Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice
Potential conflict of interest: Nothing to report.
This study was funded by NIH grants 1R01DK62277 and 1R01DK100287 and Endowed Chair for Experimental Pathology to SPSM, and NIH grant 1R01DK103775 (to K.N.B.).
Abstract
Hepatic repair is directed chiefly by the proliferation of resident mature epithelial cells. Furthermore, if predominant injury is to cholangiocytes, the hepatocytes can transdifferentiate to cholangiocytes to assist in the repair and vice versa, as shown by various fate-tracing studies. However, the molecular bases of reprogramming remain elusive. Using two models of biliary injury where repair occurs through cholangiocyte proliferation and hepatocyte transdifferentiation to cholangiocytes, we identify an important role of Wnt signaling. First we identify up-regulation of specific Wnt proteins in the cholangiocytes. Next, using conditional knockouts of Wntless and Wnt coreceptors low-density lipoprotein-related protein 5/6, transgenic mice expressing stable β-catenin, and in vitro studies, we show a role of Wnt signaling through β-catenin in hepatocyte to biliary transdifferentiation. Last, we show that specific Wnts regulate cholangiocyte proliferation, but in a β-catenin-independent manner. Conclusion: Wnt signaling regulates hepatobiliary repair after cholestatic injury in both β-catenin-dependent and -independent manners. (Hepatology 2016;64:1652-1666)
Abbreviations
-
- Alb
-
- albumin
-
- ALP
-
- alkaline phosphatase (ALP)
-
- ALT
-
- serum alanine aminotransferase
-
- BDL
-
- bile duct ligation
-
- BECs
-
- biliary epithelial cells
-
- CK19
-
- cytokeratin 19
-
- DDC
-
- 3,5-diethoxycarbonyl-1,4-dihydrocollidine
-
- ECs
-
- endothelial cells
-
- EpCAM
-
- epithelial cell adhesion molecule
-
- GS
-
- glutamine sythetase
-
- HCs
-
- hepatocytes
-
- HCC
-
- hepatocellular carcinoma
-
- HNF4α
-
- hepatocyte nuclear factor 4 alpha
-
- HSCs
-
- hepatic stellate cells
-
- IHC
-
- immunohistochemistry
-
- KO
-
- knockout
-
- Kps
-
- Kupffer cells
-
- LRP
-
- low-density lipoprotein-related protein
-
- mRNA
-
- messenger RNA
-
- PC
-
- pericentral area
-
- PCR
-
- polymerase chain reaction
-
- PMHs
-
- primary mouse hepatocytes
-
- PP
-
- periportal area
-
- PT
-
- portal triad area
-
- qRT-PCR
-
- quantitative reverse-transcriptase PCR
-
- siRNA
-
- small interfering RNA
-
- sm-cc
-
- small cholangiocyte
-
- Sox9
-
- SRY (sex determining region Y)-box 9
-
- TCF
-
- T-cell factor
-
- Wls
-
- Wntless
-
- Wls-KO
-
- liver-specific Wls KO
-
- WT
-
- wild-type
Chronic cholestasis results from impairment of bile formation or bile flow and can progress to cirrhosis and hepatocellular insufficiency requiring transplantation.1 Mice fed the biliary toxin, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), exhibit characteristic features of chronic cholestasis, such as bile duct injury, cholangitis, and periductal fibrosis.2 DDC feeding also triggers a ductular reaction composed of both proliferating cholangiocytes as well as hepatocytes (HCs) that acquire a cholangiocyte-like phenotype through a process called transdifferentiation.3 In fact, several high-profile studies in recent years have identified HC-to-cholangiocyte transdifferentiation, and vice versa, to promote hepatobiliary repair in the context of specific injuries.3-6 However, no studies to date have addressed the molecular mechanisms underlying such repair processes.
