Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia
Potential conflict of interest: Dr. Pulsoni advises, is on the speakers' bureau, and received grants from Gilead. He advises Roche and received grants from Janssen.
Supported by Fondazione Roma (to M.V.), Associazione Italiana per la Ricerca sul Cancro (fellowship to E.F., grant 17391), Istituto Toscano Tumori, and Ente Cassa di Risparmio di Firenze.
Abstract
Hepatitis C virus (HCV)-associated mixed cryoglobulinemia (MC) vasculitis commonly regresses upon virus eradication, but conventional therapy with pegylated interferon and ribavirin yields approximately 40% sustained virologic responses (SVR). We prospectively evaluated the efficacy and safety of sofosbuvir-based direct-acting antiviral therapy, individually tailored according to the latest guidelines, in a cohort of 44 consecutive patients with HCV-associated MC. In two patients MC had evolved into an indolent lymphoma with monoclonal B-cell lymphocytosis. All patients had negative HCV viremia at week 12 (SVR12) and at week 24 (SVR24) posttreatment, at which time all had a clinical response of vasculitis. The mean (±standard deviation) Birmingham Vasculitis Activity Score decreased from 5.41 (±3.53) at baseline to 2.35 (±2.25) (P < 0.001) at week 4 on treatment to 1.39 (±1.48) (P < 0.001) at SVR12 and to 1.27 (±1.68) (P < 0.001) at SVR24. The mean cryocrit value fell from 7.2 (±15.4)% at baseline to 2.9 (±7.4)% (P < 0.01) at SVR12 and to 1.8 (±5.1)% (P < 0.001) at SVR24. Intriguingly, in the 2 patients with MC and lymphoma there was a partial clinical response of vasculitis and ∼50% decrease of cryocrit, although none experienced a significant decrease of monoclonal B-cell lymphocytosis. Adverse events occurred in 59% of patients and were generally mild, with the exception of 1 patient with ribavirin-related anemia requiring blood transfusion. Conclusion: Interferon-free, guideline-tailored therapy with direct-acting antivirals is highly effective and safe for HCV-associated MC patients; the overall 100% rate of clinical response of vasculitis, on an intention-to-treat basis, opens the perspective for curing the large majority of these so far difficult-to-treat patients. (Hepatology 2016;64:1473-1482)
Abbreviations
-
- BVAS
-
- Birmingham Vasculitis Activity Score
-
- CLL
-
- chronic lymphocytic leukemia
-
- DAA
-
- direct-acting antiviral
-
- EOT
-
- end of treatment
-
- HCV
-
- hepatitis C virus
-
- IFN
-
- interferon
-
- MC
-
- mixed cryoglobulinemia
-
- NHL
-
- non-Hodgkin lymphoma
-
- PEG-IFN
-
- pegylated IFN
-
- SVR
-
- sustained virological response
Hepatitis C virus (HCV) is a major health problem worldwide and is responsible for cirrhosis, end-stage liver disease, and hepatocellular carcinoma.1 HCV also causes B-cell lymphoproliferative disorders including mixed cryoglobulinemia (MC)2-4 and non-Hodgkin's lymphoma (NHL).5, 6 MC is generally considered a benign lymphoproliferative disorder at risk for evolution into NHL7-9; a large retrospective study on 1,255 HCV-positive patients with MC estimated a rate of 660.8 new cases of NHL per 100,000 patient-years, indicating a 35 times higher risk compared to the general population.10
Monoclonal B cells of approximately 80% of patients with HCV-associated MC express a stereotyped cross-idiotype called WA,11 which is commonly but not exclusively associated with the VH1-69 heavy chain variable gene and with the VK3-20 light chain variable gene.12, 13 The monoclonal antibody G6, directed to the VH1-69 protein product, is a useful marker for investigating by flow cytometry MC monoclonal B cells putatively expressing the WA idiotype13; indeed, increased proportions of G6-positive B cells were found in 30% of patients with HCV-associated MC.14
