Human embryonic stem cell–derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection
Potential conflict of interest: Nothing to report.
Supported by the National Key Research and Development Program of China (2016YFC1101305), the National High Technology Research and Development Program of China (2012AA020501), and the National Natural Science Foundations of China (31370990, 81170388, 81471948, 31271469, and 81572398).
Abstract
Maturation of hepatic cells can be gradually acquired through multiple stages of hepatic lineage specification, while it is unclear whether hepatitis C virus (HCV) infection is maturationally lineage-dependent. We investigated the susceptibility to HCV at multiple stages of human embryonic stem cells, definitive endodermal cells, hepatic stem cells, hepatoblasts (hHBs), and mature hepatocytes. Susceptibility to infection occurred initially at the stage of human hepatic stem cells; however, hHBs proved to have the highest permissiveness and infectivity compared with all other stages. The hHBs' susceptibility to HCV correlated with the translocation of occludin, an HCV receptor, from cytoplasm to plasma membrane of HBs. Vascular endothelial cell growth factor enhanced the HCV susceptibility of hHBs through rearrangement of occludin by dephosphorylation; this minimized hHB polarization and prevented hHBs from further maturation. The transcription profiles of different hepatic lineage stages indicated that expression of innate immune response genes was correlated with hepatic maturation; interferon β played an important role in protecting hHBs from HCV infection. HCV-infected hHBs were able to engraft and integrate into the livers of Fah–/–Rag2–/– mice and maintained an hHB phenotype for over 12 weeks during the time when HCV antigen was evident. After suppression of interferon β in hHBs, HCV infection was significantly enhanced in the engrafted humanized liver tissue of host mice. Conclusion: Human embryonic stem cell–derived hHBs are the optimal hosts for HCV infectivity; the realization that HCV entry and replication occur primarily at a particular hepatic lineage stage enables us to understand the HCV infection factors, life cycle, and infection dynamics that are facets of the pathogenesis as well as suggesting targets for anti-HCV treatment. (Hepatology 2017;66:717–735).
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- ALB
-
- albumin
-
- APO
-
- apolipoprotein
-
- CD
-
- cluster of differentiation
-
- DE
-
- definitive endoderm
-
- dpi
-
- days postinfection
-
- EpCAM
-
- epithelial cell adhesion molecule
-
- F/R
-
- Fah–/–Rag2–/–
-
- HCV
-
- hepatitis C virus
-
- HCVcc
-
- HCV produced in cell culture
-
- hDEC
-
- human DE cell
-
- hESC
-
- human embryonic stem cell
-
- hHB
-
- human hepatoblast
-
- hHep
-
- human hepatocyte
-
- hHpSC
-
- human hepatic stem cell
-
- IFN
-
- interferon
-
- IHC
-
- immunohistochemical
-
- ISG
-
- IFN-stimulated gene
-
- NS5
-
- nonstructural protein 5
-
- OCLN
-
- occludin
-
- si
-
- small interfering
-
- TJ
-
- tight junction
-
- VEGF
-
- vascular endothelial growth factor
Approximately 130-150 million people globally have chronic hepatitis C virus (HCV) infection. A significant number of those will develop liver cirrhosis or liver cancer, leading to about 700,000 deaths each year.1 Antiviral medicines are highly effective at treating people with HCV infection. However, the costs of diagnosis and treatment of HCV present a major challenge for the general population. HCV infection is primarily confined to the liver. Diagnosis of HCV using liver biopsy tissue from infected humans or chimpanzees has shown that only a minority of hepatocytes are infected (estimated 7%-20%).2, 3
It is impossible to study HCV in specific lineage stages of human hepatic cells in vivo given ethical concerns and technical challenges. Primary monolayer cultures of mature primary human hepatocytes have often been used as a standard model system consisting of uniform, polarized, and primarily terminally differentiated cells. Therefore, it is not feasible to assess the effects of HCV infection on any maturational lineage stage but these terminally differentiated ones. Recently, step-wise lineage induction strategies have been used generally to guide the process of hepatic differentiation from human embryonic stem cells (hESCs) or human induced pluripotent stem cells to definitive endodermal stem cells (hDECs), hepatic stem cells (hHpSCs), hepatoblasts (hHBs), and finally to hepatocytes (hHeps). This process resembles the progressive specification of hepatic lineage determinations during embryonic liver development.4-8 As a hepatotropic virus, HCV acquires its infectivity only after cells have specialized into hepatic epithelial lineage stages.9 Several reports have shown HCV infections in hepatocyte-like cell lineage restricted from hESCs or human induced pluripotent stem cells. In these studies, induced hepatocytes were used as an HCV infection cell model,9-13 facilitating an analysis of HCV infection dynamics during the hepatic epithelial lineage restriction process. This led us to investigate the kinetics of HCV infection during each stage of the entire lineage progression from hESCs to mature hepatocytes. We hypothesized that if a given lineage stage proved optimal for HCV, it might also be supportive for other hepatitis viruses, such as HBV or hepatitis E virus.
In the current study, we systematically investigated HCV infection at all the major lineage stages from hESCs to mature hepatocytes. We confirmed that the transition to permissiveness to HCV infection occurred at the HpSC stage, at which the cells were endowed with hepatic epithelia during the hESC hepatic lineage differentiation. It was just at that stage when the key HCV entry factor occludin (OCLN)14, 15 translocated from the cytoplasm to the cell membrane. Later on, integrated, sealed tight junction (TJ) and epithelia polarity were formed gradually when the cells matured from HBs to hepatocytes. In addition, innate antiviral immunity was functionally activated. Consistently, we found that HCV preferentially infected hHBs rather than mature hepatocytes. HCV infection was found to be significantly enhanced by vascular endothelial cell growth factor (VEGF) through negative regulation of cell polarity and TJ integrity and postponing cell maturation. HCV-infected hHBs could be engrafted into the livers of Fah–/–Rag2–/– (F/R) mice; HCV levels in the humanized livers were maintained for weeks. As a part of cell innate immunity, interferon β (IFNβ) played an important role in protecting the cells from HCV infection. After suppression of IFNβ in hHBs, HCV infection was significantly enhanced in the engrafted humanized liver tissue of host mice.