The Wnt/β-catenin pathway is a critical regulator of liver development and physiology and has been investigated in a wide range of pathologies, such as hepatocellular carcinoma (HCC), alcoholic liver disease, fibrosis, acute liver injury, and others (reviewed in a previous work7). Wnt proteins are extracellular signaling molecules that trigger activation of the canonical Wnt pathway and nuclear translocation of β-catenin by binding to its cell-surface receptor, Frizzled, and coreceptor, low-density lipoprotein-related protein (LRP) 5/6. Because the posttranslational modification of Wnt proteins required for bioactivation renders them hydrophobic, a molecular chaperone is required for transport of Wnts to the cell surface. This process is mediated by Wntless (Wls), a multipass transmembrane protein that conveys Wnts from the Golgi to the membrane for secretion.8 Cell-specific Wls deletion has divulged important roles for this signaling pathway in brain and bone development.9 In the liver, inhibiting Wnt secretion from Kupffer cells (Kps) and endothelial cells (ECs) has revealed that these cell types are an important source of Wnt proteins that, in turn, activate HC β-catenin during liver regeneration.10
Although little is known about the impact of Wnt signaling in chronic liver injury, liver-specific β-catenin knockout (KO) mice exhibited significantly fewer cells staining positive for A6, a ductular and progenitor marker, after 2 weeks of DDC diet, suggesting that Wnt signaling may play a meaningful role in inducing atypical ductular proliferation during cholestasis.11 In order to explore this hypothesis, we performed a thorough characterization of Wnt signaling in mice on control and DDC diet. Through manual microdissection and liver cell isolation, we identify specific Wnts that are up-regulated during cholestasis and address the cellular source of these Wnts. Through use of in vitro model systems, we demonstrate the role of these Wnts in HC-to-biliary transdifferentiation and cholangiocyte proliferation. Finally, we utilize liver-specific KO mice lacking either all Wnt signaling, or lacking canonical Wnt/β-catenin signaling only, to validate the importance of these Wnts in the repair of cholestatic liver disease in mice. Thus, we have identified that Wnt signaling is important for biliary repair in cholestatic liver disease, and that blockade of Wnt secretion from epithelial cell adhesion molecule (EpCAM)-expressing cells disrupts this process, resulting in increased liver injury and mortality in the setting of cholestatic liver disease.
Materials and Methods
MICE
Liver-specific Wls KO (Wls-KO) mice and liver-specific LRP5/6 KO mice were generated as described.10 Mice overexpressing a stable form of β-catenin, where serine 45 is mutated to aspartic acid (S45D), have been characterized previously.12 Genotyping on all mice was performed by polymerase chain reaction (PCR) analysis using genomic DNA isolated from a tail clipping, as described.9, 10, 13, 14
Wls-KO, S45D mice, and corresponding controls were administered 0.1% DDC diet as described.11 Mice were sacrificed after 2 weeks or 1 month of diet and livers harvested for subsequent analysis. LRP5/6 KO mice and controls were subjected to bile duct ligation (BDL) by double ligating the common bile duct above the pancreas and cutting it between the ligatures.15 Mice were sacrificed 14 days postsurgery. Blood samples were collected from the orbital sinus at the time of sacrifice. Serum biochemical measurements for total bilirubin and alanine aminotransferase (ALT) were performed by the University of Pittsburgh Medical Center Clinical Chemistry laboratory.
CELL LINES
Hep3B human hepatoma cells, 293T human embryonic kidney cells, and AML12 mouse HCs were obtained from the American Type Culture Collection (Manassas, VA) and cultured as detailed in the Supporting Methods. To directly coculture 293T cells and AML12 cells (or primary hepatocytes), 293T cells were first transfected with Wnt-expressing plasmids in six-well plates using Lipofectamine 3000 (Invitrogen, Carlsbad, CA), as per the manufacturer's instructions. Cells were allowed to incubate for 4 hours, after which an equal number of AML12 cells were added to the culture wells (2.5 × 105 cells of each type). Cocultures were harvested 48 hours later for RNA analysis.
For analysis of transdifferentiation, AML12 cells and Hep3B cells were treated with recombinant human Wnt7A (Abcam, Cambridge, MA), which was added at a concentration of 500 ng/mL. Cells were harvested 48 hours later for RNA analysis or measurement of luciferase activity.
CELL SEPARATION
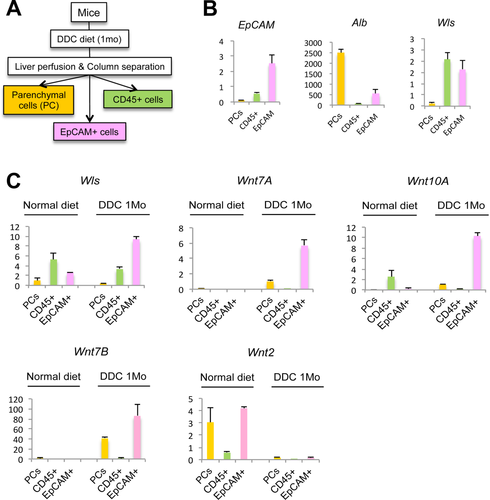
Parenchymal and nonparenchymal cells from mouse liver were isolated by a two-step collagenase perfusion.16 The parenchymal cell fraction, which consists mostly of HCs, also contains hepatic stellate cells (HSCs) attributed to their close physical association with hepatocytes in vivo. Positive selection of CD45+ cells and EpCAM+ cells from nonparenchymal cells was performed using the QuadroMACS column separation Kit (Miltenyi Biotech, Cambridge, MA).10 Cells were then purified using the Dead Cell Removal Kit (Miltenyi Biotech). Antimouse CD45 antibody, antimouse EpCAM antibody (BioLegend, San Diego, CA), and microbeads were used according to the manufacturer's instructions. To assess the purity of EpCAM+ cells, low expression of CD68 (Kp) and Tie2 (ECs) was confirmed by quantitative reverse-transcriptase PCR (qRT-PCR).
