CD14+ monocyte-derived galectin-9 induces natural killer cell cytotoxicity in chronic hepatitis C
Potential conflict of interest: Nothing to report.
This work was partly supported by a Grant-in-Aid for Research on Hepatitis from the Japan Agency for Medical Research and Development (AMED 15fk0210017h0003).
Abstract
Natural killer (NK) cell activation is associated with both liver injury and persistent infection in chronic hepatitis C (CHC); however, the detailed mechanism of this activation has not yet been fully elucidated. Because galectin-9 (Gal-9) has been reported to be increased in the serum and liver tissue of CHC patients, we investigated the function of Gal-9 in NK cell activation in CHC. First, we evaluated the function of Gal-9 on NK cytotoxicity in vitro. Gal-9 treatment resulted in increased cytotoxicity of naïve NK cells, and the Gal-9-activated NK cells demonstrated cytotoxicity toward hepatoma cells and T cells. Additionally, coculturing peripheral blood mononuclear cells (PBMCs) with JFH-1/Huh7.5.1 cells increased both Gal-9 production and NK cell cytotoxicity. Next, we investigated the source of Gal-9 and the mechanism of Gal-9 production. Deletion of CD14+ monocytes from PBMCs resulted in reduced Gal-9 production in the coculture with JFH-1/Huh7.5.1 cells. Gal-9 production was driven by coculturing of PBMCs with apoptotic hepatocytes. Blocking integrin αvβ3, a receptor for phosphatidylserine expressed on apoptotic cells, also resulted in decreased Gal-9 production. Finally, we found that serum Gal-9 levels were significantly higher in CHC patients than in healthy donors and patients who achieved sustained virologic response. Among CHC patients, serum Gal-9 levels were significantly higher in patients with elevated alanine aminotransferase (ALT) than in those with normal ALT. Conclusion: These results demonstrate that CD14+ monocyte-derived Gal-9 increases NK cell cytotoxicity in HCV infection, which might be associated with liver injury and persistent infection. (Hepatology 2017;65:18-31).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- CHC
-
- chronic hepatitis C
-
- DAA
-
- direct-acting antiviral
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- HCV
-
- hepatitis C virus
-
- HCVcc
-
- HCV in cell culture
-
- Gal-9
-
- galectin-9
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- NK
-
- natural killer
-
- PBMC
-
- peripheral blood mononuclear cell
-
- rhGal-9
-
- recombinant human Gal-9
-
- SD
-
- standard deviation
-
- SGR
-
- subgenomic replicon
-
- siRNA
-
- small interfering RNA
-
- SVR
-
- sustained virologic response
-
- Tim-3
-
- T cell immunoglobulin and mucin domain 3
Approximately 180 million people in the world are chronically infected with hepatitis C virus (HCV), an infection that leads to liver cirrhosis and hepatocellular carcinoma.1 As chronic liver damage results in liver fibrosis and carcinogenesis,2 ameliorating liver injury is important for preventing hepatocellular carcinoma. Liver injury is driven by host immune responses and leads to disease progression in chronic hepatitis C (CHC).3 Thus, elucidating the immunological mechanism of liver injury is crucial for these patients. Natural killer (NK) cells participate in the pathogenesis of CHC, whereby activation enhances their cytotoxicity, causing liver injury.4, 5 According to recent studies, activated NK cells are attracted to delete specific T cells during chronic viral infection, thereby contributing to persistent infection.6-9 Therefore, resolving the mechanism of NK cell activation in HCV infection is important to regulate disease progression in CHC patients.