Overall, our study demonstrates that the hepatoblast stage is the most susceptible for HCV infections of all analyzed lineage stages and that human pluripotent stem cell–derived HBs can be used as an optimal model system for HCV infection study both in vitro and in vivo. The understanding of HCV infection–related host factors, HCV life cycle, and pathogenesis will benefit the pharmaceutical industry in evaluations of anti-HCV treatments.
Materials and Methods
CELL CULTURE AND DIFFERENTIATION
The H9 Human ESC line was purchased and maintained following the protocols provided by the WiCell Research Institute (Madison, WI). H9 cells were passaged and grown for 1 day in MTeSR (Stem Cell, Vancouver, Canada), then cultured in Kubota Media with Activin A (100 ng/ml) and Wnt3a (50 ng/ml) for 4 days to induce cells to definitive endoderm (DE) cells. The DE cells were differentiated in Hepatocyte Culture Medium (HM, Lonza, Allendale, NJ), Wnt3a (50 ng/ml), FGF4 (30 ng/ml), retinoic acid (RA, 0.1 μM) for 6 days to achieve lineage restrictions to hHpSCs. The hHBs were obtained by treating the hHpSCs with the HM, FGF4 (30 ng/ml), BMP4 (25 ng/ml) and HGF (25 ng/ml) for another 6 days. Finally differentiation to hepatocytes was achieved with HM with HGF (25 ng/ml), OSM (10 ng/ml) and dexamethasone (Dex, 0.1 μM). Recombinant human EGF (40 ng/ml) and VEGF165 (50 ng/ml) were added from the HpSC stage to obtain functional hHePs. Matrigel, Laminin, and collagen IV were from Becton Dickinson (BD, San Jose, CA). Recombinant human Activin A, Wnt3a, FGF4, BMP4, EGF, HGF and OSM were from R&D Systems (Minneapolis, MN). Dex was from Sigma-Aldrich (Saint Louis, MI). Recombinant human VEGF165 was from PeProtech (Rocky Hill, NJ).
IMMUNOFLUORESCENT AND IMMUNOHISTOCHEMISTRY ANALYSES
Immunofluorescent and Immunohistochemistry analyses were carried out as previously described.16, 17 Details and dilutions about antibodies were listed in Supporting Table 1.
FLUORESCENCE-ACTIVATED CELL SORTING (FACS)
FACS analysis was carried out as previously described.17 Details and dilutions about anti-Albumin and anti-AFP antibody were listed in Supporting Table 1.
TRANSMISSION ELECTRON MICROSCOPY (TEM)
The cells were washed twice with PBS and fixed by incubation for 48 hours in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M PBS. After washing with PBS, the cells were post-fixed by incubation for 1 hour with 1% osmium tetroxide and dehydrated in a graded series of ethanol solutions. Then the cells were embedded in Polybed 812 epoxy resin (Polysciences, Warrington, PA). Ultrathin sections were cut and collected on 200 mesh copper grids, stained with 4% aqueous uranyl acetate for 15 minutes, and then with Reynolds' lead citrate for 7 minutes. Stained sections were examined with a H7650 transmission electron microscope (HITACHI, Tokyo, Japan).
QUANTITATIVE REAL-TIME PCR
Total RNA was extracted from cells or freshly isolated tissue using an RNeasy Micro or Mini Extraction kit (Qiagen, Hilden, Germany). Total RNA (1 µg) was used for reverse transcription with ReverTra Ace qPCR RT master mix (Toyobo, Kita-ku Osaka, Japan). The qRT-PCR analysis was performed on a Bio-Rad iQ5 System using the SYBR Green PCR Master Mix (Toyobo). The relative expression of each gene was normalized against GAPDH. Primer sequences are listed in Supporting Table 2.
FUNCTIONAL ASSAYS
Albumin Secretion and Urea Synthesis
The hESC-derived hHeps were cultured in phenol red-free media supplemented with 20 mM ammonium chloride (Sigma-Aldrich) for 24 hours, and the supernatants were collected for albumin and urea concentration assays with human albumin ELISA kit (Bethyl Laboratory, Montgomery, TX) and the QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer's instructions respectively. PHHs isolated freshly from adult livers have been used as control.
Periodic Acid Schiff Staining
For glycogen detection, the hESC-derived hHeps were stained by periodic acid-schiff (PAS, Sigma-Aldrich) following the manufacturer's instructions.
Uptake of Indocyanine Green (ICG)
The hESC-derived hHeps were exposed to Indocyanine Green (ICG, Sigma-Aldrich) for 30 minutes at 37°C, 5% CO2. Then the cells were washed with PBS several times until the dye in the rinsing medium was undetectable. Images of cells were collected every 10 minutes for 3 hours after ICG incubation for Indocyanine green uptake analysis.
Polarization Assay
The hESCs-derived hHBs and hHePs were incubated with 5 µM 5(6)-carboxy-2', 7'-dichlorofluorescein diacetate (CDFDA, Invitrogen) at 37°C for 10 minutes to allow translocation to the bile canalicular lumen. Subsequently, cells in cultures were washed with ice-cold PBS and the cell images were captured by using a confocal microscope (PerkinElmer).
