Retracted: Redox-dependent regulation of hepatocyte absent in melanoma 2 inflammasome activation in sterile liver injury in mice
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health (R01GM102146, to M.J.S., and R01HL079669, to J.F.).
Abstract
Sterile liver inflammation, such as liver ischemia-reperfusion, hemorrhagic shock after trauma, and drug-induced liver injury, is initiated and regulated by endogenous mediators including DNA and reactive oxygen species. Here, we identify a mechanism for redox-mediated regulation of absent in melanoma 2 (AIM2) inflammasome activation in hepatocytes after redox stress in mice, which occurs through interaction with cytosolic high mobility group box 1 (HMGB1). We show that in liver during hemorrhagic shock in mice and in hepatocytes after hypoxia with reoxygenation, cytosolic HMGB1 associates with AIM2 and is required for activation of caspase-1 in response to cytosolic DNA. Activation of caspase-1 through AIM2 leads to subsequent hepatoprotective responses such as autophagy. HMGB1 binds to AIM2 at a non-DNA-binding site on the hematopoietic interferon-inducible nuclear antigen domain of AIM2 to facilitate inflammasome and caspase-1 activation in hepatocytes. Furthermore, binding of HMGB1 to AIM2 is stronger with fully reduced all-thiol HMGB1 than with partially oxidized disulfide-HMGB1, and binding strength corresponds to caspase-1 activation. These data suggest that HMGB1 redox status regulates AIM2 inflammasome activation. Conclusion: These findings suggest a novel and important mechanism for regulation of AIM2 inflammasome activation in hepatocytes during redox stress and may suggest broader implications for how this and other inflammasomes are activated and how their activation is regulated during cell stress, as well as the mechanisms of inflammasome regulation in nonimmune cell types. (Hepatology 2017;65:253-268).
Abbreviations
-
- AIM2
-
- absent in melanoma 2
-
- ALT
-
- alanine aminotransferase
-
- APAP
-
- acetaminophen
-
- ASC
-
- apoptosis-associated speck-like protein containing a caspase activation and recruitment domain
-
- dsDNA
-
- double-stranded DNA
-
- GST
-
- glutathione S-transferase
-
- HC-HMGB
-
- hepatocyte-specific HMGB
-
- HIN
-
- hematopoietic interferon-inducible nuclear antigen
-
- HMGB1
-
- high mobility group box 1
-
- H-R
-
- hypoxia with reoxygenation
-
- HS/R
-
- hemorrhagic shock with resuscitation
-
- KD
-
- dissociation constant
-
- LC3
-
- microtubule-associated protein 1A/1B-light chain 3
-
- mtDNA
-
- mitochondrial DNA
-
- NLRP3
-
- nucleotide binding and oligomerization leucine-rich repeat family member with a pyrin domain-3
-
- poly(dA:dT)
-
- poly(deoxyadenylic-deoxythymidylic) acid
-
- ROS
-
- reactive oxygen species
-
- TLR
-
- Toll-like receptor
-
- WT
-
- wild type
DNA sensing in liver is essential for initiation of innate immune responses after some microbial infections, as well as after sterile injuries induced by liver ischemia-reperfusion, acetaminophen (APAP) overdose, or hepatic steatosis.1-4 Recognition of microbial DNA by intracellular pattern recognition receptors leads to production of type I interferon and interleukin-1β in immune cells, which are crucial for host defense against infections.5 Recent studies indicate that in addition to cytokine production and maturation, intracellular pattern recognition receptors can serve as regulators of cell stress responses by regulating cell death, DNA repair, and cytoprotective responses.6-8 In sterile liver injuries, these responses may be especially important for protecting liver cells from further damage and helping restore tissue homeostasis.
Inflammasomes are cytosolic sensory complexes that alarm the immune system to microbial invasion or tissue damage. In response to intracellular DNA, nucleotide binding and oligomerization, leucine-rich repeat family member with a pyrin domain-3 (NLRP3) and absent in melanoma-2 (AIM2) inflammasomes can be assembled to allow activation of caspase-1.9 AIM2 senses double-stranded DNA (dsDNA) during infection with DNA virus or intracellular bacteria such as Francisella or Listeria.10 NLRP3 inflammasome can be activated by oxidized mitochondrial DNA (mtDNA) in myeloid cells in response to chlamydial infection.11 Inflammasomes and other cytosolic DNA sensors have been primarily studied in microbial and autoimmune diseases, and their function and mechanism of activation in sterile liver injury, such as after redox stress, and their role in noninflammatory processes are still largely unknown.
High mobility group box proteins bind to all immunogenic nucleic acids and can function as universal sentinels for nucleic acid–mediated innate immune responses, promoting activation of Toll-like receptors (TLRs) 3/7/9 by their cognate nucleic acids.12 A key protein in the family, high mobility group box 1 (HMGB1), translocates from the nucleus to other cellular compartments, where it has a broad range of functions.13 Recent studies indicate that HMGB1 function depends on the redox status of cysteines at locations 23,45 (in A box domain) and 106 (in B box domain).14 Fully reduced (all-thiol) HMGB1 functions extracellularly as a chemoattractant and intracellularly can induce autophagy,15 although its role in autophagy in vivo is not as clear.16 Under mild oxidative conditions a disulfide bond forms between cysteines 23 and 45, with disulfide HMGB1 functioning extracellularly as a proinflammatory cytokine and intracellularly as a proapoptotic agent.14, 17 Hepatocytes express and activate inflammasomes,8, 18 although they often do not produce significant amounts of caspase-1-activated cytokines. We have shown that redox stress induced by hemorrhagic shock activates caspase-1 in hepatocytes to induce a protective response through induction of mitochondrial autophagy.19 In this study we show that NLRP3 is dispensable for inflammasome/caspase-1 activation in hepatocytes in response to hemorrhagic shock, a model of sterile injury with mild generalized hypoxia.20, 21 Instead, caspase-1 activation and protective mitochondrial autophagy responses were mediated by AIM2. Additionally, we show that HMGB1 plays a vital and previously unrecognized role in regulating AIM2 inflammasome activation preferentially in response to mtDNA and that AIM2 inflammasome activation in hepatocytes is regulated by redox-mediated changes to HMGB1/AIM2 binding. Our study therefore defines a novel mechanism for redox regulation of intracellular DNA signaling through AIM2 inflammasome in a nonimmune cell type during sterile inflammation.