Galectin-9 (Gal-9), known as a β-galactoside-binding lectin, exists in various tissues and is particularly abundant in the liver.10 Gal-9 functions as a ligand for T cell immunoglobulin and mucin domain 3 (Tim-3), which is related to T cell exhaustion in patients infected with human immunodeficiency virus,11 hepatitis B virus,12 and HCV.13, 14 It has been reported recently that both serum Gal-9 levels and hepatic Gal-9 expression are significantly increased in CHC patients compared with an HCV-seronegative control group and that Gal-9 elevation is linked to the apoptosis of HCV-specific cytotoxic T lymphocytes and the expansion of regulatory T cells.15 However, the effect of Gal-9 on NK cells remains controversial. Golden-Mason et al.16 reported that Gal-9 reduces cytotoxicity and cytokine production of NK cells, whereas Gleason et al.17 reported that Gal-9 increases cytokine production by NK cells, but not their cytotoxicity. Thus, the function of Gal-9 and its significance in CHC need to be fully elucidated.
In the present study, we examined the role of Gal-9 on NK cell activation in CHC. We propose that CD14+ monocyte-derived Gal-9 enhances NK cell cytotoxicity in HCV infection, which might be associated with liver injury and persistent infection.
Materials and Methods
HUMAN SUBJECTS
We analyzed the serum of 40 healthy subjects, 50 CHC patients, and 24 patients treated with interferon (IFN)-α who achieved sustained virologic response (SVR). All healthy donors were negative for HCV, hepatitis B virus, and human immunodeficiency virus infection and had no history of malignant diseases. Alanine aminotransferase (ALT) levels were significantly higher in CHC patients than in healthy subjects and patients with SVR. Liver samples were obtained from CHC patients via liver biopsy at Osaka University Hospital. The human study was approved by Osaka University Hospital Ethics Committee. We obtained written informed consent from all patients.
SEPARATION OF PBMCs, NK CELLS, MONOCYTES, AND SUBPOPULATIONS OF PBMCs
Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque gradient centrifugation. Each of the subpopulations was separated from PBMCs using magnetic microbeads according to the manufacturer's instructions (Miltenyi Biotech, CA). The purity was more than 95%, as analyzed by FACS Canto II (BD Bioscience, CA).
ELISA
Serum Gal-9 levels and supernatant Gal-9 levels were examined using Gal-9 enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (GalPharma, Japan), as reported previously.15
CELLS AND VIRUSES
Huh7.5.1 cells, JFH-1 (genotype 2a)-infected Huh7.5.1 cells (JFH-1/Huh7.5.1), and HCV subgenomic RNA replicon of Con1 strain (genotype 1b) (SGR)-cured cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, MO) containing 10% fetal calf serum (FCS). Further details are provided in the Supporting Materials and Methods.
CFSE-BASED NK CYTOTOXICITY ASSAY
Carboxyfluorescein succinimidyl ester (CFSE)-labeled target cells were cocultured for 4 hours with naïve NK cells at an effector/target ratio of 4:1 with the addition of recombinant human Gal-9 (rhGal-9), followed by staining with 7-aminoactinomycin D (7-AAD) (Biolegend, CA) for 15 minutes. Target cell lysis was monitored by means of flow cytometry, as reported previously.16 The value of NK cell cytotoxicity against target cells in coculture was calculated by subtracting the value of cytotoxicity when the target cells were cultured alone (without NK cells) from the value of cytotoxicity when the target cells and NK cells were cocultured to evaluate NK cell-specific cytotoxicity. To evaluate NK cell cytotoxicity after 24 hours of coculture with PBMCs (70 × 104/well) and Huh7.5.1 cells, JFH-1/Huh7.5.1 cells, SGR cells, or cured cells (3.5 × 104/well), CFSE-labeled K562 cells (NK cell-sensitive cell line) (10 × 104/well) were added to the coculture, followed by further incubation for 4 hours and staining with 7-AAD. The percentage of K562 cell lysis was examined by flow cytometry.
CD107a DEGRANULATION AND INTRACELLULAR IFN-γ PRODUCTION BY NK CELLS
The expression of CD107a and IFN-γ by NK cells in response to K562 cells was analyzed as described previously.5 CD107a-positive NK cells (CD3−CD56+) and IFN-γ-positive NK cells were evaluated by means of flow cytometry. (Further details are provided in the Supporting Information.)