RNA SEQUENCING
The mRNA was extracted using RNeasy Micro Kit (Qiagen). RNA sequencing was performed on HiSeq X ten (Illumina, San Diego, CA). The low quality parts of raw reads were filtered. Reads were mapped to UCSC GRCh38 reference by Tophat version 2.0.11. Read counts of each gene were summarized using HTSeq version 0.6.1 p1. DESeq2 package was employed to detect differentially expressed genes (fold change above 2 with adjusted p-values below 0.01). Original data were uploaded to the Gene Expression Omnibus database (accession number GSE101133).
HCV INFECTION IN VITRO
The HCV produced in cell culture (HCVcc, genotype 1b) in the study was a generous gift of Dr. Congyi Zheng.18 Virus stocks were prepared by transfection of plasmid pHCV-WHU-1 into the Vero cells containing recombinant vaccinia virus vTF7-3. HCVcc was collected in serum-free medium, or medium containing 10% FBS. A virus stock with a title of 109 copies/ml was used. The Huh7.5.1 cells and hESCs-derived cells at different stages progressing to mature hepatocytes were cultured for 6 hours with HCVcc at a ratio of 0.5 TCID50 unit/cell (∼1x108 copies of HCV RNA/3∼4 x106 cells). After 3 washes with PBS, cells were incubated with stage-specific culture medium. On day 6, 12, 18, 24, and 30 of differentiation from hESCs, cells were harvested to detect HCV RNA with qRT-PCR. The supernatants of the cells at each lineage stage were collected for Huh7.5.1 cells' infection to check the infectivity of the produced virions. Both intracellular and extracellular total RNAs were extracted at different points, by using an RNeasy Mini Extraction kit (Qiagen). HCV RNA was analyzed using the artus HCV QS-RGQ Kit (Qiagen) following the manufacturer's protocol.
VISUALIZATION OF INFECTED CELLS
Infected hESC-derived hHBs and hHeps were visualized using Hepatitis C Dependent Fluorescent Relocalization (HDFR). Firstly, hESC-derived hHBs and hHeps were transduced with a lentiviral vector, expressing the C-terminal region of IPS-1 (including the transmembrane domain and the NS3-4A cleavage site) fused to enhanced green fluorescenceprotein (EGFP) with the SV40 nuclear localization sequence (EGFP-NLS-IPS) in the presence of Polybrene. Then 24 hours later, the cells were infected using HCVcc (genotype 1b) and examined during the days following to assess re-localization events indicating HCV infection.
IMMUNOPRECIPITATION AND WESTERN BLOTTING
Immunoprecipitation and western blotting were carried out as previously described.16 Briefly, cells were harvested in lysis buffer and precleared with Protein G–Sepharose (GE Healthcare, Uppsala, Sweden) precoated with anti-OCLN at 4°C for 90 minutes. The beads were collected by centrifugation, washed thoroughly in lysis buffer, and the precipitated proteins eluted with Laemmli buffer. Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with anti-OCLN, anti-phosphotyrosine (Millipore, Billerica, MA) and horseradish-peroxidase- conjugated anti-rabbit or anti-mouse IgG antibodies.
TRANSPLANTATION OF HCV-INFECTED CELL INTO Fah–/–Rag2–/– MICE (F/R MICE)
The engraftment procedure was performed as follows: 2-4-month-old F/R mice were anaesthetized with Ketamine/xylazine (100 mg/kg, 10 mg/kg, i.p.). Then 30 µl medium containing 2-5 × 106 hESC-derived hepatoblasts with or without HCV-infection mixed with Matrigel were injected into the left lower lobe of the murine livers with a 28-gauge syringe. 2-nitro-4-trifluoromethylbenzoyl-1,3 cyclohexane dione (NTBC, 7.5 mg/L) was withdrawn under a stepwise schedule of colony maintenance concentrations at 100%, 25%, 12%, 6% and 0%. Two to six weeks after transplantation. The mice without cell transplantation maintained severe weight loss and died within 4-5 weeks, while mice transplanted with hESCs-derived hepatocytes were rescued from liver injury following NTBC withdrawal.
SEROLOGICAL ANALYSIS
Serum of chimeric F/R mice were harvested by retro-orbital bleeding and stored in aliquots for further analysis. Human albumin in the murine blood was assessed by ELISA (Bethyl Laboratories) according to the manufacturer's instructions. HCV RNA was extracted from 50 µl serum, and serum HCV RNA levels were analyzed by artus HCV QS-RGQ Kit (Qiagen) following the manufacturer's protocol.
HUMAN SAMPLE AND ANIMAL CARE SUBJECTS
All protocols involving human tissue were reviewed and exempted by the Beijing Institute of Transfusion Medicine. All animal protocols were approved by the Ethics Committee at the Beijing Institute of Transfusion Medicine. All surgical procedures and care applied to the animals were in accordance with IACUC guidelines.
STATISTICAL ANALYSIS
Data are shown as standard deviations (SD). For survival analysis, the Mantel-Cox log rank test was applied. Statistical analysis was performed by a one-way ANOVA test combined with a 2-tailed Student t test, with P < 0.05 considered statistically significant. For all statistics, data from at least three independent samples or repeated experiments were used.