Materials and Methods
REAGENTS
For western blotting, rabbit anti-caspase-1 was from Millipore and Cell Signaling; mouse anti–glyceraldehyde 3-phosphate dehydrogenase, rabbit anti-beclin-1, rabbit anti-HMGB1, and guinea pig anti-p62 were from Abcam; rabbit anti-AIM2 and rabbit anti–apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) were from Santa Cruz; rabbit anti-myc was from Cell Signaling; mouse anti-FLAG was from Sigma; rabbit anti–microtubule-associated protein 1A/1B-light chain 3 (LC3) was from Novus; radio immunoprecipitation assay buffer for tissue lysis was from Sigma; and cell lysis buffer was from Cell Signaling Technology for whole-cell lysis plus protease inhibitors. Western images were quantified by densitometry using ImageJ software (National Institutes of Health). Caspase-1 activity was determined using a caspase-1 activity colorimetric kit (R&D Systems). Recombinant HMGB1 was generously provided by Drs. Kevin Tracey and Huan Yang22 (Feinstein Institute for Medical Research).
HEPATOCYTE ISOLATION AND CELL CULTURE
Hepatocytes were isolated from mice by an in situ collagenase (type VI; Worthington) perfusion technique, modified as described.23 Hepatocyte purity exceeded 99% by flow cytometric assay, with viability over 95% by trypan blue exclusion. Hepatocytes (4 × 105 cells/mL, six-well plates) were plated on gelatin-coated culture plates in Williams-E medium with 10% fetal calf serum, 15 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 10−6 M insulin, 2 mM l-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin. Hypoxia with reoxygenation (H-R) treatment was performed as described.24
ANALYSIS OF CELL DEATH
Hepatocytes were cultured under hypoxia (1% O2) and then reoxygenated under normoxic conditions. Cell death was measured using an annexin V-fluorescein isothiocyanate apoptosis detection kit (BD Biosciences) according to the manufacturer's instructions and analyzed by flow cytometry using the Guava EasyCyte 8HT flow cytometer (Millipore).
MITOCHONDRIAL/CYTOSOLIC REACTIVE OXYGEN SPECIES
Hepatocytes were transfected with mitochondria-targeted HyPer-Mito or cytoplasm-targeted HyPer-Cyto (Evrogen). At 36 hours after transfection, hepatocytes were subjected to hypoxia (1% O2) for 6 hours and 1 hour of reoxygenation or kept under normoxia for 7 hours. A green fluorescence signal was observed by fluorescent microscopy in 30 random cells/treatment using the EVOS fluorescence microscope (AMG). Fluorescence intensity was assessed by ImageJ and is expressed as a fold increase to wild-type (WT) control. Experiments were repeated at least three times/treatment.
MITOCHONDRIAL DNA COPY NUMBER AND MITOCHONDRIAL VOLUME
Mitochondrial DNA copy number was measured by quantitative polymerase chain reaction as described.25 Primers (Integrated DNA Technologies) against part of mitochondrially encoded reduced nicotinamide adenine dinucleotide dehydrogenase subunit 6 were as follows: forward 5′-CCCAGCTACTACCATCATTCAAGT-3′ and reverse 5′-GATGGTTTGGGAGATTGGTTGATG-3′. Results were normalized to nuclear DNA copy number. Mitochondrial volume was determined by MitoTracker Red staining (Life Technologies) and divided by cell volume marked by calcein (BD Biosciences). Hepatocytes were loaded with 1 μM calcein and 100 μM MitoTracker Red for 30 minutes before imaging with a Zeiss LSM 510 laser-scanning confocal microscope using a 63× oil lens. Z-stacks were acquired of individual hepatocytes at 5-μm intervals. The mitochondrial volume of a random portion of cytoplasm was determined as a fraction of cytoplasm using ImageJ's 3D Object Counter macro (https://imagej.nih.gov/ij/plugins/track/objects.html).
RECOMBINANT HMGB1
Full-length complementary DNA for human HMGB1 was subcloned in-frame to the secretion signal of a modified YEpFLAG (Sigma). This vector was then transformed into protease-deficient yeast strain BJ3505. The transformed yeast were grown at 30°C for 3 days on a rotary shaker in 500 mL Expression medium (1% glucose, 3% glycerol, 1% yeast extract, 2% peptone, 100 mM potassium phosphate, pH 6.4). Proteins were further purified as described.26
HMGB1 C23S, C45S MUTATIONS
Mutants were generated using the QuickChange mutagenesis protocol (Agilent Technologies). The original HMGB1-YEpFLAG plasmid was used as template, and cysteine residues at amino acids 23, 45, and 106 were changed to serines. Plasmids were transformed into BJ3505 yeast strain, and proteins were purified as for WT protein. Myc-tagged human HMGB1 plasmid was prepared by subcloning full-length, translated complementary DNA into mammalian expression plasmid pCMV Tag5A (Agilent), placing the Myc tag in-frame at the carboxy terminus.
