Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients
Potential conflict of interest: Dr. V. Wong has received speakers' fees from Echosens. Dr. G. Wong has received speakers' fees from Echosens. Dr. H. Chan has received speakers' fees from Echosens.
This project was supported by the direct grant from The Chinese University of Hong Kong (Ref 2014.1.033).
Abstract
Although nonalcoholic fatty liver disease (NAFLD) is closely linked to obesity, around 10%-20% of nonobese Americans and Asians still develop NAFLD. Data on this special group are limited. We therefore studied the severity and clinical outcomes of nonobese NAFLD patients. Consecutive NAFLD patients who underwent liver biopsy were prospectively recruited. We used the NASH Clinical Research Network system to score the histology. The Asian body mass index cutoff of 25 kg/m2 was used to define nonobese NAFLD. Among 307 recruited NAFLD patients, 72 (23.5%) were nonobese. Compared to obese patients, nonobese patients had lower NAFLD activity score (3.3 ± 1.3 vs. 3.8 ± 1.2; P = 0.019), mainly contributed by steatosis (1.7 ± 0.8 vs. 2.0 ± 0.8; P = 0.014) and presence of hepatocyte ballooning (60.9% vs. 73.4%; P = 0.045). Similarly, nonobese patients had lower fibrosis stage (1.3 ± 1.5 vs. 1.7 ± 1.4; P = 0.004), serum cytokeratin-18 fragments (283 vs. 404 U/L; P < 0.001) and liver stiffness measurement by transient elastography (6.3 vs. 8.6 kilopascals; P < 0.001). By multivariate analysis in nonobese patients, only elevated serum triglyceride level was independently associated with higher NAFLD activity score (adjusted odds ratio [OR], 1.644; P = 0.021), whereas elevated creatinine level was the only factor associated with advanced fibrosis (adjusted OR, 1.044; P = 0.025). After a median follow-up of 49 months, 6 patients died, 2 developed hepatocellular carcinoma, and 1 had liver failure, all of whom were in the obese group. Conclusion: Nonobese NAFLD patients tend to have less-severe disease and may have a better prognosis than obese patients. Hypertriglyceridemia and higher creatinine are the key factors associated with advanced liver disease in nonobese patients. (Hepatology 2017;65:54-64)
Abbreviations
-
- BMI
-
- body mass index
-
- CK-18
-
- cytokeratin-18
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HDL
-
- high-density lipoprotein
-
- IQR
-
- interquartile range
-
- JNK
-
- c-Jun N-terminal kinase
-
- kPa
-
- kilopascals
-
- LSM
-
- liver stiffness measurement
-
- MetS
-
- metabolic syndrome
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAS
-
- NAFLD Activity Score
-
- NASH
-
- nonalcoholic steatohepatitis
-
- OR
-
- odds ratio
-
- PNPLA3
-
- patatin-like phospholipase domain-containing protein 3
-
- PPV
-
- positive predictive value
-
- TE
-
- transient elastography
-
- TM6SF2
-
- transmembrane 6 superfamily antigen 2
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the world.1 It can progress to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC).2, 3 Furthermore, patients with NAFLD have increased risk of cardiovascular events, other malignancies, and mortality.4
NAFLD is strongly associated with obesity, metabolic syndrome (MetS), and cardiovascular risk factors and is more common in obese patients.5, 6 Nonetheless, a smaller, but significant, proportion of patients develop NAFLD despite having a relatively normal body mass index (BMI). This condition is often referred to as lean or nonobese NAFLD.7, 8 Traditionally considered a condition unique in Asia, NAFLD has also been found in 10% of lean Americans in the National Health and Nutrition Examination Survey III.9 Severity, factors associated with advanced disease, and prognosis of nonobese NAFLD are not well understood. A recent international study reported that nonobese NAFLD patients might have more-severe histological necroinflammation and higher mortality than obese patients.(10) Other smaller studies reported mixed results on the disease severity.11-13
Recently, our group used proton-magnetic resonance spectroscopy and transient elastography (TE) to show that nonobese NAFLD patients were less likely to have NASH and advanced fibrosis than obese NAFLD patients in the general population.8 Although this population study minimized selection bias, few patients in the community had advanced disease. Our understanding of the impact and long-term outcomes of nonobese NAFLD remains incomplete.