IMMUNOFLUORESCENCE STAINING OF LIVER TISSUE AND JFH-1/Huh7.5.1 CELLS
Immunostaining of cryopreserved sections from biopsies of HCV-infected liver tissues and JFH-1 infected Huh7.5.1 cells cultured on glass was performed as described previously.18, 19 (Further details are provided in the Supporting Information.)
SMALL INTERFERING RNA TRANSFECTION OF PRIMARY HUMAN MONOCYTES
Negatively selected human monocytes from healthy donors were transfected with a small interfering RNA (siRNA) targeting Gal-9 (s194595, s8160, s8161) and a negative control (4390843; all from Applied Biosystems), as described previously.20 At 48 hours posttransfection, the transfected monocytes were mixed with autologous monocyte-depleted PBMCs at a ratio of 1:9 (monocytes/monocyte-depleted PBMCs), and the mixed PBMCs were cocultured for 24 hours with JFH-1/Huh7.5.1 cells. The same number of monocytes transfected with the Gal-9-specific siRNA or with the control siRNA was added to autologous monocyte-depleted PBMCs.
WESTERN BLOT ANALYSIS
Anti-Gal-9 (R&D systems) and anti-β-actin (Sigma-Aldrich) antibodies were used for immunodetection. Western blotting was performed as described previously 2.
ANNEXIN V ASSAY FOR DETECTING APOPTOTIC CELLS
To measure apoptosis, cells were suspended in annexin V binding buffer (Biolegend), stained with 7-AAD and annexin V, and subjected to flow cytometry. Annexin V (+)/ 7-AAD (−) cells were regarded as apoptotic.
TRANSWELL ASSAY, BLOCKING ASSAYS AND REAGENTS
See the Supporting Information for further details.
STATISTICAL ANALYSIS
The Mann-Whitney test or Kruskal-Wallis test was applied to detect differences between groups, and Fisher's exact test was used to compare categorical variables using the JMP statistical software (SAS Institute, NC). A P value < 0.05 was regarded as statistically significant.
Results
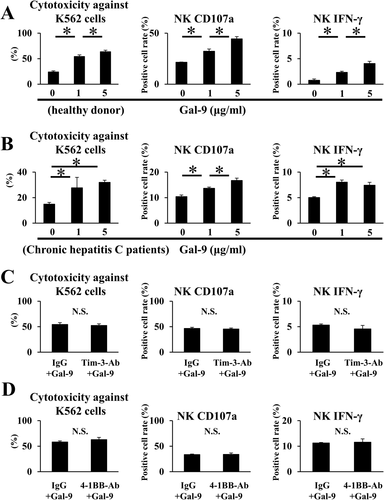
GAL-9 INCREASES THE CYTOTOXICITY OF NAÏVE NK CELLS IN A DOSE-DEPENDENT MANNER
We first evaluated whether Gal-9 could activate naïve NK cells obtained from healthy donors. The cytotoxicity of NK cells toward K562 cells was increased with the addition of rhGal-9 in a dose-dependent manner, as was the expression of CD107a, a cytotoxic marker of NK cells (Fig. 1A). IFN-γ expression of naïve NK cells was also increased by the addition of Gal-9 (Fig. 1A). We obtained similar results in NK cells from CHC patients (Fig. 1B). Tim-3 and 4-1BB have been identified as receptors for Gal-9.13, 14, 21 However, addition of a neutralizing anti-Tim-3 or 4-1BB antibody did not prevent cytotoxicity toward K562 cells or CD107a and IFN-γ expression of Gal-9-activated NK cells (Fig. 1 C, D).