Additional methods are available in the online Supporting Methods.
Results
CELLS IN THE PORTAL TRIAD EXPRESS Wnt 7A, 7B, AND 10A AFTER DDC DIET
To address the role of Wnt signaling in biliary injury and cholestasis, we subjected wild-type (WT) mice to DDC diet and then sacrificed the mice after 1 month (28 days). The pericentral area (PC), periportal area (PP), and portal triad (PT) regions were then carefully removed from formalin-fixed, paraffin-embedded liver sections by manual microdissection and subjected to real-time PCR for gene expression analysis (Fig. 1A). Measurement of glutamine synthetase (GS), which is expressed in PC HCs, and EpCAM, which is expressed by normal cholangiocytes and oval cells in the PT,17 confirms the specificity of this isolation (Fig. 1B). SRY (sex determining region Y)-box 9 (Sox9), another biliary marker, was expressed mainly in the PT; however, its presence in the PP region indicates the acquisition of a biliary-like phenotype by a population of HCs around the PT, which is a hallmark of cholestatic liver disease. Interestingly, Wls expression was also highest in the PT region, indicating that either cholangiocytes or PP HCs may be the source of Wnts in this model (Fig. 1C).
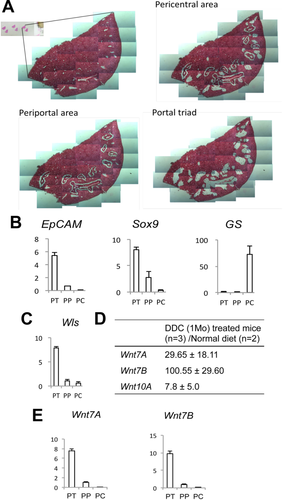
Next, expression of Wnt genes after 1 month of DDC diet was compared to normal liver. We identified three Wnts—Wnt7A, Wnt7B, and Wnt10A—to be remarkably increased after DDC diet (Fig. 1D). Further analysis revealed that expression of Wnt7A and 7B, which showed the highest fold increase after DDC, was detected mainly in the PT (Fig. 1E). Thus, during DDC-induced cholestasis, a significant up-regulation of several specific Wnts occurs in cells of the PT region, which also coincides with the site of Wls expression.
EpCAM-EXPRESSING CELLS ARE THE SOURCE OF Wnts DURING CHOLESTASIS
To determine which cell type in the PT is responsible for Wnt production during cholestasis, we isolated different cell populations from mouse livers subjected to DDC diet for 28 days. CD45+ cells (macrophages), EpCAM+ cells (cholangiocytes), and parenchymal cells (HCs) were isolated by a two-step liver perfusion and column separation technique (Fig. 2A). The purity of the cell populations obtained by isolation was verified by measuring the expression of EpCAM, which was highest in the EpCAM+ enriched cell population, and albumin (Alb), which was expressed mainly in the parenchymal cell fraction (Fig. 2B). Measurement of Wls expression in the cell fractions demonstrated that both CD45+ and EpCAM+ cells express Wls and could thus be the source of Wnt production in the PT during cholestasis. Further analysis of these cell populations before and after DDC diet, however, demonstrates a 3-fold increase in Wls expression in the EpCAM+ cells after DDC diet, whereas expression of Wls in the CD45+ population decreases, implicating EpCAM+ cells as the source of Wnts during cholestasis (Fig. 2C). Indeed, measurement of Wnt7A, 7B, and 10A, the three Wnts increased after DDC diet, shows that expression of these genes, which is virtually absent on a normal diet, is dramatically increased in the EpCAM+ cell fraction after DDC, whereas Wnt2, which is expressed in normal liver, is absent after DDC treatment, demonstrating the specificity of Wnt expression during cholestasis (Fig. 2C). Immunohistochemistry (IHC) for Wnt7A and Wnt10A also confirms the expression of these two proteins predominantly in cholangiocytes and in a few PP HCs after DDC injury (Supporting Fig. S1). Thus, EpCAM+ cells are the primary Wnt-producing cells in the PT during DDC-induced liver injury.
