Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma
Potential conflict of interest: Nothing to report.
This work was supported by the Ministry of Science and Technology (National Science Council) grants from Taiwan (NSC 101-2320-B-400-016-MY3, NSC 102-2314-B-038-028-MY3, and NSC 103-2314-B-038-059); National Health Research Institutes grant from Taiwan (CA-102-PP-41, CA-104-SP-01, CA-104-PP-12, and MOHW104-TDU-B-212-124-008), and Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare grant from Taiwan (103TMU-SHH-26 and 104TMU-SHH-01-2).
Abstract
Angiopoietin-like protein 1 (ANGPTL1) has been shown to act as a tumor suppressor by inhibiting angiogenesis, cancer invasion, and metastasis. However, little is known about the effects of ANGPTL1 on sorafenib resistance and cancer stem cell properties in hepatocellular carcinoma (HCC) and the mechanism underlying these effects. Here, we show that ANGPTL1 expression positively correlates with sorafenib sensitivity in HCC cells and human HCC tissues. ANGPTL1 significantly decreases epithelial-mesenchymal transition (EMT)-driven sorafenib resistance, cancer stemness, and tumor growth of HCC cells by repressing Slug expression. ANGPTL1 directly interacts with and inactivates MET receptor, which contributes to Slug suppression through inhibition of the extracellular receptor kinase/protein kinase B (ERK/AKT)-dependent early growth response protein 1 (Egr-1) pathway. ANGPTL1 expression inversely correlates with Slug expression, poor sorafenib responsiveness, and poor clinical outcomes in HCC patients. Conclusion: ANGPTL1 inhibits sorafenib resistance and cancer stemness in HCC cells by repressing EMT through inhibition of the MET receptor−AKT/ERK−Egr-1−Slug signaling cascade. ANGPTL1 may serve as a novel MET receptor inhibitor for advanced HCC therapy. (Hepatology 2016;64:1637-1651)
Abbreviations
-
- AKT
-
- protein kinase B
-
- ANGPTL1
-
- angiopoietin-like protein 1
-
- ANGPTLs
-
- angiopoietin-like proteins
-
- ANGs
-
- angiopoietins
-
- CA-MEK1
-
- constitutively activated mitogen-activated protein kinase kinase
-
- Co-IP
-
- coimmunoprecipitation
-
- CSCs
-
- cancer stem cells
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- EGF
-
- epidermal growth factor
-
- Egr-1
-
- early growth response protein 1
-
- EMT
-
- epithelial-mesenchymal transition
-
- ERK
-
- extracellular signal-regulated kinase
-
- GST
-
- glutathione S-transferase
-
- HA
-
- hemagglutinin
-
- HCC
-
- hepatocellular carcinoma
-
- HGF
-
- hepatocyte growth factor
-
- IC50
-
- half maximal inhibitory concentration
-
- IgG
-
- immunoglobulin G
-
- Klf-4
-
- Krüppel-like factor 4
-
- LILRB2
-
- immune-inhibitory receptor human leukocyte immunoglobulin-like receptor B2
-
- LRCC
-
- label-retaining cancer cells
-
- mRNA
-
- messenger RNA
-
- NOD/SCID
-
- nonobese diabetic/severe combined immunodeficiency
-
- Oct-4
-
- octamer-binding transcription factor 4
-
- PBS
-
- phosphate-buffered saline
-
- phospho-RTK
-
- phospho-receptor tyrosine kinase
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PIRB
-
- paired immunoglobulin-like receptor
-
- qRT-PCR
-
- quantitative reverse transcription polymerase chain reaction
-
- rANGPTL1
-
- ANGPTL1 recombinant protein
-
- SDS-PAGE
-
- sodium dodecyl sulfate/polyacrylamide gel electrophoresis
-
- sh
-
- short hairpin
-
- SOX-2
-
- SRY (sex determining region Y)-box 2
-
- SR
-
- sorafenib resistance
-
- Tie
-
- Tunica interna endothelial cell kinase
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide.1 Surgical resection, liver transplantation, and chemotherapy are the typical treatments for early-stage HCC. However, treatment success is limited because approximately 70% of patients develop tumor recurrence within 5 years.2, 3 Thus, patients with advanced HCC often face a poor prognosis.4, 5 Sorafenib, a multikinase inhibitor, is the only clinically approved drug for the treatment of advanced HCC.4 Unfortunately, although sorafenib exerts positive effects on overall survival, sorafenib responsiveness among advanced HCC patients is very low, and most patients ultimately develop drug resistance and suffer relapses.4 Therefore, more-effective therapeutic strategies and precision medicines to improve the prognoses of HCC patients with sorafenib resistance are urgently needed.