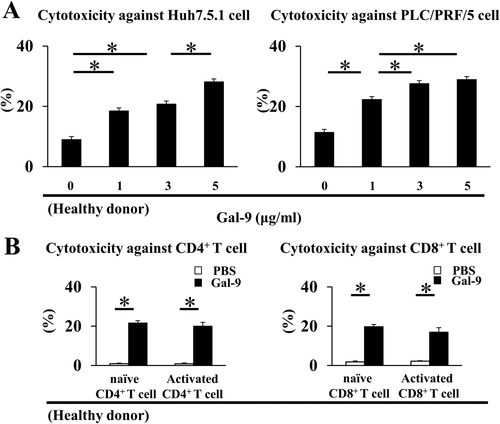
Gal-9-ACTIVATED NK CELLS LYSE BOTH HEPATOMA CELLS AND T CELLS
As both liver injury and persistent viral infection are associated with the lysis of hepatocytes and activated T cells by activated NK cells,6-9 we examined whether Gal-9-activated NK cells could lyse hepatoma cells and T cells. Addition of rhGal-9 increased the cytotoxicity of NK cells toward Huh7.5.1 cells and PLC/PRF/5 cells (Fig. 2A). Next, human CD4+ or CD8+ T cells were separated from healthy donors and stimulated with or without an anti-CD3/CD28 antibody for 24 h before co-culture. The Gal-9-activated NK cells exhibited cytotoxicity toward both naïve and activated CD4+ and CD8+ T cells (Fig. 2B).
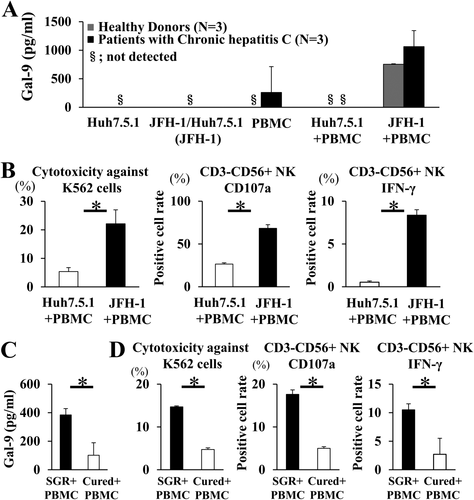
BOTH Gal-9 PRODUCTION AND NK CELL CYTOTOXICITY ARE INCREASED IN JFH-1/Huh7.5.1 CELL AND PBMC COCULTURES
PBMCs were cocultured with JFH-1/Huh7.5.1 cells. HCV infection in most Huh7.5.1 cells was confirmed by the addition of infectious HCV in cell culture (HCVcc) at an MOI of 2 (unpublished data). The Gal-9 levels in the supernatant were higher in the coculture of JFH-1/Huh7.5.1 cells with PBMCs than in the coculture of uninfected Huh7.5.1 cells with PBMCs or in the culture of PBMCs alone, including those isolated from both healthy donors and CHC patients (Fig. 3A). NK cell cytotoxicity toward K562 cells was significantly higher in the JFH-1/Huh7.5.1 cell and PBMC coculture than in that of uninfected Huh7.5.1 cells with PBMCs (Fig. 3B). CD107a and IFN-γ expression by CD3−CD56+ NK cells was also significantly higher when cocultured with JFH-1/Huh7.5.1 cells (Fig. 3B). We also examined Gal-9 production and NK cell cytotoxicity after coculture of naïve PBMCs with SGR cells or IFN-cured cells. We used SGR cells to establish the latter because JFH-1-infected Huh7.5.1 cells die shortly after infection with JFH-1.22 The supernatant Gal-9 level was significantly lower in the coculture with cured cells than in that with SGR cells (Fig. 3C). The cytotoxicity of NK cells toward K562 cells as well as CD107a and IFN-γ expression of CD3−CD56+ NK cells were also significantly lower in the coculture of cured cells with PBMCs than in the coculture of SGR cells with PBMCs (Fig. 3D).