RECOMBINANT A BOX AND B BOX
The A and B box moieties of HMGB1 were subcloned into a modified version of the bacterial expression plasmid pET23A (Novagen) to contain an amino terminus 6× His tag and an in-frame stop codon. The verified constructs were transformed into bacteria, Rosetta Gami B (Novagen). Cultures were then allowed to grow overnight at room temperature. The pelleted cells were frozen at –20°C for 1 hour, then thawed in 10 mL of 50 mM NaPO4, 0.3 M NaCl, and 0.1% Triton X-100 with protease inhibitors at pH 8.0. The suspension was sonicated at 4°C for 45 seconds, then centrifuged at 12,000g for 15 minutes. Supernatant was filtered through a 5-μm filter. Soluble protein was purified using Talon resin as above. Once purified, proteins were treated with Triton X-114 to remove endotoxin.
FLAG-AIM2 AND FLAG-HEMATOPOIETIC INTERFERON-INDUCIBLE NUCLEAR ANTIGEN PLASMID
Full-length murine AIM2 was cloned by polymerase chain reaction using Phusion Polymerase (NEB) from complementary DNA prepared from mouse liver hepatocyte messenger RNA. The amplimer was then subcloned into pCMV6 (Origene) with a FLAG tag at the C terminus. Using that construct as template, FLAG-hematopoietic interferon-inducible nuclear antigen (HIN) plasmid was prepared from amino acid residues 144-341 and subcloned back into pCMV6. FLAG tagged HIN plasmids carrying H79K, T49K, and H79KS80K mutations were generated using the QuickChange Mutagenesis Protocol (Agilent Technologies).
BIOLAYER INTERFEROMETRY
The affinity of target proteins to HMGB1 was measured using the FortéBio Octet QK platform and the default settings for the sample stage orbital rate (1,000 rpm) at 30°C. Anti–glutathione S-transferase (GST) biosensors (FortéBIO) were hydrated in phosphate-buffered saline for 10 minutes. Anti-GST biosensors were loaded with ligand solution at a concentration of 7.5 μg/mL GST-tagged AIM2 (Invitrogen) and then hydrated in Kinetics Buffer (FortéBIO). HMGB1 was introduced in solutions containing nanomole per liter concentrations of recombinant HMGB1 in the presence or absence of DNA. Dissociation was assessed by washing biosensors in kinetic buffer, and affinity to HMGB1 was calculated using the FortéBio software (Menlo Park), controlling for the buffer only sample.
HMGB1 SHIFT
Recombinant HMGB1 prepared with or without dithiothreitol was heated with or without 350 mM β-mercaptoethanol, loaded onto a 4%-16% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis gel, and revealed by western blotting against HMGB1.
AUTOPHAGIC FLUX
Primary hepatocytes were transfected with green fluorescent protein–LC3, and autophagic flux was assessed by an increase in green fluorescent protein–LC3 puncta in hepatocytes after treatment with bafilomycin (50 nM; Sigma) for 1 hour. Cells were imaged with a Zeiss LSM 510 laser scanning confocal microscope using a 63× oil lens. Green fluorescent protein puncta were counted for 30 cells/treatment. Western blot for endogenous LC3 and p62 was performed in whole-cell lysates from similar groups of bafilomycin-treated cells.
ANIMALS, HEMORRHAGIC SHOCK, AND APAP-INDUCED HEPATOTOXICITY
Male C57BL/6 (WT) and age-matched AIM2–/– mice were purchased from Jackson Laboratory. NLRP3–/– mice (Millennium Pharmaceuticals) were bred in our facility. Hepatocyte-specific HMGB1–/– (HC-HMGB1–/–) mice were generated in our lab by crossing HMGB1-flox mice with albumin-cre mice as outlined.27 Mice aged 8-12 weeks, weighing 21-30 g, were used in experiments. WT and control-flox mice bred in our facility were used as controls for genetic knockout mice bred in our facility. All experimental protocols were approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh. Experimental procedures were carried out in accordance with all regulations regarding the care and use of experimental animals (National Institutes of Health). Hemorrhagic shock surgery was performed as described.28 Hemorrhage was induced to mean arterial pressure of 25 mm Hg for 1.5 hours, followed by resuscitation with 3× shed blood volume Ringer's solution through a catheter. Mice were sacrificed at 1.5, 4.5, or 24 hours after resuscitation. Control mice were sacrificed without any procedures performed to obtain physiological baseline levels. For APAP-induced hepatotoxicity, mice fasted for 14-16 hours with free access to water. APAP solution was prepared fresh for each experiment in 0.9% saline and administered in a single intraperitoneal injection (400 mg/kg). Controls received solvent in 0.9% saline. Mice were killed 12 hours after treatment, and blood was harvested by cardiac puncture.
IN VIVO MACROPHAGE DEPLETION
Macrophage depletion was induced using clodronate-encapsulated liposomes.16 At 24 hours before hemorrhagic shock with resuscitation (HS/R), liposome containing clodronate (5 mg/mL per mouse; Encapsula Nano Sciences, Nashville, TN) or empty liposome (control) was injected intraperitoneally. Macrophage depletion was confirmed by liver Ly6G+ immunofluorescence obtained at 6 hours after HS/R. Quantification was performed using NIS Elements (Nikon).