Wnt7B AND Wnt10A PROMOTE PROLIFERATION OF SMALL CHOLANGIOCYTES
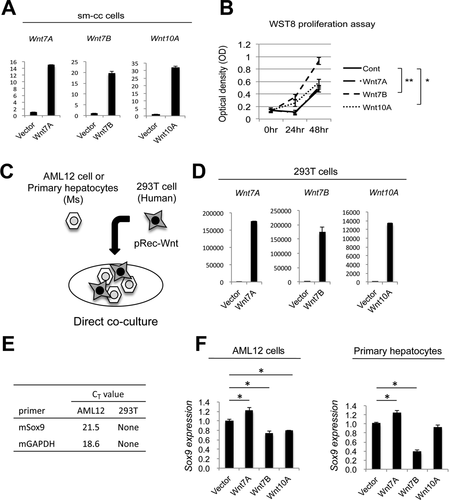
Because Wnts can act in an autocrine manner,18 and because cholangiocyte proliferation is rampant in the PT region after DDC, we then asked whether the Wnts produced by EpCAM+ cholangiocytes or progenitors had a mitogenic impact on cholangiocytes. A small mouse-SV40 cholangiocyte (sm-cc) cell line19 was transiently transfected with Wnt7A, Wnt7B, or Wnt10A plasmids; successful transfection was confirmed by up-regulation of the specific Wnt being expressed (Fig. 3A). Interestingly, Wnt7B significantly induced proliferation of sm-cc cells, whereas Wnt10A had a modest impact on proliferation and Wnt7A had no effect (Fig. 3B). Thus, EpCAM+ cells secrete Wnts that facilitate their proliferation in an autocrine fashion.
Wnt7A INDUCES Sox9 EXPRESSION IN CULTURED HCs THROUGH ACTIVATION OF β-CATENIN
Because chronic biliary injury induces the reprogramming of HCs to a biliary phenotype, we next wanted to determine whether the Wnts produced by EpCAM+ cells play any role in this process. We generated a direct coculture system where 293T human embryonic kidney cells were transfected with Wnts 7A, 7B, or 10A and cocultured with either mouse-derived AML12 cells or primary mouse hepatocytes (PMHs; Figure 3C). Transfection of 293T cells with the individual Wnt plasmids induced a robust expression of the corresponding Wnt (Fig. 3D). Primers were then designed to specifically detect expression of mouse Sox9 in the cocultures, thus excluding the contribution of any endogenous Sox9 expression in human 293T cells (Fig. 3E). Sox9 expression was modestly, but significantly, increased in both AML12 cells and PMHs cocultured with Wnt7A-expressing 293T cells. Cells cultured with 293T cells expressing Wnt7B or Wnt10A showed either no effect on Sox9 expression or a decrease in Sox9 expression (Fig. 3F).
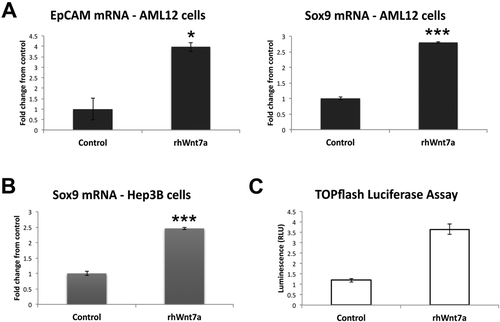
Whereas this experiment replicated in vitro the paracrine effect of Wnt7A on hepatocytes and identified a preliminary role of Wnt7A in HC transdifferentiation, we wanted to validate these findings by measuring expression of biliary markers in cultured HCs treated with recombinant Wnt7A protein. AML12 cells cultured in the presence of recombinant Wnt7A protein for 2 days showed significantly increased expression of both EpCAM and Sox9 messenger RNA (mRNA; Fig. 4A), further demonstrating that Wnt7A contributes to HC reprogramming. To demonstrate that this effect was observed across species, we utilized human hepatoma cells and confirmed that Wnt7A also increases Sox9 expression in Hep3B cells (Fig. 4B). Next, we wanted to determine whether Wnt7A acts through the canonical Wnt signaling pathway, culminating in transcriptional activation of β-catenin, in order to activate Sox9 expression. We transfected Hep3B cells with TOPflash luciferase reporter, which measures β-catenin/T-cell factor 4 (TCF4)-dependent transcriptional activation, in the presence or absence of Wnt7A. Figure 4C shows that Wnt7A significantly increases luciferase activity, suggesting that β-catenin-dependent Wnt signaling promotes reprogramming toward a biliary phenotype.