HUMAN CD14+ MONOCYTES ARE INVOLVED IN Gal-9 PRODUCTION IN JFH-1/Huh7.5.1 CELL AND PBMC COCULTURE
We examined Gal-9-producing cells in the coculture of JFH-1/Huh7.5.1 cells with PBMCs after removing specific subsets from the PBMC pool. Depletion of CD14+ monocytes from PBMCs (PBMC-CD14) significantly reduced the level of Gal-9 in the supernatant (Fig. 4A), whereas depletion of CD19+ B cells, CD56+ NK cells, or CD3+ T cells from PBMCs did not reduce the Gal-9 level. In addition, Gal-9 levels were significantly higher in PBMCs depleted of CD19+ B cells (PBMC-CD19), PBMCs depleted of CD56+ NK cells (PBMC-CD56), and PBMCs depleted of CD3+ T cells (PBMC-CD3) than in the PBMC control group. This might be due to the relative increase of proportion of CD14+ monocytes in the PBMC-CD19, PBMC-CD56, and PBMC-CD3 groups. These results suggest that CD14+ monocytes are involved in Gal-9 production.
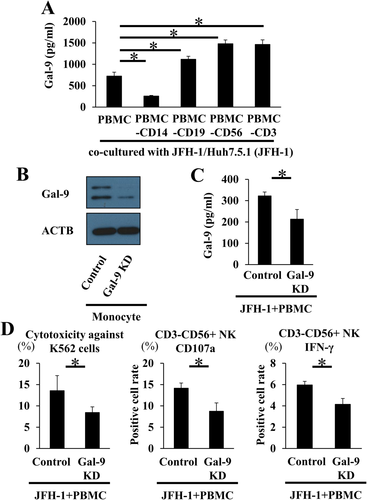
NK CELL CYTOTOXICITY IS DEPENDENT ON MONOCYTE-DERIVED Gal-9
To inhibit Gal-9 production by monocytes, isolated primary monocytes were transfected with a Gal-9-specific siRNA or nontarget siRNA control; the transfected monocytes were added to autologous monocyte-depleted PBMCs and cocultured with JFH-1/Huh7.5.1 cells, followed by evaluation of NK cell cytotoxicity. Gal-9 knockdown was confirmed by western blotting (Fig. 4B), and we also confirmed that the supernatant Gal-9 level in the Gal-9 knockdown PBMC and JFH-1/Huh7.5.1 cell coculture was significantly decreased compared with the control (Fig. 4C). Knockdown of Gal-9 decreased the cytotoxicity of NK cells and their expression of CD107a and IFN-γ compared with the control (Fig. 4D), demonstrating that NK cell activation is dependent on monocyte-derived Gal-9.
CELL-CELL CONTACT IS REQUIRED FOR Gal-9 PRODUCTION IN JFH-1/Huh7.5.1 CELL AND PBMC COCULTURE
IFN-γ has been reported to up-regulate Gal-9 production,15 and we observed increased IFN-γ expression by NK cells in response to JFH-1/Huh7.5.1 cell and PBMC coculture (Fig. 3C). Thus, we evaluated whether IFN-γ influences Gal-9 production and NK cell cytotoxicity and found that blocking IFN-γ did not alter the levels of Gal-9 in the supernatant or the cytotoxicity of NK cells when JFH-1/Huh7.5.1 cells and PBMCs were cocultured (Fig. 5A,B). To assess whether Gal-9 production requires cell-cell contact, JFH-1/Huh7.5.1 cells and PBMCs were cocultured in a transwell system. The Gal-9 levels in the supernatant were significantly lower in the transwell system than in typical coculture (Fig. 5C), suggesting that Gal-9 production requires cell-cell contact. In contrast, direct addition of infectious HCVcc to PBMCs did not increase Gal-9 production (Fig. 5D).