HISTOLOGICAL ANALYSIS
Tissue sections were analyzed using In Situ Cell Death Detection Kit tetramethylrhodamine red (Roche, Germany) following the manufacturer's instructions. Hoechst nuclear stain (Sigma; B-2883) was applied at room temperature for 30 seconds. Imaging conditions were maintained at identical settings with the original gating performed using negative control. Imaging was performed using a NikonA1 confocal microscope. Quantification was performed using NIS Elements (Nikon).
MASS-SPECTROMETRIC CHARACTERIZATION OF THE CYSTEINE OXIDATION STATUS OF HMGB1 IN THE LIVER
HMGB1 in livers of control mice and mice subjected to hemorrhagic shock and resuscitation were extracted by immunoprecipitation as described.29 The cysteine oxidation status of HMGB1 was analyzed by electrospray ionization-liquid chromatography–mass spectrometry as described.29, 30
IMMUNOFLUORESCENCE AND CONFOCAL MICROSCOPY
Liver tissue was removed after perfusion with cold phosphate-buffered saline and 2% paraformaldehyde. Tissue was fixed in 2% paraformaldehyde for 2 hours, followed by cryopreservation. Liver sections (6 μm) were permeabilized with 0.3% Triton X-100 for 20 minutes and stained as described.31 Images were taken from six random fields/section using a Fluoview2000 confocal microscope (Olympus). Imaging conditions were maintained at identical settings with the original gating performed using negative control (no primary antibody). Quantification of AIM2/HMGB1 colocalization was performed using NIS Elements (Nikon).
MOLECULAR DOCKING ANALYSIS AND SEQUENCE ALIGNMENT
Protein structural information for AIM2 (4JBM) and HMGB1 (2YRQ) was downloaded from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (www.rcsb.org/pdb) and input into ClusPro (https://cluspro.bu.edu/login.php) for molecular docking analysis. The top five potential structure orientations were analyzed by PyMOL (www.pymol.org; v1.7.6) to assess interactions closer than 5 Å. For sequence alignment of the HIN domain, 10 sequences corresponding to the HIN domain of the AIM2 protein family were downloaded from SwissProt, aligned with ClustalW, and displayed using WebLogo.
STATISTICAL ANALYSIS
Results are displayed as mean ± standard deviation or mean ± standard error of the mean from at least three independent experiments. A two-tailed Student t test was used to calculate the statistical significance of two experimental groups. P < 0.05 was considered significant.
Results
AIM2-MEDIATED ACTIVATION OF CASPASE-1 IS PROTECTIVE DURING REDOX STRESS IN HEPATOCYTES
We previously showed that caspase-1 activation protects the liver from injury during redox stress induced by HS/R through induction of mitochondrial autophagy.19, 32 Caspase-1 activation in liver during HS/R or in isolated hepatocytes exposed to H-R was not dependent on NLRP3 or on interleukins 1β/18 in our previous work.32 To investigate which inflammasome was important in initiating cellular protective responses, we assessed the role of AIM2, NLRP1, as well as NLRP3 inflammasomes by subjecting WT, AIM2–/–, NLRP3–/–, and NLRP1a-knockdown mice to HS/R. As shown, NLRP3–/– mice had similar liver damage to WT mice, measured by circulating alanine aminotransferase (ALT) levels (776 ± 184 versus 664 ± 92 IU/L, WT versus NLRP3–/–). NLRP1a-knockdowns also had similar ALT levels to control small interfering RNA-treated mice (762 ± 165 versus 798 ± 206 IU/L, control-small interfering RNA versus NLRP1a-small interfering RNA). However, AIM2–/– mice had significantly higher levels of ALT compared with WT controls after HS/R (Fig. 1A), in a pattern similar to that observed in caspase-1–/– mice19 and consistent with reduced caspase-1 activation/cleavage in livers of AIM2–/– mice as shown by western blot (Supporting Fig. S1A). ALT levels returned to near normal after 24 hours (Fig. 1A), but cell death in liver was still significantly higher in AIM2–/– mice at this time as shown by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling/tetramethylrhodamine staining (Supporting Fig. S1B,C), with tetramethylrhodamine staining corresponding to areas of cell death observed in hematoxylin and eosin staining of the same tissues (Supporting Fig. S1D). Interestingly, we did not observe differences in ALT levels between WT and AIM2–/– mice after APAP, which is a much more severe model of hepatocyte damage (Supporting Fig. S1E).
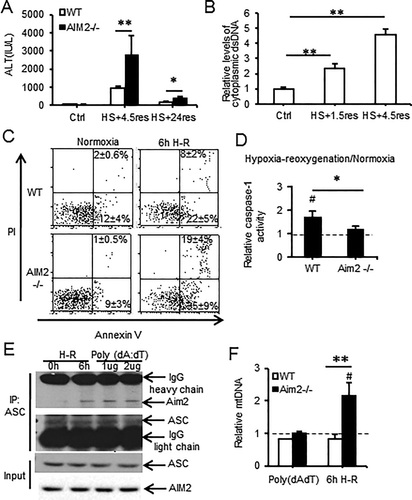
Because AIM2 is known to sense dsDNA, we measured cytosolic levels of dsDNA in liver after HS/R and observed a 2-fold increase in cytosolic dsDNA levels after 1.5 hours of resuscitation, and this increased further by 6 hours of resuscitation (Fig. 1B). We also determined the role of macrophages in liver damage after HS/R. We depleted macrophages from both WT and AIM2–/– mice using clodronate-containing liposomes and confirmed depletion by Ly6G staining of livers (Supporting Fig. S2A). There were no differences in ALT levels between depleted or undepleted WT and AIM2–/– mice or in levels of cleaved (activated) caspase-1 (Supporting Fig. S2B,C).