MC vasculitis can be severe and life-threatening15 and needs adequate treatment.16 Besides palpable purpura affecting most patients, MC syndrome is characterized by multiorgan involvement including skin ulcers, neuropathy, and glomerulonephritis.2 Sustained virological response (SVR) is the main goal in the treatment of patients with HCV-associated MC as viral clearance, using interferon (IFN)-based regimens, leads to remission of vasculitis in 88%-97% of patients.16-18 Until recently, the gold standard anti-HCV therapy for MC patients was the association of pegylated IFN (PEG-IFN) with ribavirin.16, 17 However, this treatment yielded an overall SVR rate significantly lower than that seen in patients with chronic hepatitis C.17 MC patients refractory to therapy with PEG-IFN/ribavirin need salvage treatments; rituximab, a B cell-depleting monoclonal antibody, has become a cornerstone in the therapy of MC and has been used both for treating refractory cases19 and as an adjunct to antiviral therapy for severe cases.20 HCV-associated indolent NHL may also regress following IFN-based antiviral therapy.21 Recently, Arcaini et al.22 reported an 88% rate of hematologic response in a large cohort of patients with HCV-associated indolent lymphomas treated with IFN as a first-line antilymphoma therapy. A concurrent role for the antiviral and the antiproliferative activities of IFN in lymphoma regression cannot be ruled out.23
The introduction of direct-acting antivirals (DAAs) has dramatically expanded the number of patients with chronic hepatitis C who achieved SVR, marking a new era in the therapy of HCV infection.24
The guidelines for the treatment of chronic HCV infection have been continuously updated with the release of new DAAs. In several countries, including Italy, the high cost of these drugs precludes, at present, their broad use and demands prioritization of patients for treatment. Both the American Association for the Study of Liver Diseases (http://www.hcvguidelines.org) and the European Association for the Study of the Liver (http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/recommendations-on-treatment-of-hepatitis-c-2015/report/4) recommend prioritizing DAA treatment in patients with symptomatic MC associated with HCV infection.
So far, a few studies have reported promising results on the efficacy and tolerability of DAA therapy in patients with HCV-associated MC, either in association with IFN25-28 or using IFN-free regimens,29-31 and in patients with HCV-associated NHL.32-34
In this prospective study, 44 patients with HCV-associated MC vasculitis were treated with DAA therapy individually tailored according to the latest available guidelines, representing a real-life picture of MC treatment. Safety, clinical efficacy, and virological response were evaluated in a posttherapy follow-up of at least 24 weeks.
Patients and Methods
PATIENTS
In this prospective study, 44 consecutive patients were enrolled at the outpatient clinic of the Interdepartmental Center for Systemic Manifestations of Hepatitis Viruses, University of Florence, and at the Referral Center for Mixed Cryoglobulinemia, Sapienza University of Rome. Inclusion criteria were detectable levels of serum HCV RNA, presence of an active cryoglobulinemic vasculitis diagnosed as described,35 and eligibility for treatment with a DAA according to the Italian Medicines Agency's indications: “severe HCV-associated extrahepatic manifestations (cryoglobulinemic syndrome with organ damage and B-cell lymphoproliferative syndrome).” This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki; treatments were administered on-label and therefore did not require ethical approval. Laboratory studies beyond routine clinical care were approved by the local institutional ethics committees; informed consent was obtained from all patients.
TREATMENT
Patients were treated with different sofosbuvir-based DAA combinations (Table 1) individualized according to the recommendations of the European Association for the Study of the Liver36 on which the Italian Association for the Study of the Liver and the Italian Medicines Agency guidelines are based. Drug dosages were sofosbuvir 400 mg daily, simeprevir 150 mg daily, ledipasvir 90 mg daily, daclatasvir 30 mg daily, and ribavirin 800-1200 mg daily in a weight-based dose. Details on treatment schedules are provided in Supporting Table S1. Two patients with severe vasculitis received rituximab at a reduced dosage of 250 mg/m2 given twice 1 week apart37 concurrently with DAA therapy. Patients were followed up for a median of 42 weeks (range 27-53) after the completion of therapy.