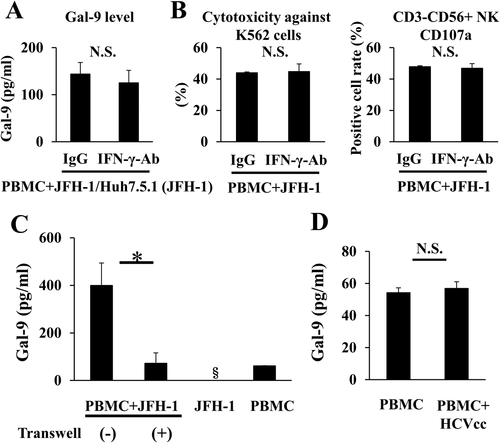
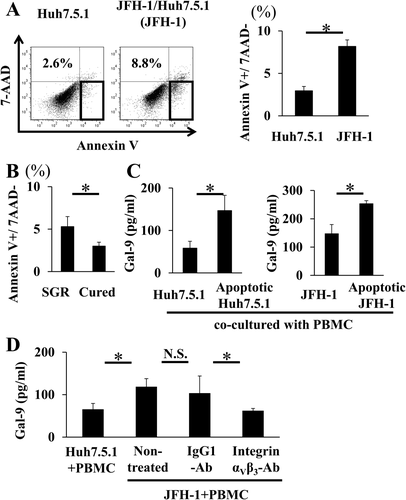
Gal-9 PRODUCTION IS PROMOTED WHEN PBMCs ARE COCULTURED WITH APOPTOTIC CELLS
HCV infection induces hepatocyte apoptosis.23 Apoptotic cells present an “eat me” signal, most likely the display of phosphatidylserine, which causes engulfment of the cells by phagocytes.24 We speculated that monocytes might recognize elevated phosphatidylserine levels on the cell surface and that these activated monocytes might produce Gal-9 in CHC. The percentage of apoptotic hepatocytes was greater among JFH-1/Huh7.5.1 cells than uninfected Huh7.5.1 cells (Fig. 6A), and the percentage of apoptotic hepatocytes was also greater among SGR cells than cured cells (Fig. 6B). Next, we cocultured PBMCs with apoptotic Huh7.5.1 cells treated with staurosporine (0.2 μM) and found that Gal-9 levels were significantly higher when PBMCs were cocultured with apoptotic Huh7.5.1 cells than when cocultured with untreated Huh7.5.1 cells (Fig. 6C). A similar result was obtained when PBMCs were cocultured with apoptotic-induced JFH-1/Huh7.5.1 cells by staurosporine (Fig. 6C). Integrin αvβ3 is one of the receptors for phosphatidylserine on phagocytes.24 Therefore, we cocultured JFH-1/Huh7.5.1 cells and PBMCs with addition of an anti-integrin αvβ3 blocking antibody. The levels of Gal-9 were decreased in the presence of the anti-integrin αvβ3 antibody (Fig. 6D), suggesting that Gal-9 production is mediated via integrin αvβ3.
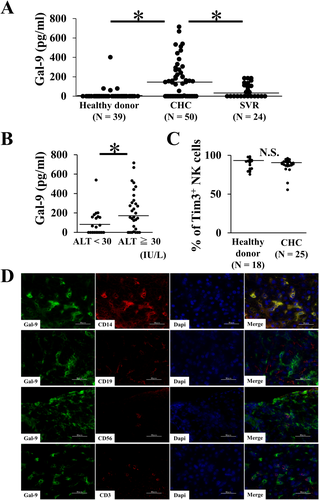
SERUM Gal-9 LEVELS AND Gal-9 EXPRESSION IN THE LIVER TISSUES OF CHC PATIENTS
The serum Gal-9 levels from 40 healthy donors, 50 CHC patients, and 24 SVR patients were analyzed by means of ELISA (Table 1), showing significant increases in CHC patients compared with healthy donors and SVR patients (Fig. 7A). Among 50 CHC patients, the levels of serum Gal-9 were significantly higher in those with elevated ALT than those with normal ALT (Fig. 7B), which suggested that Gal-9 is associated with liver injury. Next, we analyzed the expression of Tim-3, a Gal-9 receptor, on NK cells from CHC patients and healthy donors using flow cytometry, with no significant difference observed (Fig. 7C). Consistent with our in vitro results, immunofluorescence analysis of snap-frozen CHC liver tissues revealed colocalization of most Gal-9+ cells with CD14+ cells but not with CD19+ cells, CD56+ cells, and CD3+ cells (Fig. 7D).