Epithelial-mesenchymal transition (EMT) plays critical roles in development, wound healing, and tumor metastasis in many types of cancer.6 Several studies have implied an association between EMT and sorafenib resistance. Long-term exposure of liver cancer cells to sorafenib induces resistance and the EMT phenotype in HCC.7 αB-Crystallin complexes with 14-3-3ζ to activate the extracellular signal-regulated kinase (ERK) 1/2/Fos-related antigen 1/Slug signaling pathway, which induces EMT and sorafenib resistance in HCC.8 However, the mechanisms underlying acquired sorafenib resistance remain unknown. Cancer stem cells (CSCs) are a small population of cancer cells that are capable of tumor initiation, self-renewal, drug resistance, and continuous growth.9 Growing evidence suggests that CSCs possess EMT properties in various cancer types, such as lung, breast, colorectal, and liver cancer and are critical for tumor metastasis.10 A recent study showed that label-retaining cancer cells (LRCCs), a novel population of CSCs, are resistant to sorafenib in HCC.11 Enriched CD44+ and CD44+CD133+ subpopulations are observed among sorafenib-resistant cells in HCC.12 Hence, whether activation of EMT and enrichment of CSCs in advanced HCC induce sorafenib resistance must be determined.
There is a family of secreted proteins that are structurally similar to angiopoietins (ANGs). These proteins are known as angiopoietin-like proteins (ANGPTLs), which comprise eight proteins, ANGPTL1-8.13 However, ANGPTLs do not bind to the ANGs receptor, Tunica interna endothelial cell kinase (Tie) 2, or to the related protein, Tie1, which suggests that they may have different biological functions than ANGs. ANGPTLs participate in multiple biological processes, such as angiogenesis,14 hematopoietic stem cell expansion,15 cancer progression,16 and inflammation.17 Our previous study indicated that angiopoietin-like protein 1 (ANGPTL1) suppresses Slug expression, which reduces cancer cell motility and metastasis.18 However, little is known regarding whether ANGPTL1 is involved in sorafenib resistance and the CSC properties of HCC. Here, we demonstrate that ANGPTL1 inhibits sorafenib resistance, cancer stemness, and EMT by repressing MET receptor−protein kinase B (AKT)/ERK−early growth response protein 1 (Egr-1)−Slug signaling in HCC cells.
Materials and Methods
CELL LINES
Hep3B, HepG2, C3A, THLE-2, and 293T cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA), and Huh7, Huh6, and Huh1 cell lines were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). HA22T/VGH and PLC/PRF/5 cell lines were obtained from the Bioresource Collection and Research Center of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Mahlavu cells were generously donated by Dr. Michael Hsiao (Genomics Research Center, Academia Sinica, Taipei, Taiwan). HCC36 cells were generously donated by Dr. Jang-Yang Chang (National Cheng Kung University, Tainan, Taiwan). All cells were grown in regular Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen), 1,000 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. To develop sorafenib-resistant cells, Huh7 cells and HepG2 cells were treated stepwise with increasing doses of sorafenib for a long period of time. The resulting sorafenib-resistant cells, Huh7/SR and HepG2/SR, were maintained in culture medium containing sorafenib (Selleckchem, Houston, TX, USA) at a final maximum concentration of 10 (Huh7/SR) and 7.5 μM (HepG2/SR).
CELL APOPTOSIS AND CELL SORTING BY FLOW CYTOMETRY ANALYSIS
For the apoptosis assay, cells were seeded into 6-cm dishes at a density of 3 × 105 cells/dish overnight and then treated with vehicle or 10 μM of sorafenib and incubated for 48 hours. Aliquots of cells were collected and washed once with ice-cold phosphate-buffered saline (PBS) and then fixed with ice-cold 70% ethanol overnight. After fixation, cells were washed with PBS to remove residual ethanol, pelleted, and resuspended in PBS containing 1 μg/mL of propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) and 10 μg/mL of RNase A (Sigma-Aldrich), and then samples were analyzed using an FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). For stem cell marker staining and cell sorting, cells were collected and labeled with a series of primary antibodies for 30 minutes at 4°C in the dark. After being washed with PBS (Invitrogen), labeled cells were analyzed by FACSCalibur flow cytometer (BD Biosciences) for stem cell marker population staining and a FACSAria (BD Biosciences) for cell sorting.