MICE OVEREXPRESSING Ser-45 MUTANT β-CATENIN SHOW INCREASED HC-TO-BILIARY TRANSDIFFERENTIATION AFTER DDC DIET
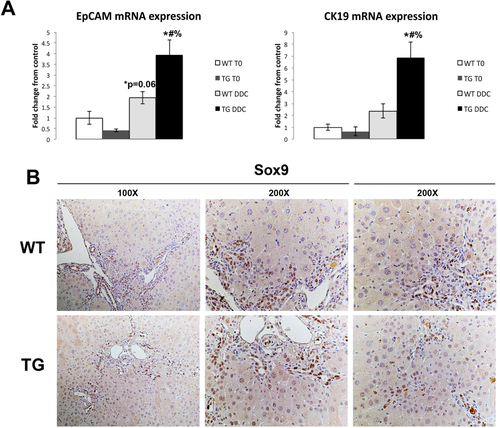
We have previously published that mice expressing a mutated nondegradable form of β-catenin (S45D) in liver have a significant number of HCs expressing cholangiocyte markers after long-term exposure to DDC compared to age-matched WT mice.20 In order to verify the role of Wnt/β-catenin signaling in HC reprogramming in vivo, we performed a detailed analysis of livers from WT and S45D mice on DDC diet. We observed that increased expression of biliary markers EpCAM and cytokeratin 19 (CK19) occurs as early as 28 days after DDC in S45D mice (Fig. 5A). Furthermore, S45D hepatocytes in the PP region also begin to express Sox9, a transcription factor normally restricted to bile ducts, as shown by IHC (Fig. 5B).21 Therefore, activation of β-catenin enhances hepatobiliary repair after DDC injury by inducing transdifferentiation of HCs to cholangiocytes.
Wls-KO MICE SHOW DECREASED DUCTULAR RESPONSE AND INCREASED MORTALITY FOLLOWING DDC DIET
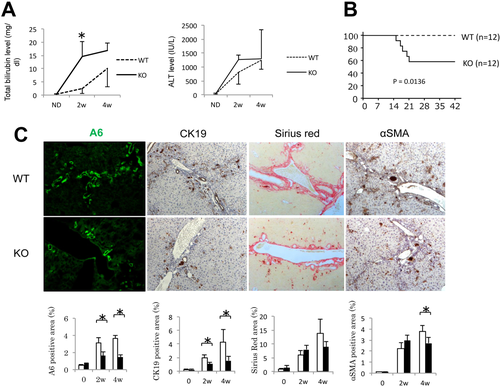
In order to assess the impact of inhibiting Wnt signaling during cholestasis, we generated liver-specific conditional Wls-KO mice and performed a detailed analysis of Wls gene expression in various cell populations at baseline. After confirming deletion of Wls in the KO liver (Supporting Fig. S2A), we separated nonparenchymal cells—containing Kps, ECs, and cholangiocytes, or biliary epithelial cells (BECs)—from parenchymal cells, which contain HCs as well as HSCs (Supporting Fig. S2B), and measured Wls expression in these two cell populations. Wls expression is much higher in nonparenchymal cells than in parenchymal cells (Supporting Fig. S2C); however, parenchymal cells from KO livers have significantly less Wls expression than WT livers, confirming efficient knockdown of Wls gene expression in HCs. Given the previous observation that the Alb-Cre system deletes floxed target genes in cholangiocytes as well,11 we looked at Wls expression in EpCAM+ cells. Indeed, Wls expression is remarkably suppressed in EpCAM+ cells isolated from the KO as compared to WT (Supporting Fig. S3A). This was confirmed by Western blotting showing loss of Wls in EpCAM+ cells, which also express CK19 (Supporting Fig. S3B).
Next, we administered DDC diet to WT and Wls-KO mice for 28 days. Analysis of serum biochemistry from both groups of animals revealed higher total bilirubin and ALT levels in KO as early as 2 weeks after DDC diet (Fig. 6A). These findings corresponded with increased mortality in Wls-KO mice fed DDC diet (Fig. 6B). Histological analysis demonstrated that KO had fewer cells positive for A6, fewer CK19-positive cells, and fewer myofibroblasts, although there was no overall difference in fibrosis between the two groups (Fig. 6C). Thus, blocking Wnt secretion from HCs and cholangiocytes resulted in fewer bile ducts, which negatively affected overall survival.