| Control | CHC | SVR | P | |
|---|---|---|---|---|
| Number of patients | 40 | 50 | 24 | |
| Sex, male/female | 22/18 | 17/33 | 12/12 | NS |
| Age, years, median (range) | 67 (48-78) | 65 (29-81) | 63 (25-73) | NS |
| ALT, IU/L, median (range) | 17 (9-30) | 39 (10-313) | 18 (6-50) | <0.05 |
| HCV-RNA, Log IU/mL, median (range) | Not tested | 6.4 (2.4-7.4) | Not tested | — |
- Abbreviation: NS, not significant.
Discussion
We demonstrated that the addition of rhGal-9 could activate the cytotoxicity of naïve NK cells. Previous reports have demonstrated that Gal-9 decreased the cytotoxicity of interleukin (IL)-stimulated NK cells.16 The discrepancy between our results and those of Golden-Mason might result from the condition of the NK cells: we used naïve NK cells, whereas Golden-Mason et al. used IL-stimulated fully activated NK cells. Our results show the opposite function of Gal-9 on NK cells; the addition of rhGal-9 increased the cytotoxicity of naïve NK cells, whereas it decreased the cytotoxicity of IL-stimulated NK cells (Supporting Fig. S1). Another lectin, Siglec-2, inhibits B-cell receptor signaling and can also activate alternative signaling pathways, resulting in B cell activation.25 Similar to Siglec-2, the condition of NK cells might influence Gal-9 binding to these cells or the activation of signaling pathways when adding Gal-9. Both Tim-3 and 4-1BB are known receptors of Gal-9. In our study, blocking Tim-3 and 4-1BB did not change the cytotoxicity of Gal-9-activated NK cells, indicating that the activation of naïve NK cells does not depend on the interaction between Gal-9 and Tim-3 or 4-1BB. Recent reports have shown that the function of Gal-9 is independent of Tim-3,16, 26 suggesting that NK activation might depend on interaction between Gal-9 and an unknown receptor.
We demonstrated that Gal-9-activated NK cells were able to lyse CD4+ and CD8+ T cells. Recent reports have shown that activated NK cells regulate T cell activities and that this regulation contributes to persistent viral infection.7, 9 The levels of serum Gal-9 in CHC patients were higher than in healthy subjects or SVR patients, indicating that Gal-9 elevation might be related to persistent infection of HCV. Our data raise the possibility that Gal-9-activated NK cells might be involved in persistent HCV infection. Ahlenstiel et al.5 suggested that HCV-induced IFN-α causes NK cell activation,5 and we investigated whether cytokine-stimulated NK cells (IFN-α-activated, IL-2-activated, and IL-12+15-activated NK cells) could lyse T cells. As shown in Fig. 2B, Gal-9-activated NK cells were able to lyse both CD4+ and CD8+ T cells, with a cytotoxic capacity of approximately 20%. In contrast, although IL-12+15-activated NK cells could lyse CD8+ T cells, the observed cytotoxicity was less than 5%. In addition, the majority of activated-NK cells did not lyse CD4+ or CD8+ T cells (Supporting Fig. S2). These results suggest that Gal-9 might be associated with NK cell-mediated deletion of T cells in CHC.