Results
hESCs DIFFERENTIATED TO HEPATOCYTE-LIKE CELLS THROUGH MULTIPLE LINEAGE STAGES AND IN A STEP-WISE PROCESS
We previously set up protocols of a step-wise differentiation procedure with serum-free media to efficiently and induce either hESCs16 or multipotent stem cells17 into several hepatic lineages efficiently and reproducibly (Fig. 1A). Following this differentiation procedure, we observed dynamic morphological changes from hESCs to hHeps (Fig. 1B). The expression of lineage-specific markers was used to define empirically each lineage stage and to evaluate the differentiation efficiency. Under the undifferentiated hESC maintenance conditions, almost all of the cells expressed pluripotency markers OCT4 and NANOG. After 4 days of culture in the defined medium used to lineage-restrict the hESCs to hDECs, they lost expression of the pluripotency markers, while >90% of them expressed DE-specific markers SOX17 and FOXA2 (Fig. 1B). The culture medium was changed into a defined medium to lineage-restrict these hDECs to hHpSCs within 6 days, which resulted in high expression of the stem cell marker epithelial cell adhesion molecule (EpCAM); the liver-specific transcription factor hepatocyte nuclear factor 4α exhibited a high level of expression complemented by the activation of albumin (ALB). The specification markers indicative of hepatic lineage cells were robust after treatment with the induction medium for hHBs for another 6 days. About 70% of cells were doubly positive for ALB and alpha-fetoprotein (AFP) (Fig. 1D). Hepatocyte growth factor, dexamethasone, and oncostatin M were used as the final induction factors for at least 6-20 days to generate functional hHeps. At the final stage of maturation, about 80% of the cells were positive for ALB, negative for AFP, and polygonal in shape, all traits consistent with maturation to hepatocytes. Quantitative RT-PCR (Fig. 1C) also confirmed the transition from pluripotent stem cells to SOX17-positive and FOXA2-positive hDECs, followed by induction to AFP-positive hHBs and finally to ALB-secreting, AFP-negative hHeps. The hHeps were also found to display the known hepatocyte functions including glycogen storage, indocyanine green uptake, and urea secretion (Fig. 1E). Ultrastructurally, the hESC-derived hHeps resembled primary human hepatocytes with accumulation of glycogen rosettes, mitochondria, endoplasmic reticulum, canaliculi, and exocytotic vesicles (Fig. 1F). These results indicate that this protocol is highly efficient for generating differentiated hepatocytes from hESCs.
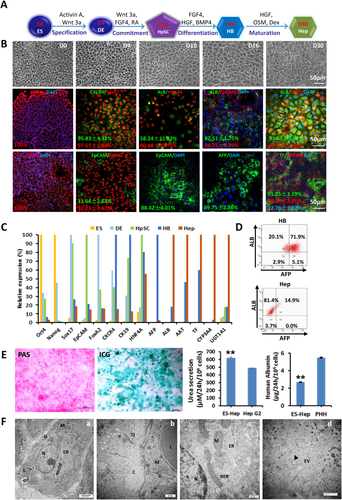
IN VITRO DIFFERENTIATED hHBs WERE THE MOST SUSCEPTIBLE LINEAGE STAGE TO HCV INFECTION
We divided the entire hepatic lineage restriction process into five stages: hESC, hDE, hHpSC, hHB, and adult hHep. These stages were used to define the potential for HCV infections. We further subdivided the stages into pre-Hep and Hep stages after the cells had gone through the hHB stage and were about to enter the hepatocyte stage. To demonstrate the kinetics of HCV infection during the hepatic lineage differentiation process, we inoculated a genotype 1b HCV produced in cell culture (HCVcc), the most common HCV genotype in China, generated with Vero-E6 cells18 at each stage (Fig. 2A). In agreement with previous work, there was no detectable HCV RNA in hESCs or in hDECs. HCV permissiveness started during hepatic specification at the hHpSC stage (Fig. 2B,C,F). Quantitative RT-PCR on cell lysates at 5 days postinfection (dpi) showed that HCV genomes were most abundant in hHBs. This result was consistent with immunofluorescent staining and western blotting (Fig. 2B,C; Supporting Fig. S1). Intracellular HCV RNA levels generally showed an inverted S shape and reached a peak, followed by a decrease and then a relatively stable phase when lineage-restricted cells acquired mature functions (Fig. 2F).
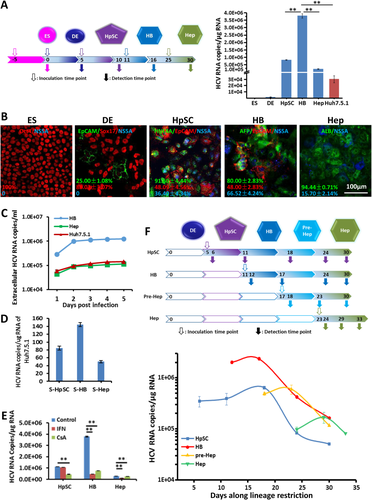
Compared to the classical HCV culture system Huh7.5.1 cells, hHBs were more suitable for intracellular HCV replication. The intracellular HCV from hHBs achieved almost 4 × 106 copies/μg RNA compared to 3 × 104 copies/μg Huh7.5.1 cell RNA. In addition, the supernatant from HCV-infected hHBs contained much more infectious virions that were able to infect Huh7.5.1 cells at a titer of ∼200 RNA copies/μg, 2-3 times that from mature hepatocytes or from Huh7.5.1 cells (Fig. 2C,D). Treatment of infected cells with IFN or cyclosporine A for 2 days inhibited 88% or 80% of HCV replication correspondingly in the hHB stage, confirming that this is authentic HCV replication. HCV-infected hHpSCs and hHeps were not as sensitive as those at the hHB stage to IFN or cyclosporine A treatment (Fig. 2E).