BLOCKING Wnt SECRETION FROM CHOLANGIOCYTES DECREASED CHOLANGIOCYTE PROLIFERATION AND ABROGATED TRANSDIFFERENTIATION OF HCs AFTER DDC DIET
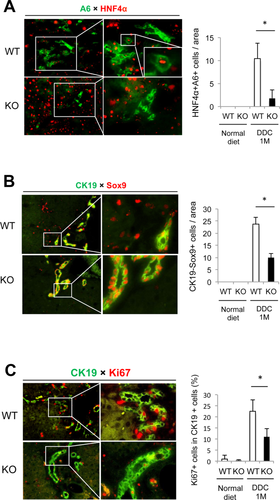
To determine which functions of Wnt signaling were impaired in Wls-KO mice during cholestasis, we performed double immunofluorescence for HC and biliary markers, as well as markers of proliferation, on WT and KO livers after DDC diet. There was a significant decrease in the numbers of transdifferentiating HCs, which stained for both A6 and hepatocyte nuclear factor 4 alpha (HNF4α), in Wls-KO compared to the control (Fig. 7A). The number of Sox9+CK19— cells, which also represents the subset of HCs acquiring a biliary phenotype, is likewise decreased in KO (Fig. 7B). Finally, the number of actively proliferating cholangiocytes, as determined by dual labeling for CK19 and Ki67, is decreased as well in KO, further substantiating the mitogenic role of Wnts in inducing biliary proliferation (Fig. 7C).
Wnt7A, 7B, AND 10A ARE OVEREXPRESSED IN MULTIPLE CHOLESTATIC INJURY MODELS ACROSS SPECIES
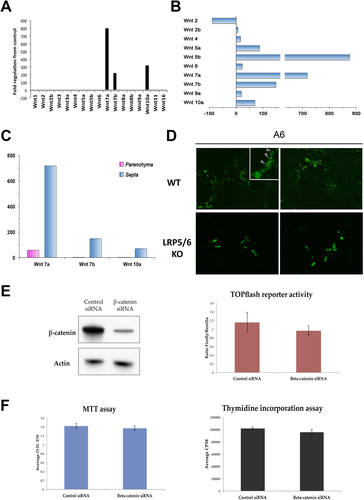
We next validated the role of Wnt signaling in BDL, a surgical obstruction that results in cholestasis, intrahepatic accumulation of toxic bile acids, and proliferation of cholangiocytes. Expression of various Wnts was measured by gene array 14 days after BDL in WT mice, which is comparable to 4 weeks of DDC diet. Intriguingly, Figure 8A shows that expression of Wnt7A, Wnt7B, and Wnt10A—the same three Wnts up-regulated after 28 days of DDC diet—were also dramatically increased in whole livers after BDL. Moreover, we also analyzed Wnt gene expression in a rat model of biliary fibrosis induced by BDL. RNA was isolated from laser capture microdissected fibrotic septa and surrounding parenchymal tissue regions, as described.22 A notable increase in Wnts7A, 7B, and 10A, in addition to Wnts 5A and B, was evident in fibrotic septa as compared to the parenchyma (Fig. 8B,C), demonstrating the relevance of these three Wnt genes in cholestatic injury across species.
Because of the commonality in Wnt gene signatures, we hypothesized that specific signaling pathways were activated during cholestasis that would differentially induce expression of these Wnts. The ChIP-X database was queried for transcription factors that are predicted to regulate Wnt gene expression.23 Although we identified six transcription factors that bind upstream of Wnts 7A, 7B, and 10A, none are exclusive to these three alone (Supporting Fig. S4), suggesting that there is a complex interaction between transcription factor binding and the existing chromatin landscape. Thus, additional studies would be required to further characterize the mechanism of Wnt up-regulation during cholestasis.
Wnt-INDUCED TRANSDIFFERENTIATION OF HCs TO CHOLANGIOCYTES AFTER BDL IS β-CATENIN DEPENDENT, WHEREAS PROLIFERATION OF CHOLANGIOCYTES IS β-CATENIN INDEPENDENT
To determine whether Wnt signaling plays a similar role in BDL as in DDC diet, we then performed BDL on liver-specific LRP5/6 KO mice, in which Wnt signaling is disrupted but β-catenin expression is intact,10 as well as littermate controls. Although we found no difference in the total number of A6-positive atypical ductules between the two groups, we did detect the presence of A6-positive hepatocytes in WT after 14 days of BDL, which were absent in LRP5/6 KO (Fig. 8D). Thus, as in DDC, Wnt/β-catenin signaling in the context of BDL may facilitate the transdifferentiation of hepatocytes to cholangiocytes.