| n = 44 | |
|---|---|
| Mean age (years)a | 65.3 ± 10.1 |
| Gender (male/female) | 16/28 |
| METAVIR scoreb | |
| F0-F1 | 20 |
| F2 | 3 |
| F3 | 4 |
| F4 | 17 |
| Child Pugh-score (A/B) | 13/4 |
| Mean Model for End-Stage Liver Disease scorea | 9.9 ± 2.5 |
| Mean alanine aminotransferase (U/L)a | 77.7 ± 77.2 |
| Mean aspartate aminotransferase (U/L)a | 55.2 ± 60.4 |
| Mean HCV RNA titer (IU/mL × 106)a | 2.9 ± 3.6 |
| HCV genotype | |
| 1a | 2 |
| 1b | 21 |
| 2 | 13 |
| 3 | 5 |
| 4 | 3 |
| Previous antiviral treatment response | |
| Naive | 19 |
| No response | 18 |
| Relapse | 7 |
| DAA treatment | |
| Sofosbuvir + ribavirin | 18 |
| Sofosbuvir + simeprevir (+ ribavirin) | 12 (6/12) |
| Sofosbuvir + daclatasvir (+ ribavirin) | 4 (1/4) |
| Sofosbuvir + ledipasvir (+ ribavirin) | 10 (3/10) |
| MC type | |
| II | 29 |
| III | 15 |
| Laboratory | |
| Cryocrit (%)a | 7.2 ± 15.4 |
| Rheumatoid factore | 15/21c |
| Reduced C4f | 14/24d |
- a Based on liver stiffness assessed by FibroScan.
- b Data are expressed as mean ± standard deviation.
- c Data available for 21 patients.
- d Data available for 24 patients. Normal range for alanine aminotransferase 12-65 U/L; normal range for aspartate aminotransferase 15-37 U/L.
- e Rheumatoid factor: elevated rheumatoid factor levels over normal values (<20 IU/mL).
- f Reduced C4: complement C4 levels below normal values (0.1-0.4 g/L).
CLINICAL AND LABORATORY ASSESSMENTS
HCV infection was proven by detecting circulating anti-HCV antibodies (EIA-2 and RIBA-2; Ortho Diagnostic Systems, Raritan, NJ) and HCV RNA (AMPLICOR HCV Test, v2.0; Roche Diagnostics, Alameda, CA). The HCV genotype was assessed by the VERSANT HCV Genotype 2.0 assay (Siemens Healthcare Diagnostics, Deerfield, IL). SVR was defined as undetectable serum HCV RNA at weeks 12 (SVR12) and 24 (SVR24) after the end of treatment.
Liver disease was evaluated by noninvasive methods including liver elastography using FibroScan (Echosens, Paris, France), imaging, clinical presentation, and laboratory data; the METAVIR score as well as the Model for End-Stage Liver Disease scoring system were used to assess the severity of liver disease.
Clinical response of cryoglobulinemic vasculitis was evaluated by two different methods. One method was a modified version of a classification of clinical response of cryoglobulinemic vasculitis.17, 38-40 Thus, we classified as “full-complete responders” (criterion 1) patients with disappearance of all the baseline symptoms, as “complete responders” (criterion 2) those with improvement of all the baseline symptoms, as “partial responders” (criterion 3) those with disappearance or improvement of at least half of the baseline symptoms, and as “nonresponders” (criterion 4) patients with disappearance or improvement of fewer than half of the baseline symptoms. Clinical response of cryoglobulinemic vasculitis was also evaluated by the Birmingham Vasculitis Activity Score (BVAS), version 3.41
Immunological parameters for evaluating MC response included quantification of cryoglobulins (expressed as percent cryocrit) and measurement of the C3 and C4 complement fractions and of rheumatoid factor. The immunological response was defined as “complete” in case of disappearance of cryoglobulins, “partial” for a decrease of cryocrit to <50% of baseline level, and “null” in all other cases.17, 37, 40 Monoclonal B-cell lymphocytosis was evaluated by flow cytometry, as described.13
All patients were evaluated for clinical and laboratory parameters monthly during the first 2 months of treatment, at the end of treatment (EOT), and at weeks 12 and 24 after the end of treatment.