Together, the dynamic HCV infections during hESC hepatic lineage restriction have been outlined; hESC-derived hHB proved to be the most suitable stage for HCV entry and replication, as well as showing the best anti-HCV drug response, compared with cells at the HpSC and Hep stages. Clinically, the liver immunohistochemical (IHC) data of HCV patients also revealed that HCV nonstructural protein 5 (NS5) was always located in and around the AFP+/EpCAM+ early hepatic epithelial cells that appeared in the regenerating pseudo-lobules (Supporting Fig. S2), which is consistent with the in vitro results.
DISTINCT EXPRESSION LEVELS OF HCV-RELATED CELLULAR DETERMINANTS WERE FOUND DURING MATURATION OF hESCs TO HEPATOCYTES
Subsequently, we identified the cellular determinants that correlated with HCV infection during hESC differentiation. Expression and localization of the viral receptors are the important determinants for cell susceptibility to infection and viral tropism of particular tissues. The expression of at least 10 reported receptors (e.g., LDLR, SRBI, claudin 1, epidermal growth factor receptor)19, 20 related to HCV entry or infection was checked during the whole differentiation process from hESCs to hHeps by quantitative RT-PCR. The results indicated that epidermal growth factor receptor, OCLN, and claudin 1 might play more important roles in HCV permissiveness (Supporting Fig. S3A).
Among all of these HCV entry factors, cluster of differentiation 81 (CD81) and OCLN are the minimal necessary human-specific HCV entry factors.15 The quantitative RT-PCR results showed that the expression of CD81 was maintained at a stable level, whereas OCLN gradually increased during lineage restriction through hepatic stages (Supporting Fig. S3A). Immunostaining results indicated that CD81 is at the surface throughout the maturational lineage stages. By contrast, OCLN showed a gradual translocation process from cytoplasm at the ES and DE stages, to the apical poles little by little, to the membranes when cells entered the hHpSC stage. They finally settled stably on the lateral cell membranes of hHBs and hHeps. OCLN localization occurred increasingly at the cell apex, paralleling the formation of hepatic epithelial polarization. As a TJ protein, the amount of OCLN on the cell surface was reported to be correlated with cellular polarity formation.21, 22 During the maturation of induced hepatocytes, more and more OCLN protein transferred to plasma membrane and formed intermittent belts at the hHB stage and sealed circles at the mature hHep stage (Fig. 3A). These data suggested that the location of OCLN, rather than its expression, might play an important role for hepatic epithelia in the transition from nonsusceptibility to susceptibility of HCV infection. In our study, epithelial polarization and TJ formation occurred with hESC maturation to hepatocytes and were speculated to be barriers to HCV infection, which was consistent with the published data of reduced susceptibility to infection following polarization.23-25
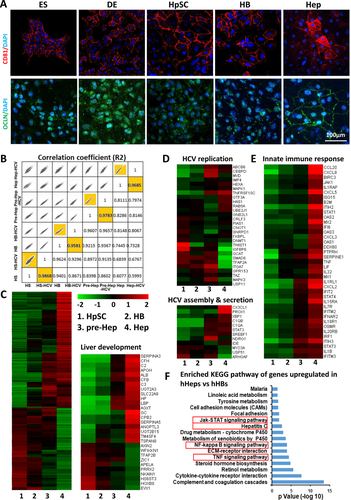
To further explore the host factors involved in HCV replication, assembly, and secretion, genome-wide gene expression in cells at different stages was compared using RNA sequencing. In general, the gene expression profile of the cells at each stage clustered closer to the HCV-infected cells at the same stage, which indicated that the genome profile of lineage specifications could not be affected significantly by HCV infection (Fig. 3B). Genes involved in the regulation of liver development were up-regulated along with processes of maturation (Fig. 3C). Among the genes positively associated with HCV replication,26 ABCB6, CEBPD, MAPK1, RAB5A, and UBE2J1 were highly expressed in mature hHeps, while CRLF3, ITGA7, and TFAP2A were highly expressed in the progenitor hHB stage (Fig. 3D), which indicated that HCV might exploit stage-specific host signal pathways that support its replication in immature and mature cells, respectively. HCV assembly and secretion have been reported to be associated with the lipoprotein secretion pathway and particularly with pathways mediated by apolipoprotein B (APOB) and APOE.20, 27 The expression of both APOB and APOE increased as demonstrated by quantitative RT-PCR and enzyme-linked immunosorbent assay (Supporting Fig. S3). Most of the 66 genes known to be involved in HCV assembly and secretion26 remained unchanged, except that CX3CL1 and PROX1 increased in mature hepatocytes, while MYO3A increased in immature hepatic cells.
Families of viral restriction factors involved in HCV infection10, 26, 28 were significantly enriched in hHeps (Fig. 3E), including IFN-stimulated effector genes (ISGs, e.g., IFIT1, oligoadenylate synthetase 2), leukocyte recruiting chemokines (e.g., chemokine [C-X-C motif] ligands 3 and 8), inflammatory genes (e.g., interleukin 1β, tumor necrosis factor), and recently reported protein tyrosine phosphatase family genes (e.g., PTPRH). Furthermore, 1,101 genes significantly up-regulated in hHeps versus hHBs were analyzed by the Kyoto Encyclopedia of Genes and Genomes. We found that the tumor necrosis factor, nuclear factor-κB, and Janus kinase–signal transducer and activator of transcription signaling pathways, which mediate host antiviral immune responses, were enriched. Interestingly, hepatitis C pathway genes were also significantly up-regulated in hHeps versus hHBs. This pathway contained five claudin family genes and 14 innate immune genes, including IFN pathway genes (e.g., IFNAR2, IFN regulatory factor 9, Janus kinase 1, signal transducer and activator of transcription 1, oligoadenylate synthetase 2) and inflammatory genes (e.g., tumor necrosis factor, chemokine [C-X-C motif] ligand 8) (Fig. 3F). Together, except for the specific physiological characteristics of the hepatic host cells at different lineage status, it was the comprehensive effect on both acceptance and defense to the virus (proviral and antiviral effects) that resulted in different HCV infection features. Low level of cell polarity, integrated TJ, and innate immunity might comprise the main reasons for hHB being the optimal stage of HCV infection.