To confirm the role of AIM2 in caspase-1 activation and hepatocyte protection after redox stress, we subjected hepatocytes isolated from WT and AIM2–/– mice to H-R. Consistent with in vivo results showing increased liver cell death after HS/R, AIM2–/– hepatocytes exhibited higher levels of apoptosis and necrosis together with lower levels of caspase-1 activity after H-R compared with WT cells (Fig. 1C,D). We further confirmed AIM2 inflammasome activation by assessing AIM2 association with the inflammasome component ASC by immunoprecipitation in WT hepatocytes exposed to H-R. AIM2 and ASC association was induced in hepatocytes after H-R as well as by stimulation with the known AIM2 inflammasome activator poly(deoxyadenylic-deoxythymidylic) acid (poly[dA:dT]) (Fig. 1E). Collectively, these data suggest that caspase-1-mediated protection in hepatocytes is dependent on AIM2 after redox injury.
We have shown that caspase-1 activation in hepatocytes protects against cell death after redox stress through up-regulation of mitochondrial autophagy, with subsequent enhanced clearance of damaged mitochondria.19 Consistent with this relationship, we found that AIM2–/– hepatocytes had defective autophagic flux in response to H-R (Supporting Fig. S3A-C). AIM2–/– cells also failed to clear mitochondria as measured by increased cellular mtDNA levels (Fig. 1F) and increased mitochondrial volume (Supporting Fig. S3D) after redox stress. This was combined with increased mitochondrial reactive oxygen species (ROS) production and similar cytosolic ROS levels in AIM2–/– hepatocytes compared with WT after H-R (Supporting Fig. S3E,F), consistent with a central role for caspase-1 in regulating mitochondrial ROS production. These data together with our previous findings confirm that AIM2 inflammasome, and not other known damage-associated molecular pattern–induced inflammasomes, activates caspase-1 in response to increased cytosolic dsDNA released after hypoxic cell stress in hepatocytes.
AIM2-MEDIATED CASPASE-1 ACTIVATION IN HEPATOCYTES IS DEPENDENT ON HMGB1
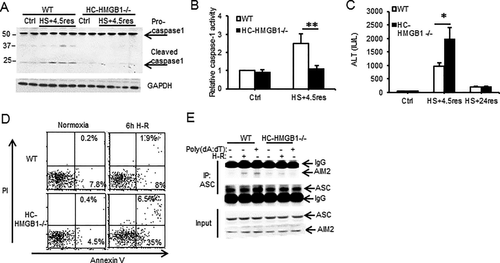
HMGB1 is known to associate with nucleic acids and can translocate to the cytosol from the nucleus during redox stress induced by ischemia-reperfusion.33 We therefore hypothesized that HMGB1 facilitates DNA-mediated AIM2 inflammasome activation in hepatocytes. To test this hypothesis, we used HC-HMGB1–/–27 mice to assess caspase-1 activation and liver damage after HS/R. Caspase-1 was cleaved/activated after HS/R in liver from WT, but not HC-HMGB1–/–, mice (Fig. 2A,B); and serum ALT levels were significantly increased in HC-HMGB1–/– mice compared with WT mice at 6 hours, but ALTs in both groups dropped to near normal levels by 24 hours (Fig. 2C) consistent with a deficient caspase-1 protective response. Hepatocytes isolated from HC-HMGB1–/– mice also had higher levels of apoptosis and necrosis compared to WT cells after H-R (Fig. 2D), less autophagic flux (Supporting Fig. S4A,B), and higher mitochondrial ROS generation (Supporting Fig. S4C,D). Our new findings linking HMGB1 with caspase-1-mediated autophagy are consistent with previous work showing a protective role for HMGB1 after ischemia-reperfusion injury27 as well as a role for HMGB1 in promoting autophagy.15 To determine if the absence of HMGB1 reduced inflammasome formation, we immunoprecipitated ASC from WT or HC-HMGB1–/– hepatocytes after no treatment, H-R, or poly(dA:dT) and assessed pull-down of AIM2. AIM2 associated with ASC in inflammasomes in WT cells after H-R or after poly(dA:dT), but this association was not seen in HC-HMGB1–/– cells (Fig. 2E). These data taken together suggest that HMGB1 plays an integral role in AIM2 inflammasome formation and subsequent caspase-1 activation during sterile injury.
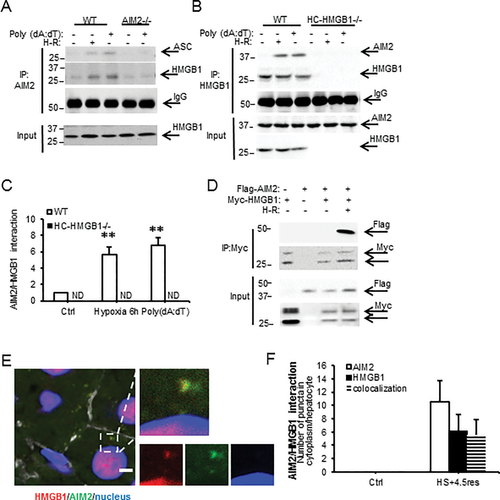
HMGB1 BINDS AIM2 AND FACILITATES INFLAMMASOME ACTIVATION AFTER REDOX STRESS
We next wanted to determine if HMGB1 associates directly with the AIM2 inflammasome and investigated this by immunoprecipitation. In WT cells, but not AIM2–/– or HC-HMGB1–/– cells, HMGB1 associated with AIM2 after hypoxia or poly(dA:dT) treatments (Fig. 3A-C), suggesting direct interaction of HMGB1 with AIM2 inflammasome during redox stress. We also overexpressed Flag-tagged AIM2 and Myc-tagged HMGB1 in HC-HMGB1–/– hepatocytes and were similarly able to pull down AIM2 and HMGB1 in these cells after H-R (Fig. 3D). Immunofluorescence studies confirmed colocalization of AIM2 and HMGB1 in liver tissue in vivo after HS/R (Fig. 3E,F).