The molecular analysis of B-cell receptors is described in Supporting Information.
STATISTICAL METHODS
Statistics were performed using the Student t test and Wilcoxon test for quantitative data, chi-squared test, Fisher's exact test for qualitative variables, and Spearman's rank test for correlation between variables. Categorical variables are expressed as count and percentages and quantitative ones as mean ± standard deviation. All statistical tests were two-tailed with a significance level of 0.05 (Stata, version 9.0, and SPSS, version 17.0).
Results
From February 2015 to August 2015, 44 consecutive HCV-infected patients with active MC vasculitis (16 males and 28 females, mean age 65.3 ± 10.1 years) were prospectively recruited for treatment with sofosbuvir-based DAA combinations. Twenty-nine patients (66%) had type 2 MC and the remaining 15 (34%) type 3; two patients had MC complicated by indolent B-cell lymphoma, chronic lymphocytic leukemia (CLL)-like in one case and marginal zone lymphoma in the other. The majority of patients (57%) failed previous treatments with IFN-based regimens. The main demographic, clinical, and virological characteristics of the study population at baseline are summarized in Table 1. DAA combination and treatment duration were individualized on the basis of HCV genotype, liver disease severity, and previous treatment experience and tolerance to ribavirin, according to the recommendation of the European Association for the Study of the Liver36 on which the Italian Association for the Study of the Liver and the Italian Medicines Agency guidelines are based (Table 1; Supporting Table S1).
Main clinical manifestations of MC vasculitis included palpable purpura (73%), arthralgia (59%), weakness (77%), peripheral neuropathy (63%), Raynaud's phenomenon (32%), renal involvement (9%), sicca syndrome (41%), and skin ulcers (14%). Among the 4 patients with kidney involvement, 1 had a renal biopsy showing membranoproliferative glomerulonephritis with hypertension, microhematuria, and proteinuria; 1 had a nephrotic syndrome with proteinuria, edema, and dyslipidemia, requiring the administration of albumin and diuretics; and 2 had reduced glomerular filtration rate and proteinuria.
HEPATOVIROLOGICAL RESPONSE
In all patients HCV RNA was undetectable at week 4 of treatment and remained negative throughout the follow-up, with SVR12 and SVR24 rates of 100%. An overall improvement was observed for the main liver function parameters: the mean alanine aminotransferase level decreased from 77.7 ± 77.2 IU/L at baseline to 27.4 ± 10.3 IU/L at SVR12 (P < 0.0001) and to 27.3 ± 10.3 IU/L at SVR24 (P < 0.0001). The mean aspartate aminotransferase decreased from 55.2 ± 60.4 IU/L to 21.3 ± 9.1 IU/L (P < 0.0001) at SVR12 and to 22.6 ± 8.3 IU/L (P < 0.001) at SVR24. A slight decrease of the Model for End-Stage Liver Disease score was observed in the 17 patients with F4 fibrosis from a mean of 9.9 ± 2.5 at baseline to 8.8 ± 2.7 at SVR12 and to 7.3 ± 1.9 at SVR24.
CLINICAL EFFICACY
Among the 44 patients, at SVR12, 34% were full-complete responders and cleared all manifestations of vasculitis (criterion 1); 32% were complete responders, with improvement of all the manifestations of vasculitis (criterion 2); 27% were partial responders, with disappearance or improvement of at least half of the manifestations of vasculitis (criterion 3); and 7% were nonresponders, with disappearance or improvement of fewer than half of the baseline symptoms (criterion 4) (Fig. 1A). At SVR24, all 44 patients had a clinical response of vasculitis, with 36% full-complete responders, 41% complete responders, and 23% partial responders (Fig. 1A). In no patients did the vasculitis manifestations maintain the same pretreatment severity or worsen. The mean BVAS decreased from 5.41 (±3.53) at baseline to 2.35 (±2.25) (P < 0.001) at week 4 on treatment, to 1.39 (±1.48) (P < 0.001) at SVR12, and to 1.27 (±1.68) (P < 0.001) at SVR24 (Fig. 1B). Because the presence of type 2 cryoglobulins may qualitatively and quantitatively correlate with MC vasculitis,42 we compared clinical presentation and response to therapy in patients with type 2 (n = 29) or type 3 (n = 15) in our series. We could not detect any significant difference between the two groups either in the prevalence of specific clinical manifestations at baseline (data not shown) or in the clinical response to therapy; in fact, at SVR24 of the 29 patients with type 2 MC, 35% fulfilled criterion 1, 41% criterion 2, and 24% criterion 3, while these figures were 40%, 40%, and 20%, respectively, among the 15 patients with type 3 MC. Also, we did not notice any trend to a different outcome in patients previously treated with IFN-based therapy, infected with specific HCV genotypes or receiving specific DAA combinations (data not shown).