VEGF ENHANCED HCV INFECTION BY MINIMIZING hHB POLARIZATION AND OBVIATING FURTHER MATURATION
Because HCV infectivity occurs optimally in hHBs, optimizing conditions for keeping the cells at the hHB stage should facilitate a highly effective HCV infection cell model system. Mature hepatocytes are highly polarized, with TJs separating the basolateral (sinusoidal) and apical (canalicular) domains. VEGF and the VEGF–receptor 2 pathway were reported to inhibit hepatocellular TJ integrity and cell polarity in the hepatocellular carcinoma cell line HepG2, through reorganization of OCLN.24 The VEGF–VEGFR2 pathway has also been reported to play an important role in liver regeneration, which suggests that the VEGF pathway could be significant for hepatic epithelial proliferation and differentiation.29 We hypothesized that VEGF might regulate HCV infection through managing cell polarity status.
RNA was extracted from HCV-infected cells treated with VEGF at 5 dpi or not treated. We found that upon VEGF treatment, the expression of intracellular HCV increased during each stage of hepatic lineage restriction (Fig. 4A). Infectivity assays using Huh7.5.1 cells infected with supernatants collected from cells of each stage showed that VEGF also enhanced the production of infectious HCV particles (Fig. 4B). HCV replication in cells was confirmed with immunofluorescence staining (Fig. 4C). We also used HCV-dependent relocalization assays30 to indicate HCV-infected hHBs and hHeps induced by the differentiation protocol with or without VEGF. hHBs and hHeps were transduced with IPS-NLS-EGFP reporters and then infected with HCV. Infected cells exhibited relocalization of fluorescence reported by protease NS3-4A activity. More translocation could be observed in VEGF-treated cells as early as 2 dpi (Fig. 4D). Transmission electron microscopy showed that VEGF increased the area occupied by the membranous web in HCVcc-infected HBs, without altering the morphology of the double-membrane vesicles, the site of genome replication (Fig. 4E),31 which meant that VEGF also could enhance HCV replication. Further mechanisms are expected in the future on this phenomenon.
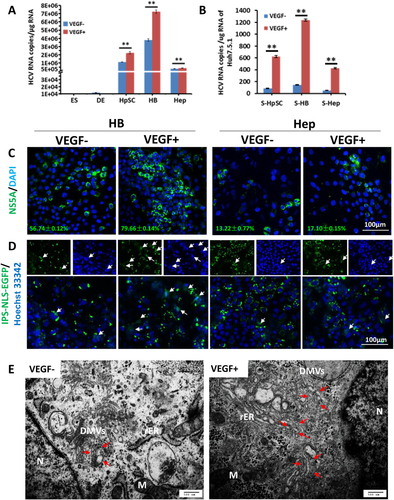
The 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate assay and canaliculus marker multidrug resistance protein 2 immunostaining were used to check the status of cell polarity. Less cell polarity was found in VEGF-treated cells at both the HB and hepatocyte stages (Fig. 5A). Immunoprecipitation assays showed there was more phosphorylated OCLN protein at the hepatocyte stage than at the HB stage, though a similar level of OCLN existed at both cell stages (Fig. 5B). In addition, when we added VEGF to the serum-free, stage-specific defined medium following the step-wise differentiation, the expression of fetal-specific AFP was up-regulated, while the hepatocyte functional genes, e.g., ALB, transferrin, AAT, and UGT1A3, were suppressed in both hHB and hHep cells by quantitative RT-PCR (Fig. 5C). Immunofluorescence staining showed that VEGF increased AFP-positive cells in all stages of the hepatic lineage cells, consistent with RNA level, and reduced the percentage of cytokeratin 18–positive cells, which indicated that VEGF could postpone hepatic maturation (Fig. 5D). Thus, VEGF treatment might inhibit cell polarity through phosphorylation of OCLN and, secondarily, minimize hepatic differentiation, maintain the cells at an immature stage, and enhance HCV infectivity.

SUSTAINED EXPRESSION OF HCV PROTEIN IN THE LIVER PARENCHYMA OF ENGRAFTED F/R MICE
Wild-type primary hepatocyte transplantation has been proven to rescue liver failure and death of Fah–/– mice when they are removed from being fed with 2-nitro-4-trifluoromethylbenzoyl-1,3 cyclohexane dione, which provides an ideal model to characterize in vivo repopulation and functions of hepatic cells as well as construction of a humanized murine model for HCV infection.32 In our study, we used immune-deficient F/R mice33 for transplantation to avoid immune rejection and enhance the chimera efficiency. We injected hESC-derived hHBs infected with HCV or not into the left and right lower lobes directly. Immunosuppressor FK506 was used to raise engrafted cell repopulation.34 The survival rate and hALB in the sera of the recipient mice were monitored to evaluate the engraftment procedures. F/R mice without transplantation were all dead within 5 weeks after withdrawal of 2-nitro-4-trifluoromethylbenzoyl-1,3 cyclohexane dione. In contrast, 12 out of 16 F/R mice transplanted with hHBs were alive for more than 12 weeks, no matter the transplanted hHBs with or without infected HCV (Fig. 6A). The results indicated that hESC-derived hHBs can be functionally integrated into F/R mice and can rescue them from liver failure.