To further characterize HMGB1 binding to AIM2, we next assessed whether recombinant AIM2 binds recombinant HMGB1 using biolayer interferometry. Recombinant GST-tagged AIM2 was captured onto the surface of FortéBio anti-GST biosensor tips, and association/dissociation curves for AIM2 along with a dilution series of HMGB1 were used to determine the global fit for the equilibrium dissociation constants (KD) (Supporting Fig. S5 and Table S1). AIM2 bound directly to HMGB1 with a KD of (2.3 × 10–8) ± 0.59 mol/L, and binding was concentration-dependent (Supporting Fig. S5). Binding affinity was not affected by pretreating recombinant HMGB1 with deoxyribonuclease to degrade any DNA that may have been bound to HMGB1 (Table), suggesting that HMGB1 can directly associate with AIM2 even in the absence of DNA.
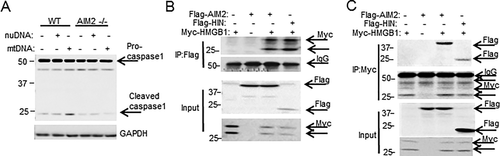
Both nuclear DNA and mtDNA are released during oxidative stress,34 so we assessed whether either DNA type could affect the interaction between AIM2 and HMGB1 to activate caspase-1. We premixed HMGB1 with either nuclear or mtDNA and tested binding affinity with AIM2 as before. The HMGB1–nuclear DNA complex bound to AIM2 at a KD of (2.0 × 10–8) ± 0.42 mol/L, a level similar to AIM2/HMGB1 binding alone. In contrast, HMGB1 mixed with DNA purified from hepatocyte mitochondria showed 2-fold to 3-fold higher binding affinity with AIM2 compared with nuclear DNA (Table 1). To determine if this enhanced binding resulted in increased activation of inflammasome and caspase-1 cleavage, we transfected WT and AIM2–/– hepatocytes with either nuclear or mtDNA. Intracellular mtDNA triggered AIM2-dependent caspase-1 activation in hepatocytes to a greater extent than nuclear DNA (or lipofectamine control) (Fig. 4A), which is consistent with observed stronger mtDNA binding. These data suggest that HMGB1 is able to function as a sensor for mtDNA to induce activation of the AIM2 inflammasome.
| Protein Coupled to Biosensor | Protein in Solution | Kon (1/Ms × 104) | Koff (1/s × 10–4) | KD (M × 10–8) | P |
|---|---|---|---|---|---|
| AIM2 | HMGB1 | 1.4 | 3.4 | 2.3 ± 0.59 | — |
| AIM2-DNase | HMGB1-DNase | 1.3 | 3.3 | 2.5 ± 0.45 | ns |
| AIM2 | HMGB1-nuclear DNA | 1.7 | 3.4 | 2.0 ± 0.42 | ns |
| AIM2 | HMGB1-mtDNA | 1.7 | 1.4 | 0.8 ± 0.19a | <0.05a |
| AIM2 | HMGB1-DTT | 3.0 | 3.8 | 1.3 ± 0.31a | <0.05a |
| AIM2 | A box | 6.4 | 26.1 | 4.1 ± 0.79a | <0.05a |
| AIM2 | A box-DTT | 2.1 | 2.2 | 1.0 ± 0.15b | <0.05b |
| AIM2 | HMGB1(C23S) | 2.5 | 3.0 | 1.2 ± 0.21a | <0.05a |
| AIM2 | HMGB1(C45S) | 2.7 | 3.6 | 1.3 ± 0.20a | <0.05a |
| AIM2 | BSA | Not detectable | Not detectable | Not detectable | — |
- a P value versus KD of AIM2 with HMGB1.
- b P value versus KD of AIM2 with A box.
- Abbreviations: DNase, deoxyribonuclease; DTT, dithiothreitol; ns, not significant.
We then further investigated whether specific domains of AIM2 preferentially associate with HMGB1. The HIN domain of AIM2 is thought to contain the dsDNA binding site, leading to subsequent pyrin domain association with ASC to form the inflammasome and activate caspase-1.35 To assess the involvement of AIM2-HIN in HMGB1 binding in vitro, we generated myc-tagged HMGB1, FLAG-tagged AIM2, and FLAG-tagged AIM2-HIN domain fusion proteins. Coexpression of myc-HMGB1 and FLAG-AIM2 in HEK293A cells followed by immunoprecipitation confirmed the physical association between HMGB1 and AIM2 (Fig. 4B,C). Similarly, there was an association between FLAG-HIN and myc-HMGB1, suggesting that HMGB1 binds to AIM2 at its HIN domain.