SPHERE FORMATION ASSAY
Single cells were seeded on ultralow attachment plates (Corning Inc., Corning, NY, USA) at a concentration of 1,000 cells/mL in DMEM supplemented with 50 ng/mL of epidermal growth factor (EGF), 20 ng/mL of basic fibroblast growth factor, and 1× B27. Fresh medium was added every week. Spheres were counted at 21 days after seeding (>100 μm in diameter).
SPECIMENS
Tissues used for sorafenib treatment were obtained from National Cheng Kung University (Tainan, Taiwan) with institutional review board approval (A-ER-103-174). Patient specimens had been fixed in formalin and embedded in paraffin before they were archived. Sorafenib responses of patients were defined according to the Response Evaluation Criteria in Solid Tumors and were classified as PD (progressive disease) and SD (stable disease) + PR (partial response).19 We analyzed data for all patients whose tumor, nodes, metastasis and survival data were available (n = 92) through the Taiwan Liver Cancer Network with institutional review board approval and divided the patients into two groups in accord with their mean ANGPTL1 and Slug messenger RNA (mRNA) expression levels.
MICE XENOGRAFT ASSAY
All animal experiments were conducted in accord with a protocol approved by the Laboratory Animal Center of the National Health Research Institutes (Zhunan, Taiwan). Female nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (6-8 weeks old) were used for tumor growth in xenograft studies. Approximately 1 × 107 cells/mouse suspended in 100 μL of PBS and Matrigel (1:1; BD Biosciences) were subcutaneously injected into each flank. When tumor volumes reached approximately 200 mm3, as determined by measuring tumor lengths and widths using calipers and calculating their volumes with the formula (1/2[length × width2]) after inoculation, mice were randomly assigned to groups in which they received thrice-weekly oral doses of ether vehicle solution or 25 mg/kg of sorafenib (Selleckchem) for 4 weeks. Mice were treated intravenously with 100 μg/kg of ANGPTL1 recombinant protein (rANGPTL1) twice a week. Tumor volumes were measured every 3 days, and tumor tissues were extracted at indicated times for further analysis.
IN VITRO BINDING ASSAY
Two micrograms of MET-His protein (MET extracellular domain 1-932 amino acids) was mixed with 2 μg of ANGPTL1-GST (glutathione S-transferase) protein in binding buffer and incubated at 4°C for 4 hours, with gentle rotation. Forty microliters of Glutathione Sepharose 4B beads (GE Healthcare Life Sciences, MA, USA) were added to the mixture and incubated at 4°C for 2 hours, with gentle rotation. Beads were washed extensively with binding buffer and boiled. Then, proteins were resolved on sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by western blotting.
IMMUNOPRECIPITATION
Cells were lysed by brief sonication in coimmunoprecipitation (Co-IP) buffer (20 mM of Tris [pH 7.4], 150 mM of NaCl, 1 mM of ethylenediaminetetraacetic acid, and 0.5% nonyl phenoxypolyethoxylethanol) supplemented with protease inhibitor cocktails (Sigma-Aldrich). Lysates were centrifuged for 20 minutes at 14,500 rpm, and the resulting supernatant was precleared by incubation with immobilized Protein A/G gel (20 μL; Pierce, Dallas, TX, USA) for 1 hour at 4°C. The precleared supernatant was subjected to overnight immunoprecipitation at 4°C using the indicated antibodies (Supporting Table S1) or control immunoglobulin G (IgG) antibodies. The next day, protein complexes were collected by incubation with 35 μL of immobilized Protein A/G gel for 2 hours at 4°C. Protein complexes were washed five times with Co-IP buffer and eluted by boiling in protein sample buffer under reducing conditions, after which proteins were resolved by SDS-PAGE and analyzed by western blotting.
STATISTICAL ANALYSIS
All statistical analyses were carried out using Prism software (GraphPad, La Jolla, CA, USA). Data are presented as the mean ± SE of at least three independent experiments. A chi-squared test was used to compare clinicopathological data. Comparisons of groups were performed using one-way analysis of variance. A two-tailed Student t test was used to compare data between two groups. P values <0.05 were considered statistically significant.