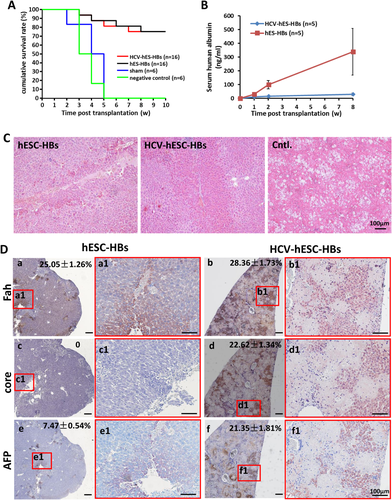
Hematoxylin/eosin staining of liver sections from mice receiving hHBs showed clusters of donor hepatic cells over the entire lobes, demonstrating that engrafted hHBs could repopulate the F/R murine livers effectively, consistent with findings from a previous study35; vacuolar degeneration appeared in liver sections from mice in the negative control group (Fig. 6C). IHC staining of human Fah showed that HCV-infected or uninfected hHBs repopulated 28.36 ± 1.73% and 25.05 ± 1.26% of the liver parenchyma, respectively, in the surviving mice. Though the repopulation rates were similar, most of the engrafted HCV-hHBs were hAFP-positive, indicating a fetal phenotype rather than a mature one over the time in the mouse liver. By contrast, hAFP-positive cells were fewer in recipient mice transplanted with uninfected hHBs (Fig. 6D), which was consistent with the higher hALB in serum of those mice (Fig. 6B). Together, the results suggested that the maturation of HCV-infected hHBs was much slower than that of the uninfected hHBs in vivo. We found a similar phenomenon in the liver tissues of HCV patients (Supporting Fig. S2). In mice, Fah-positive cells were positively stained for the HCV core protein after repopulation, which means that the HCV persisted in the engrafted HCV-hHBs. However, HCV RNA and protein are undetectable in the sera or liver tissue. Together, our data suggest that HCV-infected HBs can repopulate F/R livers and ameliorate impaired liver functions caused by Fah deficiency. HCV was maintained in engrafted cells for at least 12 weeks.
IFNβ-MEDIATED INNATE IMMUNE RESPONSES SUPPRESSED THE INFECTIVITY OF HCV AND SMALL INTERFERING hIFNβ ENHANCED HCV INFECTION IN THE LIVERS OF ENGRAFTED F/R MICE
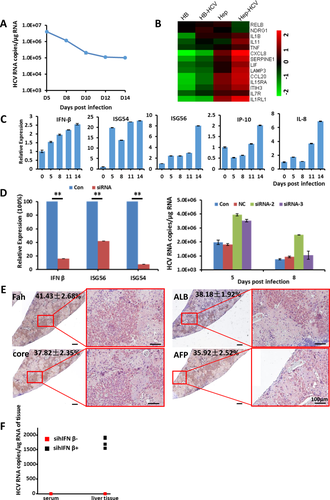
Although cells at the hHB stage were susceptible to HCV infection, the replication of HCV in hHBs kept decreasing, with HCV RNA copies stabilizing at around 104 after 14 days of infection. hHB cells could be well maintained without shifting to any other stages by using the defined medium (Fig. 7A). The host antiviral immunity was often regarded to be the major reason for the decrease of HCV replication. In addition to the increase of immune response genes and the related pathways shown in Figs. 3E and 7B, expression of IFN-inducible protein 10, interleukin-8, IFNβ, ISG54, and ISG56 increased in the hHBs after infection with HCV, which indicated a gradual activation of inflammation stimulation and IFN responses, respectively (Fig. 7C). Spreading of the HCV infection in primary human fetal liver cells was also reported to be limited by the IFN-stimulated mechanisms.36 To enhance HCV replication in hHBs, we suppressed the expression of IFNβ with small interfering RNA (siRNA) to counteract IFN induction and antiviral signaling. The addition of siRNA suppressed the induction of hIFNβ and downstream genes ISG54 and ISG56, and intracellular HCV RNA was enhanced in hHBs (Fig. 7D; Supporting Fig. S4). As expected, HCV RNA in mouse liver tissue engrafted with HCV-infected hHBs was detectable, which showed enhanced HCV infection in vivo (Fig. 7E,F). Taken together, introduction of sihIFNβ could improve HCV infection in vitro and in vivo.
Discussion
Distinctions in phenotypic traits of maturational lineage stages of cells progressing from pluripotent stem cells (e.g., embryonic stem or induced pluripotent stem cells) to mature hepatic cells have been shown to be relevant to susceptibility to infection by HCV. We used lineage restriction of hESCs sequentially through the major maturational lineage stages of liver organogenesis to provide host cells that were then assessed for their vulnerability to HCV infection. Neither hESCs nor hESC-derived hDECs could be infected by HCV. The earliest stage at which HCV permissiveness occurred was at the hHpSC stage. The state identified as most permissive for HCV infection proved to be hHBs and declined in prehepatocytes and mature hepatocytes (Pre-Heps and Heps). Both hHpSCs (EpCAM+, ALB±, AFP–) and hHeps (EpCAM–, ALB+, AFP–) were less susceptible to HCVcc when compared to the hHBs (EpCAM+, ALB+, AFP++) in vitro. Also, engrafted HCV-infected hHBs were able to keep HCV as a persistent infection in vivo for over 12 weeks. Such an optimal stage selection of HCV infection was tightly associated with hepatic linage stage–specific host functions. The location of clusters of HCV-infected cells in liver tissue has been found to overlap with reactive ductule containing EpCAM+ cells.37, 38 This implicates hepatic stem/progenitor cells that express EpCAM+ as candidates for HCV infection in the livers of patients.