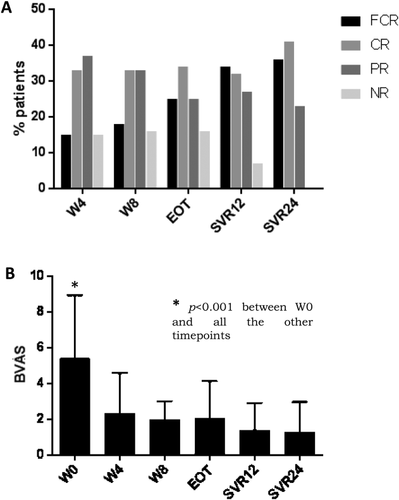
The relative increase of complete versus partial responders and the disappearance of nonresponders at SVR24 compared to SVR12 (Fig. 1A) suggest that some manifestations of MC vasculitis slowly respond to eradication of HCV. Indeed, the improvement of fatigue, sicca syndrome, and peripheral neuropathy was relatively slow, whereas other manifestations such as palpable purpura, kidney disease, and skin ulcers tended to respond more rapidly (Table 2). Six patients presented with multiple skin ulcers; in 4 of them the ulcers were chronic and resistant to intensive medication and antibiotics, whereas in 2 other cases they occurred early during treatment. In all of these patients ulcers disappeared by SVR24.
| EOT | SVR12 | SVR24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Baseline n |
Disappearance n (%) |
Improvement n (%) |
Persistence n (%) |
Disappearance n (%) |
Improvement n (%) |
Persistence n (%) |
Disappearance n (%) |
Improvement n (%) |
Persistence n (%) |
| Purpura | 32 | 23 (72) | 4 (12) | 5 (16) | 27 (84.5) | 3 (9.5) | 2 (6) | 28 (87.5) | 2 (6.25) | 2 (6.25) |
| Arthralgias | 26 | 9 (34.5) | 8 (31) | 9 (34.5) | 11 (42.5) | 6 (23) | 9 (34.5) | 12 (46) | 6 (23) | 8 (31) |
| Weakness | 34 | 9 (26) | 7 (21) | 18 (53) | 19 (56) | 9 (26) | 6 (18) | 19 (56) | 10 (29) | 5 (15) |
| Peripheral neuropathy | 28 | 8 (28.5) | 8 (28.5) | 12 (43) | 13 (46.5) | 11 (39.25) | 4 (14.25) | 14 (50) | 10 (36) | 4 (14) |
| Raynaud phenomenon | 14 | 8 (57) | — | 6 (43) | 9 (64.25) | 2 (14.25) | 3 (21.5) | 11 (79) | 1 (7) | 2 (14) |
| Renal involvement | 4 | 3 (75) | 1 (25) | — | 3 (75) | 1 (25) | — | 3 (75) | 1 (25) | - |
| Sicca syndrome | 18 | 7 (39) | 3 (17) | 8 (44) | 8 (44.5) | 6 (33.25) | 4 (22.25) | 8 (44.5) | 6 (33.25) | 4 (22.25) |
| Ulcer | 6 | 4 (67) | — | 2 (33) | 5 (83) | — | 1(17) | 6 (100) | - | - |
Among the 4 patients with renal involvement, the 1 with membranoproliferative glomerulonephritis experienced an increase in the glomerular filtration rate up to normal values, subsiding of proteinuria, and normalization of blood pressure; another patient had a complete remission of the nephrotic syndrome with no further requirement for albumin and diuretics, and the remaining two patients showed a marked improvement of the glomerular filtration rate with normalization of proteinuria.