Results
ELEVATED EXPRESSION OF ANGPTL1 ENHANCES THE SORAFENIB SENSITIVITY OF HCC IN VIVO AND IN VITRO
To determine whether ANGPTL1 is associated with sorafenib resistance in HCC, we examined expression of ANGPTL1 and the half maximal inhibitory concentration (IC50) of sorafenib in HCC cell lines, including Huh7 cells and their sorafenib-resistant counterparts, Huh7/SR (sorafenib resistance) cells (Supporting Fig. S1A,B). A negative correlation between ANGPTL1 expression and the IC50 value of sorafenib was observed in HCC cell lines by enzyme-linked immunosorbent assay (Fig. 1A). To determine the impact of ANGPTL1 on sorafenib sensitivity, we overexpressed ANGPTL1 in relative sorafenib-resistant cells (Huh7/SR and Mahlavu) and found that ANGPTL1 significantly decreased sorafenib resistance (Fig. 1B and Supporting Fig. S1C-E). We also found that knockdown of ANGPTL1 in Huh7 and HepG2 cells increased sorafenib resistance (Fig. 1C and Supporting Fig. S1F-H). To further investigate the role of ANGPTL1 in sorafenib resistance in HCC cells in vivo, HCC cells were genetically modulated of ANGPTL1 and subcutaneously inoculated into NOD/SCID mice. The results showed that tumor expression of ANGPTL1 was more sensitive to sorafenib treatment than control tumors (Fig. 1D and Supporting Fig. S1I, left panel). Tumor xenografts dissected from each mouse were used to confirm ANGPTL1 expression by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis (Fig. 1D and Supporting Fig. S1I, right panel). These results demonstrate that ANGPTL1 can overcome sorafenib resistance in HCC.
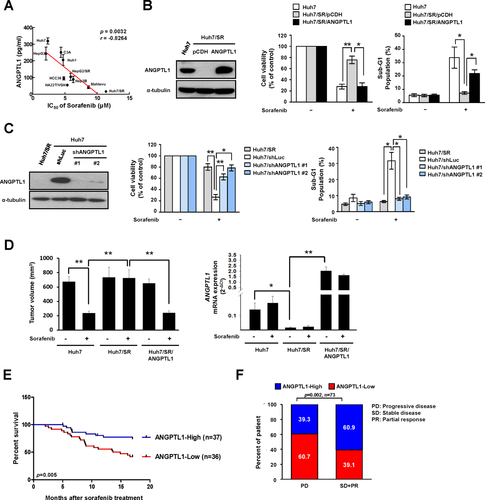
To determine the clinical significance of ANGPTL1 in HCC patients, we analyzed ANGPTL1 expression in a cohort of 92 HCC specimens (cohort 1). The results showed that ANGPTL1 expression was inversely correlated with tumor size, tumor stage, tumor grade, vascular invasion, and poor prognosis (Table 1; Supporting Fig. S2). We also collected another cohort of 73 HCC specimens (cohort 2) with sorafenib treatment and found that elevated expression of ANGPTL1 was associated with longer survival after sorafenib treatment (Fig. 1E) and was positively correlated with sorafenib responsiveness (Fig. 1F). Taken together, these results indicate that ANGPTL1 expression is inversely correlated with poor sorafenib responsiveness and poor prognosis in patients with HCC.
| Characteristic |
Low ANGPTL1 Expression |
High ANGPTL1 Expression |
P Value |
|---|---|---|---|
| No. of patients | 46 | 46 | |
| Age (mean ± SD) (in years) | 60.02 ± 16.16 | 58.33 ± 13.66 | 0.59 |
| Sex | |||
| Male | 33 | 38 | |
| Female | 13 | 8 | 0.21 |
| Tumor size (cm) | |||
| ≦5 | 16 | 26 | |
| >5 | 30 | 20 | 0.04a |
| Stage | |||
| I | 11 | 22 | |
| II-IV | 35 | 24 | 0.02a |
| Grade | |||
| Low (grade 1-2) | 26 | 41 | |
| High (grade 3-4) | 20 | 5 | p<0.01b |
| AFP | |||
| ≦200 | 24 | 31 | |
| >200 | 22 | 15 | 0.14 |
| Vascular invasion | |||
| No | 11 | 22 | |
| Yes | 35 | 24 | 0.02a |
- Significances of association were determined using a chi-square test.
- a P < 0.05.
- b P < 0.01.