We therefore aimed to study the severity of NAFLD in nonobese and obese patients by liver biopsy and noninvasive tests of NASH and fibrosis, and to evaluate factors associated with severe disease. We also aimed to study the prognosis of nonobese and obese NAFLD patients.
Patients and Methods
PATIENTS
This was a prospective cohort study. Consecutive patients who underwent liver biopsy for the evaluation of NAFLD at the Prince of Wales Hospital, Hong Kong, were evaluated. The main indications for liver biopsy included assessment of unexplained elevation of liver enzymes, evaluation of NAFLD severity, and participation in clinical trials. We included adult patients aged 18 years or above with histology-proven NAFLD. Patients with positive hepatitis B surface antigen or anti-hepatitis C virus (HCV) antibody with detectable HCV RNA, excessive alcohol consumption (≥20 g/day in men or ≥10 g/day in women), secondary fatty liver (e.g., use of systemic steroids or tamoxifen), or malignancies before baseline were excluded. Although not one of the exclusion criteria, none of the patients had liver decompensation at baseline. All patients provided informed written consent for liver biopsy, blood sample storage, and prospective follow-up. The study protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee.
CLINICAL ASSESSMENT
Clinical assessment, blood taking, and TE were performed 1 day before liver biopsy. Drug history, alcohol consumption, and past medical history were recorded using a standardized questionnaire. BMI was calculated as weight (kg) divided by height (m) squared; a BMI less than 25 kg/m2 was used to define the nonobese Asian population.14 The nonobese population was further divided into lean (<23 kg/m2) and overweight (23-25 kg/m2). Waist circumference was measured at a level midway between the lower rib margin and iliac crest with the tape all around the body in the horizontal position. After overnight fasting for at least 8 hours, blood samples were taken for liver biochemistry, glucose, insulin, and lipids. The patatin-like-phospholipase domain-containing protein 3 (PNPLA3) and the transmembrane 6 superfamily antigen 2 (TM6SF2) polymorphisms were performed using the TaqMan SNP Genotyping Assay (Life Technologies, Grand Island, NY).
MetS was defined according to the ethnic specific criteria by the International Diabetes Federation, which was modified from the National Cholesterol Education Program, Adult Treatment Panel III Guidelines, as the presence of three or more of the following: (1) central obesity (waist circumference ≥ 90 cm in men and ≥80 cm in women for Asians); (2) triglycerides > 1.7 mmol/L; (3) reduced high-density lipoprotein (HDL) cholesterol (<1.03 mmol/L in men and <1.29 mmol/L in women); (4) blood pressure ≥ 130/85 mm Hg; and (5) fasting plasma glucose ≥ 5.6 mmol/L or receiving treatment for the above metabolic problems.15
ASSESSMENT OF DISEASE SEVERITY
Liver biopsy was performed using a 16G Temno needle. Two passes were obtained for each patient, on average, with an aim to get a liver specimen of ≥15 mm in length. All liver specimens were read by two experienced pathologists (A.W.-H.C., P.C.-L.C.) who were blinded to all clinical data. In case of discrepancy, the pathologists read the slides together and reached a consensus score. We used the NASH Clinical Research Network system to score the histological features.16 In particular, NASH was defined as the presence of steatosis, lobular inflammation, and hepatocyte ballooning.17 Fibrosis was staged from F0 (no fibrosis) to F4 (cirrhosis), with F3-F4 considered as advanced fibrosis.