Two patients, 1 with chronic skin ulcers and 1 with kidney disease, were treated with rituximab, 250 mg/m2 given twice 1 week apart, in association with a DAA. In the first patient ulcers completely healed by week 8, and in the other patient complete remission of the nephrotic syndrome was observed at week 4 of treatment. In both patients the remission of vasculitis persisted throughout a 24-week follow-up after EOT.
To clear our data of the confounding effects of rituximab cotreatment, we separately evaluated the 42 patients who received DAA only. The results of this restricted analysis did not substantially differ from those of the analysis performed on the entire cohort, with 33% full-complete responses, 43% complete responses, and 24% partial responses at SVR24 and a decrease of cryocrit from 7.1 ± 15.8% to 3.0 ± 7.5% at SVR12 and to 1.9 ± 5.2% at SVR24 (P < 0.001).
Interestingly, in two patients with MC and lymphoma there was a dissociation of the hematologic response and the response of MC vasculitis. In fact, while in both cases there was a partial regression of the symptoms of vasculitis and a decrease of cryocrit at SVR24, in neither case was there a reduction of monoclonal B-cell lymphocytosis. Notably, in 1 of these patients, who had a CLL-like disorder, treatment with PEG-IFN done 18 months earlier had induced a virologic response associated with regression of both MC vasculitis and monoclonal B-cell lymphocytosis; later virologic relapse was associated with relapse of MC vasculitis and of monoclonal lymphocytosis, leading to treatment with a DAA. We tested whether there were differences in the B-cell receptors expressed by circulating monoclonal B cells or if there were oligoclonal rather than monoclonal expansions before treatment with PEG-IFN or with DAA; at both time points there was a single population of monoclonal B cells expressing an idiotype encoded by somatically mutated IGHV3-7 heavy chain and IGKV3-15 light chain variable genes, homologous to the WA cross-idiotype.43, 44 Thus, different responsiveness of monoclonal B-cell lymphocytosis to PEG-IFN compared to DAA therapy appears to be more likely related to the type of treatment than to changes in the characteristics of lymphoma cells. The other patient, who had marginal zone lymphoma with plasmacytic differentiation, had a single circulating monoclonal B-cell population expressing an idiotype encoded by IGHV2-5 and IGKV1-5. Detailed information on these patients is provided in Supporting Information.
IMMUNOLOGICAL EFFICACY
The mean cryocrit level rapidly decreased from 7.2 ± 15.4% at baseline to 2.9 ± 9.0% at week 4 on treatment (P < 0.05) and remained substantially stable up to SVR12 to eventually decrease to 1.8 ± 5.1% (P < 0.001) at SVR24 (Fig. 2A). The immunological response at SVR12 was complete (disappearance of cryoglobulins) in 32% of cases, partial in 39%, and null (<50% decrease of cryocrit) in 29%. At SVR24, cryocrit was available for 38/44 patients and there were 39.5% complete responders, 34% partial responders, and 26.5% null responders; in patients with null response, cryocrit values decreased at least 38% from baseline. The serum C4 levels were serially measured only in the 14 patients out of 24 who had low values at baseline (Fig. 2B); in 45% of these patients C4 levels had returned to normal at SVR12. Five of the 11 patients studied at SVR24 had persistently low C4 levels, and 4 of them had persistent cryoglobulins. Rheumatoid factor levels decreased from 131.2 ± 252.9 at baseline to 66.2 ± 91.8 at SVR12 and to 39.0 ± 29.8 at SVR24 (Fig. 2C).