ANGPTL1 OVERCOMES SORAFENIB RESISTANCE BY SUPPRESSING Slug
Previous studies have shown that EMT is crucial for sorafenib resistance in HCC.7, 8, 20 We found that Huh7/SR cells exhibited EMT characteristics and a spindle-like, fibroblastic morphology (Supporting Fig. S3A). Expression of the epithelial marker, E-cadherin, was lower in Huh7/SR cells, whereas expression of the mesenchymal marker, Vimentin, and the EMT-related transcription factors, Slug and Twist, were higher in Huh7/SR cells than in parental cells (Supporting Fig. S3B). We subsequently investigated whether Slug or Twist is involved in ANGPTL1-mediated sorafenib sensitization. Knockdown of ANGPTL1 increased Slug mRNA and protein expression, but not Snail and Twist (Fig. 2A,C). Overexpression of ANGPTL1 in Huh7/SR cells consistently decreased Slug expression (Fig. 2B,C). Moreover, we found that sorafenib resistance was abolished by Slug knockdown in Huh7/short hairpin (sh)ANGPTL1 cells (Fig. 2D). ANGPTL1-induced sensitization to sorafenib in Huh7/SR cells was repressed by Slug reexpression (Fig. 2E). Similar results were found in HepG2 and Mahlavu cells (Supporting Fig. S3C-H). To further elucidate the physiological role of Slug in sorafenib resistance, we silenced Slug and found recovery of sorafenib sensitivity in Huh7/SR cells (Supporting Fig. S4A-C). Moreover, high Slug expression was positively correlated with poor prognosis in cohort 1 (Supporting Fig. S4D,E) and was associated with shorter survival rates and poorer responses to sorafenib treatment in cohort 2 (Supporting Fig. S4F,G).
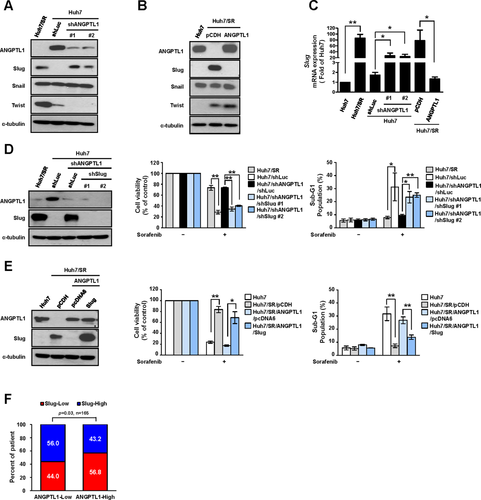
We next analyzed the correlation between ANGPTL1 and Slug expression in two cohorts and found that ANGPTL1 expression was inversely correlated with Slug expression (Fig. 2F and Supporting Fig. S5A). Additionally, the Gene Expression Omnibus and Oncomine databases21, 22 also demonstrated an inverse correlation between ANGPTL1 and Slug expression (Supporting Fig. S5B,C). These results suggest that ANGPTL1 overcomes sorafenib resistance by repressing Slug in HCC cells.
Slug RESCUES ANGPTL1-MEDIATED REPRESSION OF CANCER STEMNESS AND TUMOR GROWTH IN HCC CELLS
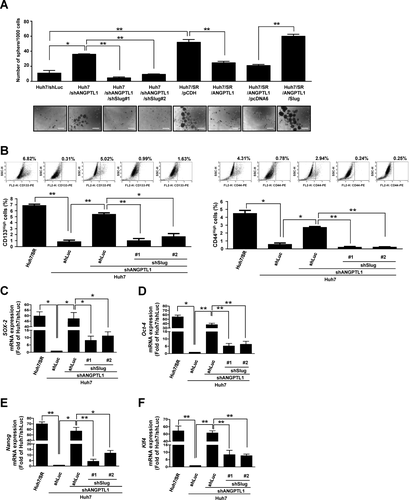
Emerging evidence suggests that CSC-mediated EMT in HCC cells is critical for tumor metastasis and sorafenib resistance.11, 12 To clarify the effects of ANGPTL1 on CSCs, we examined the sphere-forming abilities of Huh7, Huh7/SR, and HepG2 cells and their derivatives and found that sphere formation was repressed by Slug knockdown (Fig. 3A and Supporting Fig. S6A). Treatment with rANGPTL1 significantly inhibited sphere formation and sorafenib-resistant tumor growth (Supporting Fig. S7A,B). ANGPTL1-mediated repression of sphere formation in Huh/SR/ANGPTL1 cells was reversed by Slug overexpression (Fig. 3A). It is known that CD133 and CD44 are CSC markers of HCC.23, 24 Thus, Huh7/SR cells with high and low CD44 and CD133 expression levels were sorted to determine sorafenib sensitivity. Notably, our data demonstrated that Huh7/SR cells expressing low levels of CD44 or CD133 showed greater sensitivity to sorafenib than parental cells and CD44High/CD133High Huh7/SR cells (Supporting Fig. S8). Furthermore, Slug knockdown reduced shANGPTL1-mediated CD133High and CD44High cell population and CSC marker (SRY [sex determining region Y]-box 2 [SOX-2], octamer-binding transcription factor 4 [Oct-4], Nanog, and Krüppel-like factor 4 [Klf-4]) expression (Fig. 3B-F and Supporting Fig. S6B-G). Taken together, these results indicate that ANGPTL1 decreases cancer stemness and sorafenib resistance in HCC by suppressing Slug.
