofosbuvir. Possible application of G-Rg3 for other viral infections is presently being investigated.38, 39
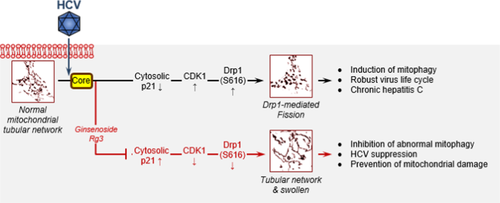
Schematic of the function of G-Rg3 in mitochondrial dynamics perturbed by HCV infection. HCV infections induce endoplasmic reticulum stress and trigger calcium leakage from the endoplasmic reticulum, resulting in mitochondrial oxidative stress and an altered membrane potential, which in turn causes mitochondrial depolarization and induces mitochondrial fission initiated by the mitochondrial translocation of p-Drp1. HCV core protein induces degradation of cytosolic p21 expression that leads to up-regulation in CDK1 and p-Drp1 that can be modulated by G-Rg3 treatment.
Despite the promising potential of G-Rg3 as a multifunctional drug candidate including anticancer,40 antiangiogenesis,41 liver protection,42 immune enhancement, and hypertension management, with a neuroprotective effect,43 there could be some concern regarding its metabolic consequences. For instance, G-Rg3 can be quickly metabolized to protopanaxadiol and G-Rh2 by bacteria in the intestinal tract with diverse intestinal microbial flora,44, 45 resulting in different clinical output depending upon the individual. In addition, G-Rh2 is known to have an anticancer effect but can be highly toxic to cells (Fig. 2E). Therefore, the use of G-Rg3 compound as an antiviral should be evaluated with caution.
Acknowledgment
We thank Dr. Takaji Wakita (National Institute of Infectious Disease, Japan) for providing the HCV phosphorylated Japanese fulminant hepatitis 1 plasmid, Dr. Francis V. Chisari (The Scripps Research Institute, La Jolla, CA) for providing Huh7.5.1 cells, Dr. Mansun Law (The Scripps Research Institute, La Jolla, CA) for providing the HCV E2 (AR3A) antibody, and Dr. Jong-Won Oh (Yonsei University, Seoul, Korea) for providing the R-1 HCV subgenomic replicon cells.