It was intriguing to note that the number of ductular structures was equivalent in WT and LRP5/6 KO. Because deletion of LRP5/6 only inhibits Wnt/β-catenin signaling, leaving noncanonical Wnt signaling intact, we hypothesized that Wnt-stimulated cholangiocyte proliferation was independent of β-catenin. To test this, we transfected sm-cc cells with small interfering RNA (siRNA) against β-catenin, which effectively suppressed protein expression (Fig. 8E). Interestingly, TOPflash assay revealed that there is minimal β-catenin transcriptional activity in sm-cc cells at baseline, which is not further decreased by inhibition of β-catenin (Fig. 8E). Furthermore, both viability and proliferation of sm-cc cells is unchanged in the presence of β-catenin siRNA, suggesting that Wnt-induced proliferation in cholestasis may be independent of β-catenin activation (Fig. 8F).
Discussion
Our lab has recently described the critical role of Wnt signaling in regulation of β-catenin activity during liver regeneration. Utilizing genetic mouse models, we deleted Wls in epithelial cells, HSCs, macrophages, and ECs. Disruption of Wnt signaling in these hepatic cell types revealed that Kps and ECs are required for activation of β-catenin and progression of the cell cycle in HCs, a finding that has also been described by others.10, 24 The development of these model systems has afforded us the opportunity to extend our knowledge of Wnt signaling and β-catenin activation to other liver injury models. Previous work in our laboratory has revealed an important role for β-catenin in repair of DDC-induced liver injury.11, 20 However, because β-catenin can be activated by multiple signaling pathways, including protein kinase A and receptor tyrosine kinases, the regulatory mechanism remained unknown. In this study, we sought to address whether β-catenin activation is Wnt-signaling dependent by characterizing the source and role of Wnts in cholestasis.
Tissue microdissection revealed a dramatic up-regulation of Wnt7A, Wnt7B, and Wnt10A in the PP region of mouse livers subjected to DDC diet for 28 days. Expression of these Wnts is very low in the normal liver, suggesting that they are necessary only under specific circumstances, such as cholestatic liver disease. Intriguingly, expression of these same three Wnts were also increased in livers of mice and rats subjected to BDL, indicating a common signaling mechanism in cholestatic liver injury regardless of origin. Our findings were consistent with previous reports that demonstrated increased expression of these same three Wnts after DDC treatment.25 Moreover, cell-specific analysis revealed that the EpCAM-enriched population is the primary source of these Wnts during biliary injury. Cholangiocytes have been shown to play an important role during cholestatic liver injury, secreting factors pivotal for bile duct repair.26 Similarly, in this study, we have shown that Wnts secreted by proliferating biliary cells are necessary for repair of cholestatic liver injury.
Interestingly, the function of Wnt7B and Wnt10A in our model is different from that of Wnt7A, although all three are secreted by EpCAM+ cells. Both Wnt7B and Wnt10A induce the proliferation of small cholangiocytes in an autocrine fashion. Previous studies have also identified a role for Wnt10A and Wnt7B in proliferation, regeneration, and self-renewal.27-29 Interestingly, Wnt7B was also found to be expressed in poorly differentiated HCC cell lines, which have higher proliferative indices than well-differentiated HCC.30, 31
Wnt7A, on the other hand, acts in a paracrine fashion on neighboring HCs to induce cellular reprogramming. Notably, Wnt7A has been shown to induce TCF-mediated transcriptional activation when added in combination with R-spondin, a modulator of Wnt signaling, which is in line with our findings that Wnt7A signals through the canonical Wnt pathway.32 Interestingly, a previous study demonstrated that depletion of macrophages, a cellular source of Wnt7A, resulted in loss of HC specification and formation of biliary structures during hepatocellular injury and regeneration.33 Although these results differ from ours, it is important to note that the cellular source of Wnt7A and the type of liver injury differ, and thus activity of Wnt7A in liver may be context specific.