ANGPTL1 REPRESSES Slug-MEDIATED SORAFENIB RESISTANCE THROUGH INHIBITION OF THE MET RECEPTOR−AKT/ERK SIGNALING PATHWAY
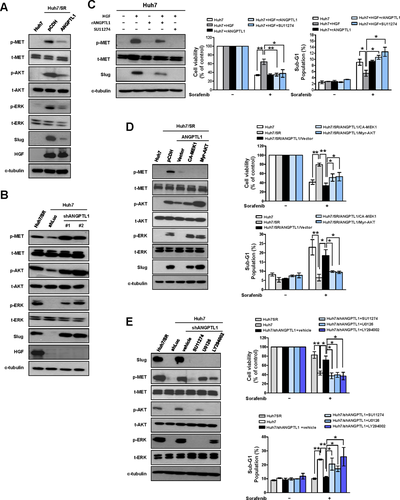
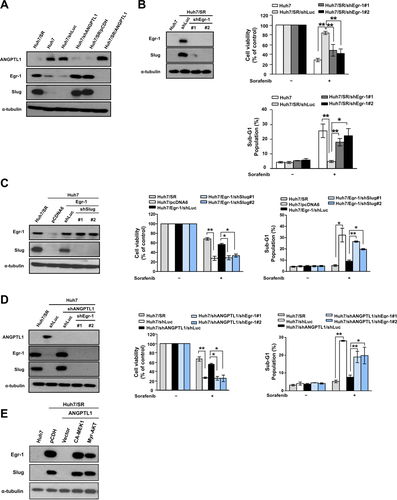
To identify the mechanisms underlying the regulation of sorafenib resistance in HCC by ANGPTL1, we used a phospho-receptor tyrosine kinase (phospho-RTK) array to analyze the alterations of phospho-RTKs in Hun7/shANGPTL1 cells, Huh/SR/ANGPTL1 cells, and their control cells. The results showed that phosphorylation of the MET receptor was significantly increased in Huh7/shANGPTL1 cells compared with Huh7/shLuc cells. ANGPTL1 overexpression reduced MET receptor phosphorylation in Huh7/SR cells (Supporting Fig. S9A). To determine whether the MET receptor signaling pathway is associated with ANGPTL1-mediated sorafenib resistance, we examined the phosphorylation of AKT and ERK, which are downstream molecules of the MET receptor pathway, and found that these proteins were negatively regulated by ANGPTL1 (Fig. 4A,B). We also found that Huh7/SR cells express higher levels of HGF than Huh7 cells (Fig. 4A,B). However, HGF expression was not affected by ANGPTL1. Next, we found that rANGPTL1 and SU11274 (a MET inhibitor) blocked hepatocyte growth factor (HGF)-induced MET phosphorylation, Slug expression, and sorafenib resistance (Fig. 4C and Supporting Fig. S9B). An inverse correlation between ANGPTL1 and HGF was observed in HCC cell lines (Supporting Fig. S10A-C). We also found that the HGF/MET signaling pathway positively regulates ANGPTL1 expression (Supporting Fig. S10D,E). However, ANGPTL1 did not alter HGF expression (Supporting Fig. S10F). ANGPTL1 expression was also regulated by different stimuli, such as serum, HGF, EGF, and hypoxia, in normal and malignant liver cells (Supporting Fig. S11A-F), but the mechanism underlying this regulation requires further investigation. To confirm the role of MET signaling in the regulation of Slug expression and sorafenib resistance, constitutively active forms of mitogen-activated protein kinase kinase (CA-MEK1) and AKT (Myr-AKT) were transfected into Huh7/SR/ANGPTL1 cells. The results showed that ANGPTL1-mediated repression of sorafenib resistance and Slug expression in Huh7/SR/ANGPTL1 cells was recovered by CA-MEK1 and Myr-AKT (Fig. 4D). We found that sorafenib resistance and Slug expression were reduced by treatment with SU11274, U0126 (a MEK inhibitor), and LY294002 (a phosphoinositide 3-kinase [PI3K] inhibitor) in Huh7/shANGPTL1 cells (Fig. 4E). These data indicate that ANGPTL1 is a negative regulator of the MET receptor-AKT/ERK signaling pathway, which is associated with Slug regulation and sorafenib resistance.