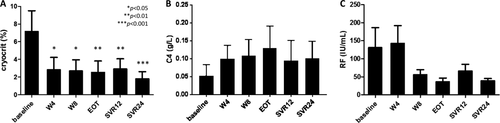
Reduction of cryocrit, expressed as a percent decrease from baseline, did not correlate with the degree of clinical response, expressed as a percent decrease of BVAS, either at SVR12 (rho = 0.059 by Spearman rank correlation) or at SVR24 (rho = 0.29). Disappearance of cryoglobulins and regression of all manifestations of vasculitis (criterion 1) did not appear to be associated; in fact, at SVR24 there were only 8 negative cryocrits out of the 16 patients fulfilling criterion 1.
TREATMENT SAFETY
Adverse events related to DAA occurred in 26/44 (59%) patients; they were generally mild and in no instance led to discontinuation of DAA treatment. Most patients (22/26) had more than one adverse event, with anemia, fatigue, and nausea being the most complained. Anemia occurred in 13 patients, all receiving ribavirin. In 8 patients with decrease of hemoglobin to <10 g/dL (>8.5 g/dL) reduction of ribavirin by 200 mg resulted in no further decline. In 4 additional patients hemoglobin decreased further despite the reduction of the ribavirin dose; 3 patients were treated with erythropoietin and completed ribavirin treatment at reduced dosage, while in 1 patient ribavirin was withdrawn while continuing sofosbuvir plus simeprevir. In another patient, an abrupt fall of hemoglobin to 7.5 g/dL required blood transfusion; reduction of ribavirin by 200 mg was followed by stable hemoglobin levels. In 2 patients (1 needing reduction and 1 withdrawal of ribavirin) hyperbilirubinemia also occurred. Further details on adverse events are reported in Table 3.
| Type of Adverse Event | No. patients |
|---|---|
| Anemia | 13 |
| Ribavirin reduction | 8/13 |
| Ribavirin suspension | 1/13 |
| Erythropoietin | 3/13 |
| Blood transfusion | 1/13 |
| Hyperbilirubinemia | 2 |
| Fatigue | 15 |
| Nausea | 7 |
| Irritability | 4 |
| Pruritus | 4 |
| Diarrhea | 3 |
| Headache | 3 |
| Insomnia | 2 |
| Depression | 1 |
| Vertigo | 1 |
| Rash | 1 |
Discussion
To our knowledge, this is the first prospective study of therapy of HCV-associated MC with sofosbuvir-based DAA therapy individually tailored according to the available guidelines.
We observed a rapid decrease of viremia with all patients being negative for HCV RNA at week 4 on treatment; the rate of SVR12 was 100% with no on-therapy viral breakthrough; thus, unlike with IFN-based regimens, the presence of MC vasculitis seems to not represent a risk factor for virological nonresponse. The negative effect of MC presence in HCV patients has been shown in both PEG-IFN plus ribavirin and triple first-wave DAA-based therapy.17, 25 In fact, even if the first wave of DAAs used in association with PEG-IFN and ribavirin led to an overall improvement of SVR rates,45 the presence of MC remained a negative prognostic factor with a lower rate of virological response compared to controls and a high frequency of severe adverse events.25
The advent of the second-generation DAAs determined a further increase of antiviral efficacy, and mostly, the IFN-free regimens minimized the side effects. For these reasons, it was expected that these new therapeutic approaches would be optimal for patients not tolerating IFN-based regimens, due to multiple side effects, and at the same time would increase the response rate.