For a specific cell type, the expression of viral receptors is typically associated with viral tropism. HCV enters host cells through a coordinated cascade of pathways involving multiple entry factors. In this study, we mainly focused on HCV receptors CD81 and OCLN, the minimal requirements for HCV entry into human cells.15 OCLN-knockout Huh7.5.1-8 cells were reported to be completely resistant to HCV infection.39 We also found that the translocation of OCLN from cytoplasm to cell surface happened consistently with the time point of HCV susceptibility transition during the hepatic specification. The result verified that not only the expression but the location of OCLN proteins were the key factors for HCV infection.
HCV has been reported to infect early–lineage stage cells from human fetal liver but then generated and secreted mature virions only when the cells entered a mature stage,40 which was verified by Yamane et al.31 This indicates that mature hepatocytes might provide more function for HCV assembly and secretion than immature cells, which is also supported by the fact that more APO100 protein was secreted into the media along the differentiation in our study and others. However, at least two factors will counteract this effect: (1) cell polarity (with an epithelia-specific character, cell polarity provided a physical barrier for viral access to receptors; mature hepatic epithelial cells had sealed and functional TJs, thus protecting the hepatic epithelia from HCV infection) and (2) innate immunity (as the cell defense system, mature innate immunity is essential to viral clearance). There was a significant enrichment of innate immune genes and signaling pathways in hHeps compared to hHBs, which provided an obstacle to HCV propagation.
The epidemiological data showed that similar percentages of chronic HCV infections were found in both adults and children41; however, the numbers of infected hepatocytes were significantly higher in liver tissues of pediatric patients than in those of adults.42 It was found that clusters of HCV-infected cells often present close in proximity to fibrous septae that appeared to bridge portal tracts3 where EpCAM+ progenitor cells are mainly located38; IHC staining with HCV NS5 protein indicated that HCV prefers regeneration conditions, comprised of higher numbers of EpCAM+ cells located in the pseudo-lobules in HCV-infected adult liver tissue. These regenerating progenitor cells are in small numbers but contain high levels of HCV and may serve as an HCV reservoir. Consistent with this result, much lower permissivity (1%-3%) to HCV infections, detected with HCV pseudoparticles, occurred in micropatterned adult human hepatocytes.43 Liang and coworkers have shown that a low proportion (7%-20%) of ex vivo patient hepatocytes express viral antigens.3 All these studies contribute to the composite reality of chronic hepatitis in vivo as being in a subset of parenchymal cells.
Although high levels of HCV RNA were detected in hHBs in vitro and we were able to detect HCV genomic RNA in the liver of HCV-infected, hHB-engrafted mice, we could not detect significant HCV viremia. The reason for the low level of viral replication in vivo might be several-fold. Firstly, assembly and secretion of progeny virions involved the lipoprotein secretion pathway. We found that the secreted APOB100-containing lipoprotein from hHBs was much lower than that from mature hepatocytes, which indicated that the secretion of HCV depended on the maturational status of hepatic cells. No viremia might be caused by the prolonged immature phenotype of engrafted hHBs. Secondly, HCV infection could stimulate innate antiviral IFN and inflammatory responses in hESC-derived hHBs. Suppression of IFNβ could enhance HCV infection in murine livers transplanted with HCV-infected hHBs. Finally, the host animals were F/R mice that lacked mature T and B cells but retained both natural killer cells and macrophages. The natural killer cells were regarded as first-line effectors of the innate immune response and played a distinct role against HCV infection.15, 44 The loss of HCV from engrafted hHBs could be due partially to the existing innate immune cells. In future, we will improve the hESC-derived HBs engrafted mouse model through removing natural killer cells by antibody treatments along with other forms of immunosuppression for susceptibility or enhanced HCV replication.
Although the results of our present study indicated that the hepatoblast was the optimal stage for HCV infection during the hepatic lineage restriction from stem cells, the strategies we employed could define stages optimal for other hepatitis viruses such as HBV or hepatitis E virus. Technically, we benefited from the well-controlled process of step-wise lineage restriction of ES cells to a hepatic fate using wholly defined conditions. Being able to use a pluripotent stem cell line, which is experimentally manipulated by the medium conditions through the lineage stages, proved a far more facile model system than trying to isolate these stages from freshly isolated cell suspensions. In addition, all derived hepatic cells at different lineage stages were from the same original stem cells and thus had exactly the same genetic background. Therefore, our experimental strategies were able to reduce the variables influencing the results.
In summary, we interpreted HCV entry and replication at particular hepatic lineage stages from the perspective of the HCV infection dynamic. hESC-derived hepatoblasts proved the optimal stage for HCV infection. Based on that finding, the use of additional variables such as VEGF can help sustain the cells at this stage and thus further elevate HCV expression. The model system comprises entirely defined in vitro conditions and in vivo ones using F/R mice as hosts. hHBs infected with HCV can be engrafted into the livers of F/R mice, resulting in humanization of the host livers and persistence of HCV infections. These approaches should prove valuable in studies of HCV infections both in vitro and in vivo, benefiting efforts to develop anti-HCV therapies.
Acknowledgment
We thank Dr. Congyi Zheng for his generous gift of HCVcc (1b); Dr. Zheng Zhang for his help with clinical samples; Drs. Lola Reid and Yue J. Wang for critical review; Drs. Zhenbo Liu, Wei Zhu, and Yanchang Li for carrying out RNA sequencing and data analysis; and Dr. Jinhua Qin and Mr. Kai Wang for confocal data processing and analysis.
REFERENCES
Author names in bold designate shared co-first authorship