We showed that Gal-9-activated NK cells lysed Huh7.5.1 cells and PLC/PRF/5 cells and that coculture of PBMCs with JFH-1/Huh7.5.1 increased Gal-9 production and NK cell cytotoxicity compared with the control. Consistent with these results, serum Gal-9 levels were higher in patients with elevated ALT than in patients with normal ALT. These results indicated that Gal-9-activated NK cells might induce liver injury. Moreover, we found that coculture of PBMCs with cured cells decreased both Gal-9 levels and NK cell cytotoxicity, which supports the idea that HCV infection induced Gal-9 production. We also demonstrated that the main source of Gal-9 is CD14+ monocytes. Immunofluorescence analysis revealed that CD14+ cells in the liver, which represent Kupffer cells,27 produce Gal-9, in accordance with previous reports.12, 15, 18 These results were consistent with the in vitro results of Gal-9 production and NK cell cytotoxicity during HCV infection. Knockdown of Gal-9 in monocytes reduced the cytotoxicity of NK cells in JFH-1/Huh7.5.1 cell and PBMC coculture. These results suggest that monocyte-derived Gal-9 increases NK cell cytotoxicity in CHC.
We demonstrated that IFN-γ production by NK cells increases in response to JFH-1/Huh7.5.1 cells, as reported previously.28 Furthermore, because IFN-γ is known to up-regulate Gal-9,15 we examined whether IFN-γ influences Gal-9 production and NK cell cytotoxicity. However, blocking IFN-γ did not alter the supernatant levels of Gal-9 or the cytotoxicity of NK cells when JFH-1/Huh7.5.1 cells and PBMCs were cocultured. Transwell assays revealed that the supernatant Gal-9 level largely depends on cell-cell contact rather than cytokines in JFH-1/Huh7.5.1 cell and PBMC coculture. Thus, we submit that IFN-γ does not affect Gal-9 production or NK cell cytotoxicity.
Gal-9 is produced by CD14+ monocytes, which are parts of the phagocyte pool. Phagocytes recognize apoptotic cells through phosphatidylserine expressing on the surface of apoptotic cells, and HCV infection induces hepatocyte apoptosis.23 Therefore, we speculated that Gal-9 might be produced by CD14+ monocytes that recognize apoptotic hepatocytes. We demonstrated that Gal-9 levels in coculture of PBMCs with apoptotic Huh7.5.1 cells were significantly higher than in coculture of PBMCs with nonapoptotic Huh7.5.1 cells. We also showed that HCV infection resulted in increasing apoptosis in vitro. Blocking integrin αvβ3, one of the receptors for phosphatidylserine on phagocytes, decreased the Gal-9 level when JFH-1/Huh7.5.1 cells and PBMCs were cocultured. These results suggest that Gal-9 was produced via integrin αvβ3 in PBMC coculture with HCV infection-induced apoptotic cells.
Recently, HCV treatment has changed dramatically. IFN-free direct-acting antivirals (DAAs) have made it possible to achieve HCV clearance in more than 95% of patients. However, there are still patients who fail to achieve sustained viral response in DAA treatment, and continuous liver damage might increase the development of liver cirrhosis and hepatocellular carcinoma. Gal-9 might be a potential therapeutic target for liver injury in those DAA-resistant patients. In addition, we showed that the increase in hepatocyte apoptosis induces Gal-9 production, which might be associated with liver injury by regulating NK cell cytotoxicity. Hepatocyte apoptosis occurs in CHC as well as in other liver diseases, such as chronic hepatitis B and nonalcoholic steatohepatitis. Therefore, apoptosis is a possible cause of secondary liver injury, and Gal-9 might have a potent target for liver injury in other liver diseases. We also confirmed Gal-9 expression, colocalized with CD14+ cells, in the liver tissue of patients with chronic hepatitis B and NASH (unpublished data).
In this study, we focused on the function of Gal-9 in CHC and demonstrated that CD14+ monocyte-derived Gal-9 increased NK cell cytotoxicity, which might be associated with liver injury and persistent HCV infection.