Two previous studies reported 74%29 and 83%30 SVR12 rates in 24 and 12 MC-patients treated with IFN-free DAA therapy, respectively; and an additional study31 reported promising results at week 8 of treatment in 17 patients. These earlier studies, however, suffered either from a suboptimal treatment schedule using a sofosbuvir/ribavirin combination in a cohort of patients infected by various HCV genotypes, predominantly including HCV genotype 1,29 from including a relatively small number of patients,30 or from a short follow-up.31 The present observation of 100% SVR12 rate in 44 patients, although to be taken with caution in the wait for larger and controlled confirmatory studies, strongly suggests that updated treatment protocols may yield the same rate of virological results in MC patients as in HCV subjects without MC.46 These results, once confirmed, would represent an extraordinary breakthrough for a difficult-to-treat condition such as HCV-associated MC vasculitis.16, 17 The clinical response of vasculitis was also surprisingly high in our study, with an overall 100% clinical response at SVR24 including 36% of cases experiencing complete healing of all symptoms. We noticed a trend to persistence of certain disease manifestations including sicca syndrome and peripheral neuropathy. Irreversible damage to salivary glands or to peripheral nerves47 may account for refractoriness of sicca syndrome and peripheral neuropathy in some patients. By contrast, we observed rather rapid responses of skin ulcers and, similar to the findings of Sise et al.,30 of glomerulonephritis. This outcome is of particular relevance because these manifestations of MC vasculitis appear to be associated with increased mortality and high health care costs.15, 48 Finally, while a correlation between decrease of cryocrit and response of MC vasculitis has been reported in patients treated with IFN-based therapy,11 we failed to clearly show this trend. Dissociation between clinical response and cryocrit reduction has been noticed in MC patients treated with rituximab49; in addition, the susceptibility of cryoglobulin quantitation, especially with low levels, to variables such as blood sample handling, tissue deposition, and other pitfalls35, 50 might have contributed to obscure a clinical-immunological correlation in the present study.
Recent case reports have suggested the efficacy of IFN-free DAA therapy for treating HCV-associated lymphomas.32-34 In a larger study, Arcaini et al.22 recorded 12 hematologic responses (8 complete and 4 partial, all marginal zone lymphomas) in 20 patients (60%) who achieved SVR after IFN-free DAA therapy; 60% of patients cleared cryoglobulins after therapy. In this study, we observed a dichotomous response of cryoglobulinemia and of lymphoma in two patients, who had clinical and laboratory improvement of MC but stable monoclonal B-cell lymphocytosis. The molecular identification in both patients of only one monoclonal B-cell population argues against the possibility that they had oligoclonal B-cell expansions,44 although the possibility of an additional clone(s) not circulating through peripheral blood cannot be ruled out. Data in 1 patient with MC and CLL-like disorder were particularly intriguing. In this patient, both MC and monoclonal B-cell lymphocytosis had previously regressed upon virologic response to PEG-IFN, but after relapse, the hematologic disorder failed to regress following the virologic response obtained with IFN-free therapy. This patient's circulating monoclonal B cells expressed, stably over time, a B-cell receptor encoded by IGHV3-7 and IGKV3-15. Stereotyped B-cell receptors encoded by these variable genes recur in CLL and in HCV-associated lymphomas and possess rheumatoid factor activity that renders CLL cells able to proliferate in response to stimulation with human immunoglobulin G.51 It is difficult to explain the different response to IFN and to IFN-free DAA of this patient's CLL-like disorder; one possibility is that the antiproliferative activity of IFN, together with the silencing of antigenic stimulation by HCV, allowed the constraint of both clonal expansion and cryoglobulin production, while the sole elimination of the viral stimulus with DAA could have left monoclonal B cells capable of proliferating upon binding of immunoglobulin G but less proficient in the production of cryoglobulins. In the case of the patient with marginal zone lymphoma, it is possible that the IGHV2-5/IGKV1-5 B-cell receptor of the circulating clone was unrelated to HCV, while another tissue-based, HCV-dependent B-cell clone was responsible for the production of cryoglobulins.
In summary, the present study demonstrates that an IFN-free therapy optimized from the virological point of view may yield, in patients with HCV-associated MC, very high virological and clinical response rates. Our observation that all MC patients achieved, on an intention-to-treat basis, a clinical response of vasculitis opens the perspective of curing this so far difficult-to-treat disorder in the large majority of cases. Association with rituximab might be of value for cases with refractory lymphoproliferative disorders or with severe or obstinate manifestations of cryoglobulinemic vasculitis. Furthermore, this study strongly supports the opportunity of an early eradication of HCV, before MC-related tissue damage becomes irreversible and/or the lymphomagnetic process becomes independent from the etiologic agent.
REFERENCES
Author names in bold designate shared co-first authorship.