ANGPTL1 REPRESSES Slug-INDUCED SORAFENIB RESISTANCE IN AN Egr-1-DEPENDENT MANNER
A previous study showed that AKT and ERK1/2 are associated with EGF-induced Slug expression through Egr-1 in ovarian cancer cells.25 Our findings indicated that ANGPTL1 represses Egr-1 and Slug expression (Fig. 5A). To determine whether Egr-1 is involved in Slug expression and the subsequent development of sorafenib resistance, Egr-1 expression was knocked down in Huh7/SR cells, which resulted in significant repression of Slug expression and sorafenib resistance (Fig. 5B). Egr-1-induced sorafenib resistance in Huh7 cells was abrogated by Slug knockdown (Fig. 5C). We also found that inhibition of Erg-1 expression in Huh7/shANGPTL1 cells suppressed Slug expression and sorafenib resistance (Fig. 5D). To confirm the role of the MET signaling pathway in Egr-1 regulation, we transfected CA-MEK1 and Myr-AKT into Huh7/SR/ANGPTL1 cells and found that ANGPTL1-mediated repression of Egr-1 expression was recovered by CA-MEK1 and Myr-AKT (Fig. 5E). These results indicate that ANGPTL1-mediated repression of Slug expression occurs through the AKT/ERK-regulated Egr-1 pathway.
ANGPTL1 INTERACTS WITH THE MET RECEPTOR AND COMPETES WITH HGF FOR MET BINDING
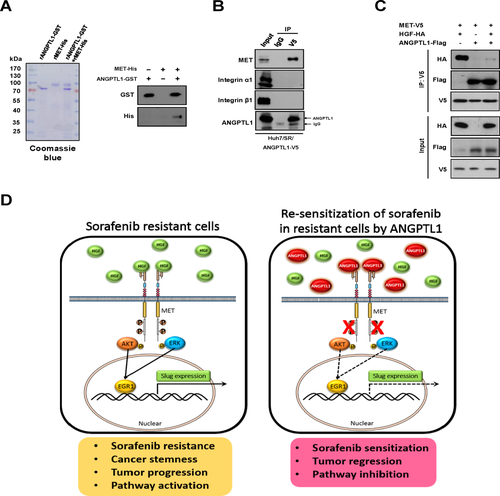
ANGPTLs exert their biological effects by binding to integrins in various cancers,16, 26 but the functional receptor of ANGPTL1 in HCC is unknown. To determine whether integrins are associated with ANGPTL1-mediated repression of sorafenib resistance, we first analyzed expression of integrins by qRT-PCR. We found that integrin β1 expression was reduced in ANGPTL1-overexpressing Huh7/SR cells (Supporting Fig. S12A-I). We also found that integrin β1 knockdown in Huh7/SR/ANGPTL1 cells did not abolish ANGPTL1-mediated repression of sorafenib resistance (Supporting Fig. S12J-M), suggesting that ANGPTL1-mediated sorafenib resistance may not through integrin β1.
To determine whether ANGPTL1 interacts with MET to exert its effects, we performed an in vitro binding assay and confirmed that the rANGPTL1-GST protein binds directly to the His-tagged recombinant MET extracellular domain (1-932 amino acids) protein (Fig. 6A). Co-IP assay showed that overexpressed exogenous ANGPTL1-V5 interacted with endogenous MET, but not integrin α1 and integrin β1, in Huh7/SR cells (Fig. 6B). To determine whether ANGPTL1 competes with HGF for MET binding, Co-IP assay was performed. Analysis of immunoprecipitates from 293T cells transfected with ANGPTL1-flag, MET-V5, and HGF-HA (hemagglutinin) revealed that ANGPTL1 interacts with the MET receptor and competes with HGF for MET binding (Fig. 6C). These results indicate that ANGPTL1 represses MET phosphorylation through competition with HGF.