We next used computer modeling to determine putative sites for AIM2-HIN interaction with HMGB1 based on protein structures available in the Research Collaboratory for Structural Bioinformatics Protein Data Bank. We performed molecular docking analysis using ClusPro36-38 to determine the most likely protein interaction sites within a distance of 5 Å. All of the top five most likely interaction models showed interaction at amino acid residues 79 (histidine) and 80 (serine) on AIM2-HIN (4JBM: His65/Ser66 in this protein sequence), which associated with the N terminus domain of the A box of HMGB1 (2YRQ) separate from the known disulfide bridge structure (Fig. 5A). Both His79 and Ser80 are highly conserved among vertebrates (Fig. 5B), further suggesting an important role in maintaining the function of AIM2. To determine if this proposed interaction site was actually involved in the HMGB1 association with AIM2-HIN, we generated mutant Flag-tagged AIM2-HIN constructs, substituting lysine for histidine at position 79 (H79K) or for threonine at position 49 (T49K, a site away from the proposed association site) or a double substitution for both histidine and serine at positions 79 and 80 (H79KS80K); and we overexpressed these in HEK293A cells together with myc-HMGB1. We confirmed by immunoprecipitation for myc that Flag-HIN associated with myc-HMGB1 as before, with similar association between Flag-HIN(H79K) or Flag-HIN(T49K) and myc-HMGB1 (Fig. 5C). However, mutation of both H79 and S80 (Flag-HIN[H79KS80K]) almost completely abrogated association with myc-HMGB1, suggesting that this is an important site for specific interaction between HMGB1 and AIM2-HIN. To confirm whether mutating AIM2-HIN at H79/S80 also affected downstream caspase-1 activation, we overexpressed the same Flag-tagged proteins as above into AIM2–/– hepatocytes, followed by H-R. Supporting our association studies of AIM2 and HMGB1, caspase-1 activation was reduced in Flag-HIN(H79KS80K)-expressing cells compared with Flag-HIN expressing cells (Fig. 5D).
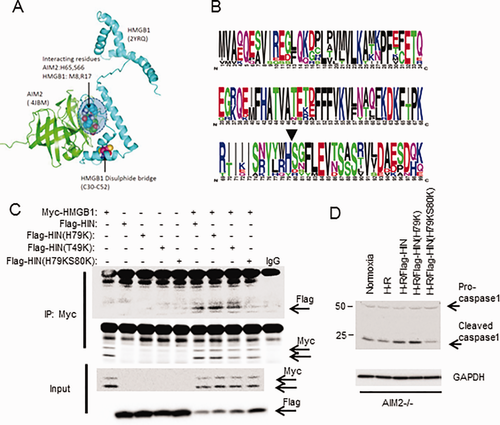
REDOX STATUS OF HMGB1 AFFECTS ITS BINDING TO AIM2
Given the different roles of HMGB1 depending on its redox status, we hypothesized that HMGB1 binding to AIM2 is redox-dependent and tested this by biolayer interferometry as before. After fully reducing HMGB1 to its all-thiol form by adding 5 mM dithiothreitol, binding affinity with AIM2 was significantly greater compared with disulfide HMGB1 (Table 1). We confirmed the redox status of HMGB1 using a mobility shift assay39 (Supporting Fig. S6). We also used mass spectrometry to determine the redox status of HMGB1 in the liver at baseline and at time points after HS/R. As expected, at baseline HMGB1 was nonacetylated (nucleic) and fully reduced (all thiol) (Fig. 6A). After hemorrhagic shock with 1.5-hour resuscitation HMGB1 becomes increasing acetylated, suggesting movement out of the nucleus into the cytosol; but mostly HMGB1 remains fully reduced, with a small part become partially oxidized (disulfide) (Fig. 6B). By 4.5-hour resuscitation there are increasing amounts of disulfide HMGB1 and some is now fully oxidized (Fig. 6C). Levels are quantified in Fig. 6D.
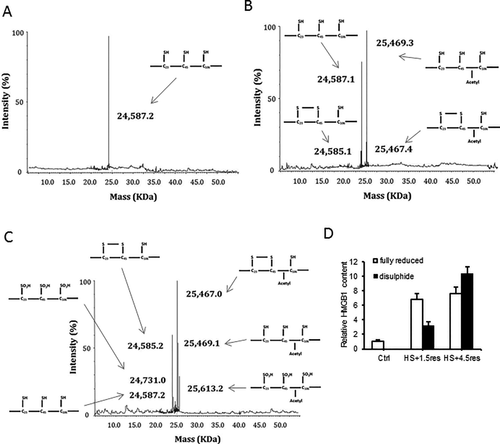
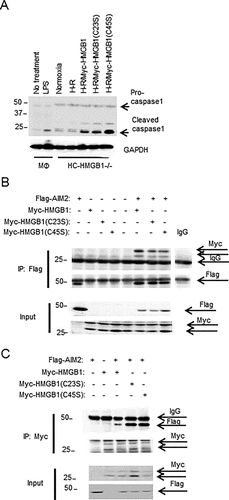
To confirm our binding results, we generated recombinant HMGB1-A box protein. Interestingly, fully reduced A box HMGB1 also had increased AIM2 binding affinity compared with its disulfide form (Table 1), suggesting that the disulfide bond reduces AIM2 binding. We further explored the role of each cysteine using HMGB1 cysteine-to-serine mutants (C23S and C45S). Both C23S and C45S HMGB1 had significantly enhanced binding affinity with AIM2 compared with WT HMGB1 (Table 1), further suggesting that disulfide bond formation impeded AIM2 and HMGB1 interactions. We also tested the ability of cysteine mutants of HMGB1 to activate inflammasome by overexpressing them in HC-HMGB1–/– hepatocytes and then subjecting cells to H-R. As expected, expression of HMGB1 restored caspase-1 activation in HC-HMGB1–/– cells, and caspase-1 activation was increased with HMGB1(C45S) expression (Fig. 7A), supporting a functional role for redox status of HMGB1 in inflammasome activation. The results were further confirmed by assessing the association between Flag-AIM2 and Myc-HMGB1, myc-HMGB1(C23S), and myc-HMGB1(C45S) when overexpressed in HEK293A cells (Fig. 7B,C). Whereas all HMGB1 cysteine mutants associated with AIM2 under physiological conditions (Fig. 7B), C23S and C45S HMGB1 showed increased binding to AIM2 in comparison with WT HMGB1 after redox stress induced by H2O2 treatment (Fig. 7C), again confirming that disulfide HMGB1 has reduced binding capacity to AIM2.