We have previously shown that the number of A6-positive cells is decreased in β-catenin KO mice at early stages (2 weeks) of DDC compared to WT littermate controls, although there is a partial recovery at 4 weeks suggesting compensatory mechanisms.11 In the current study, Wls-KO also showed a paucity of proliferating cholangiocytes after DDC, verifying an essential role for the Wnt/β-catenin axis in biliary repair. However, because, in this model, all epithelial-derived Wnts are inhibited regardless of function, we were unable to determine whether the primary role of Wnt signaling after cholestatic liver injury is to stimulate cholangiocyte proliferation or HC-to-cholangiocyte transdifferentiation. Furthermore, it was unclear whether β-catenin is required for Wnt-mediated cholangiocyte proliferation as well as HC transdifferentiation. Thus, the LRP5/6 KO mouse afforded us an opportunity to address the extent to which these processes are β-catenin dependent, given that noncanonical Wnt signaling is intact in this model. We utilized BDL as a secondary model of cholestasis and ductular proliferation to further validate the findings in our DDC model. Because transdifferentiation was decreased, but the number of ductular structures was unchanged, in the LRP5/6 KO after BDL, we hypothesize that the Wnts involved in inducing cholangiocyte proliferation are independent of β-catenin and function through noncanonical signaling mechanisms. This was further supported by in vitro studies demonstrating that proliferation and cell viability were unimpaired in sm-cc cells after β-catenin suppression. Thus, the rebound in ductular response observed in β-catenin KO during later stages of DDC diet may be attributed to increased β-catenin-independent cholangiocyte proliferation, which is compensating for absent β-catenin-dependent HC transdifferentiation. Indeed, equivalent CK19 mRNA expression is observed in β-catenin KO and WT after 4 weeks of DDC diet (data not shown). Future experiments utilizing lineage tracing and Wnt-specific KOs will help further eludicate the relative contribution of these two mechanisms to hepatobiliary repair. However, it is important to note that whereas Wls-KO showed significant mortality after 4 weeks of DDC diet, all β-catenin KO mice survived DDC diet. Thus, Wnt-driven cholangiocyte proliferation appears to be indispensable for hepatobiliary repair during cholestasis.
It is becoming increasingly apparent that HCs are capable of transdifferentiating into cholangiocytes in models where biliary injury is the predominant insult to the liver. Early studies utilized in vitro organoid culture systems to demonstrate that isolated HCs exposed to a defined culture medium organize into a defined histological architecture, with cholangiocytes covering the surface of the tissue exposed to media.34, 35 Additional studies using strain-tagged cells demonstrated that the cholangiocytes were derived from HCs that have undergone cellular reprogramming.4, 36 More-recent work utilizing genetic mouse models and lineage tracing has confirmed that HCs are capable of converting to a biliary lineage under conditions that induce chronic biliary injury.3, 5, 37 These results suggest that the number of HCs expressing biliary markers increases over time during biliary injury, and that as cholestasis progresses, more and more HCs are “recruited” to compensate for damage to or loss of the biliary epithelium.
β-catenin and its downstream targets have been shown to be up-regulated in hepatic organoid cultures under conditions that promote HC-to-cholangiocyte transdifferentiation,38 implicating a role for Wnt signaling in this process. Underscoring the functional significance of these observations, we found that S45D mice, which express excess β-catenin in liver, had an increased number of A6-positive HCs compared to WT when subjected to long-term cholestatic injury.20 Importantly, this finding was coincident with a decrease in markers of biliary injury, as assessed by serum alkaline phosphatase (ALP) levels. Further analysis of the S45D mice at earlier time points after DDC has shown an up-regulation of biliary markers EpCAM, CK19, and Sox9. Interestingly, though loss of LRP5/6 from liver led to fewer A6-positive HCs after BDL, we do not see a corresponding decrease in markers of cholestasis, such as serum ALP, which may be attributed to the relatively short duration of the injury.
Previous studies and we have demonstrated that transdifferentiation of HCs expressing Sox9 occurs primarily in the PP area.3, 39 In light of our findings, it is likely that the concentration of Wnt7A, and thus transdifferentiation of HCs, is highest in the PP region attributed to the continuous injury to the bile ducts. The injury provides a stimulus for compensatory proliferation of cholangiocytes, which are also simultaneously secreting Wnt7A to recruit surrounding HCs to participate in biliary repair.
In conclusion, our results have demonstrated that β-catenin signaling regulates hepatobiliary repair in a Wnt-dependent manner. These findings mandate a comprehensive analysis of the role of Wnt/β-catenin signaling in transdifferentiation and amelioration of cholestasis and may provide novel therapeutics for treating cholestatic liver disease.
Acknowledgments
We thank Sucha Singh for invaluable technical assistance. We also thank Dr. Valentina Factor and Dr. Gianfranco Alpini for generously providing essential reagents for this study.
REFERENCES
Author names in bold designate shared co-first authorship.