Discussion
ANGPTL1 has been shown to be an antiangiogenesis protein in vitro and in vivo.27, 28 Recently, we reported that ANGPTL1 reduces lung cancer cell motility and metastasis by repressing Slug.18 However, whether ANGPTL1 influences other biological functions is unclear. Here, we demonstrated that ANGPTL1 enhances sorafenib sensitivity in HCC and represses CSCs. Moreover, we demonstrated the clinical significance of ANGPTL1, given that its expression was inversely correlated with sorafenib resistance and poor patient prognoses. ANGPTL1 competes with HGF for binding on the MET receptor, which results in decreased MET phosphorylation and subsequent inhibition of Slug expression through the AKT/ERK-regulated Egr-1 pathway (Fig. 6D).
Vascular invasion is a prognostic factor that is associated with early HCC recurrence.29 The EMT regulators, Twist and Snail, have been found to enhance HCC cell invasiveness.30, 31 In addition to promoting EMT, CSCs also promote the invasive abilities of HCC cells.12 Previous studies have been shown that EMT activation and CSC enrichment are associated with sorafenib resistance in HCC.7, 12 Our in vitro and in vivo results indicate that ANGPTL1 can overcome sorafenib resistance and that ANGPTL1 expression is inversely correlated with vascular invasion, which suggests that ANGPTL1 may facilitate suppression of tumor invasiveness and metastasis and sorafenib resistance in advanced HCC.
Integrins are functional receptors of ANGPTLs that activate or inhibit downstream signaling.16, 26 ANGPTL1 binds to integrin α1β1 and represses the downstream focal adhesion kinase/ERK pathway, inhibiting cancer cell motility and metastasis.18 A recent study showed that immune-inhibitory receptor human leukocyte immunoglobulin-like receptor B2 (LILRB2) and its mouse orthologue, paired immunoglobulin-like receptor (PIRB), are receptors for several ANGPTLs in hematopoietic stem cells and leukemia cells.32 These studies indicate that ANGPTLs have several binding partners, enabling them to perform their multiphysiological functions. Notably, our results showed that ANGPTL1 interacts with the MET receptor in HCC cells. The MET receptor (also called HGF receptor) has been shown to play a critical role in cancer progression and drug resistance,33, 34 especially in HCC.35 In addition to HGF, which is a native MET ligand, Fas ligand36 and leukocyte cell-derived chemotaxin 237 have been found to act as MET ligands to exert oncogenic and tumor-suppressive effects, respectively. Thus, our data suggest that MET is a novel binding partner for ANGPTL1 and participates in sorafenib responsiveness of HCC.
Recently, emerging evidence demonstrated that HGF/MET-induced signaling pathways, such as the PI3K/AKT, ERK, and phospho-vascular endothelial growth factor receptor 2 pathways, are up-regulated in sorafenib-resistant HCC cells and advanced HCC patients.38-40 Numerous HGF/MET inhibitors have been developed for study in preclinical therapeutic models and clinical trials, including tyrosine kinase inhibitors, monoclonal antibodies, and decoy MET and HGF antagonists.41, 42 Trivantinib, a selective MET inhibitor, is being tested in an ongoing phase III trial based on the results of a phase II study of advanced HCC.43 AMG102, an anti-HGF antibody, failed to improve advanced gastric cancer outcomes in a phase III trial. MetMab, an anti-MET antibody, failed a phase III trial for advanced non-small-cell lung cancer treatments. DN30, an antibody against the extracellular domain of MET, and NK4, an HGF-derived factor, have been shown to exert their antitumor effects in mouse models.44, 45 Interestingly, we found that ANGPTL1 significantly inhibits p-MET and downstream AKT/ERK-Egr-1-Slug signaling, which suppresses sorafenib resistance in HCC cells. Taken together, our findings indicate that ANGPTL1 is a potential target that may be used to overcome sorafenib resistance by inhibiting the MET receptor.
In conclusion, we showed that ANGPTL1 represses sorafenib resistance and cancer stemness by regulating the MET receptor-AKT/ERK-Egr-1-Slug signaling axis and demonstrated the clinical significance of ANGPTL1 in HCC. These data imply that ANGPTL1 may be a therapeutic target in the treatment of advanced HCC (e.g., sorafenib resistance) and a novel biomarker for predicting responsiveness to sorafenib treatment.
Acknowledgments
We thank the National RNAi Core Facility (Academia Sinica, Taiwan) for providing specific shRNAs. We thank Ms. Fang-Yu Tsai, Dr. I-Shou Chang, and Dr. Shih-Sheng Jiang of Taiwan Bioinformatics Institute Core Facility for assistances on using the Oncomine database (National Core Facility Program for Biotechnology, NSC-100-2319-B-400-001).
REFERENCES
Author names in bold designate shared co-first authorship.