Discussion
Our data suggest that AIM2 inflammasome activation is regulated by the intracellular redox status of HMGB1 and driven by increased mtDNA translocation to the cytosol under stress conditions. Formation of the AIM2 inflammasome in hepatocytes leads to caspase-1 activation and subsequently enhanced mitophagy to clear dysfunctional mitochondria and prevent cell damage. Our molecular docking and mutagenesis studies demonstrate that HMGB1 specifically binds to AIM2 at His79/Ser80, a site separate from the proposed DNA binding site on AIM2-HIN. Binding of HMGB1 at this location is essential for caspase-1 activation after redox stress in hepatocytes. Furthermore, disulfide bond formation in HMGB1 in response to excessive or prolonged oxidative stress reduced its binding to AIM2, suggesting a novel mechanism of regulation of AIM2 inflammasome activity. Our data therefore propose a previously unappreciated mechanism for regulation of hepatocyte protective responses that may have important consequences for liver function in the setting of sterile inflammation.
DNA is a major damage-associated molecular pattern in sterile liver injuries, including nonalcoholic steatohepatitis,1 liver ischemia-reperfusion,40 and drug-induced liver injury.3, 4 DNA released from apoptotic or necrotic cells triggers sterile inflammation by activating membrane-bound receptors including TLR3, TLR8, and TLR9 in immune cells. DNA responses in APAP have been shown to be mediated by TLR9 and NLRP3,4 which fits with our data suggesting that AIM2 inflammasome activation is not important in this model. Extracellular HMGB1 has also been shown to potentiate the effects of intracellular DNA through interactions with receptor for advanced glycation end products on the surface of monocytes.41 Although the role of circulating extracellular dsDNA has been extensively studied in these models, whether cytosolic DNA sensors can be activated in response to sterile liver injury remains largely unknown. Our data provide an important novel mechanism for the regulation of activation of AIM2 inflammasome by cytoplasmic dsDNA in hepatocytes after redox stress. Our data suggest that the release of a sublethal amount of DNA into the cytosol can lead to the activation of AIM2 and subsequent clearance of mitochondria through mitophagy, which can in turn limit further mtDNA and ROS release. These findings fit with a recently described role for AIM2 in autophagosome assembly in macrophages,6 which together with our findings in hepatocytes may suggest that nucleic acid sensing by AIM2 is a more generalized mechanism to regulate stress-induced autophagy as a hepatoprotective mechanism.
Our data show that the redox status of translocated HMGB1 plays an essential role in regulating inflammasome formation and caspase-1 activation. HMGB1 remains in reduced form inside cells, given the reducing environment.39 Therefore, it seems reasonable to suppose that when redox stress is not overwhelming, all-thiol (reduced) HMGB1 coordinates with AIM2 to survey the cytosol for mtDNA released from dysfunctional mitochondria to up-regulate cell stress responses such as mitophagy. However, in response to excessive or prolonged redox stress, oxidized disulfide HMGB1 becomes more prevalent. Disulfide HMGB1 has reduced binding to AIM2 and, thus, reduces inflammasome activation and decreases the effect of protective mechanisms. In this way the balance of cytosolic oxidized and reduced HMGB1 may represent an important tipping point for regulation of hepatocellular cell death or survival in response to redox stress.
In this study we have defined for the first time a mechanism for redox-mediated regulation of inflammasome activation through the direct binding of an intermediary protein. The direct association between HMGB1 and AIM2 is a novel finding. Our data suggest that His79 and Ser80 on AIM2-HIN are essential for interaction with HMGB1, and this proposed HMGB1 binding site is on the opposite side of the structure from the proposed DNA binding site. Furthermore, the site for DNA binding is thought to be obscured by the pyrin domain of AIM2 until inflammasome formation is initiated.35, 42 It is therefore interesting to speculate that HMGB1 binding may facilitate opening of the AIM2 structure to expose the DNA binding site and allow subsequent DNA binding and inflammasome activation. HMGB1 may also act as a dsDNA chaperone that facilitates interactions with AIM2. It is also possible there are inflammasome-independent effects of AIM2 as these have been recently described.43
Our findings suggest a novel and important mechanism for regulation of AIM2 inflammasome activation in hepatocytes during redox stress. Our study may also suggest broader implications for how AIM2 and other inflammasomes are activated and how their activation is fine-tuned during cell stress. Our findings may also shed light on the design of new therapies and treatments for sterile liver injuries through modulation of AIM2 inflammasome activation in hepatocytes.
Acknowledgment
We thank Hong Liao, Alicia Frank, Lauren Kohut, Danielle Reiser, and Aaron Walters for technical assistance.