To further support our histological interpretation, severity of NASH and liver fibrosis was also assessed by noninvasive methods. Serum cytokeratin-18 (CK-18) fragments, a marker of hepatocyte apoptosis and NASH, were measured by the M30-Apoptosense enzyme-linked immunosorbent assay kit (PEVIVA, Bromma, Sweden). At a cutoff of 338 U/L, CK-18 fragments have a 66% sensitivity and 66% specificity for NASH.18 As for liver fibrosis, liver stiffness measurement (LSM) by TE with FibroScan (Echosens, Paris, France) was performed on the same day of clinical assessment according to the instructions and training provided by the manufacturer. All operators had performed at least 200 examinations before this study and were blinded to all clinical data of the subjects. LSM was considered reliable if 10 successful acquisitions were obtained and the IQR (interquartile range)-to-median ratio of the 10 acquisitions was < 0.3. The cut-off value of 9.6 kilopascals (kPa) was used to estimate the number of subjects with advanced fibrosis or cirrhosis (F3 or F4 disease). This cut-off value has a specificity of 92% when validated against liver histology.19
LONG-TERM OUTCOMES
Patients were prospectively followed at least yearly after the baseline liver biopsy and more frequently as clinically indicated. During each visit, clinical events were recorded, and the anthropometric measurements and metabolic assessments were repeated. Follow-up duration was calculated as the time between the biopsy date and the most recent follow-up. Only events that occurred during this follow-up period were considered. The clinical events considered and their definitions are listed in Supporting Table S1. Apart from follow-up records, we also cross-checked the diagnostic codes and mortality of all patients by the Hong Kong Hospital Authority's Electronic Patient Record system, which captures data from all local public hospitals (accounting for > 80% of the local hospitalizations) and is linked to the Hong Kong Death Registry. Because of the relatively low event rate, a composite endpoint of cardiovascular events, liver-related events, malignancies, and mortality was used in the primary analysis, but individual outcomes were also reported.
STATISTICAL ANALYSIS
Statistical tests were conducted using SPSS Statistics software (version 22; IBM Corp, Armonk, NY). Continuous variables and ordinal variables are expressed in mean ± SD or median (IQR) and compared using unpaired t test and Mann-Whitney U test, as appropriate. Categorical variables were compared using the χ2 test. Independent variables associated with NAFLD Activity Score (NAS) in nonobese patients were identified using the ordinal logistic regression model, whereas independent variables associated with advanced liver fibrosis were identified using the binary logistic regression model. Clinical outcomes were illustrated by Kaplan-Meier curves and compared between nonobese and obese patients using the log-rank test. A two-sided P value of < 0.05 was considered significant. In our previous studies, NASH was found in around 40%-50% of patients undergoing liver biopsy.18 Assuming 40% of nonobese patients and 60% of obese patients had NASH and that one quarter of NAFLD patients were nonobese, a total sample size of 284 patients would achieve 80% power to detect the difference at a 5% significance level.
Results
From July 2006 to February 2015, 342 patients underwent liver biopsy. After excluding patients with missing BMI data and biopsies that contained less than 5% steatosis, 307 NAFLD patients were included in the analysis (Supporting Fig. S1). The mean age of all subjects was 51 ± 11 years, and 171 patients (55.7%) were male. A total of 170 patients had diabetes and hypertension (55.4%), and 186 patients (60.6%) had MetS (Table 1). Seventy-two patients (23.5%) were nonobese with a BMI <25kg/m2. All patients were ethnic Chinese.
| Characteristics | All | BMI <25 kg/m2 | BMI ≥25 kg/m2 | P Value |
|---|---|---|---|---|
| N | 307 | 72 | 235 | |
| Age (years) | 51 ± 11 | 54 ± 11 | 51 ± 12 | 0.075 |
| Male sex, n (%) | 171 (55.7) | 33 (45.8) | 138 (58.7) | 0.054 |
| Waist circumference (cm) | 95 ± 14 | 83 ± 7 | 99 ± 13 | <0.001 |
| Men | 98 ± 12 | 86 ± 7 | 101 ± 11 | <0.001 |
| Women | 92 ± 15 | 81 ± 6 | 96 ± 15 | <0.001 |
| Waist-to-hip ratiob | 0.96 ± 0.17 | 0.90 ± 0.06 | 0.98 ± 0.19 | 0.001 |
| Men | 0.96 ± 0.09 | 0.92 ± 0.05 | 0.97 ± 0.10 | 0.008 |
| Women | 0.96 ± 0.23 | 0.89 ± 0.06 | 0.98 ± 0.27 | 0.001 |
| BMI (kg/m2) | 27.6 (25.2-30.5) | 23.5 (22.5-24.3) | 28.9 (26.8-31.4) | <0.001 |
| Systolic blood pressure (mm Hg) | 136 ± 18 | 134 ± 17 | 136 ± 19 | 0.379 |
| Diastolic blood pressure (mm Hg) | 79 ± 11 | 77 ± 11 | 80 ± 11 | 0.020 |
| Creatinine (µmol) | 78 ± 23 | 74 ± 22 | 79 ± 23 | 0.148 |
| Total bilirubin (mmol/L) | 13 ± 6 | 12 ± 6 | 13 ± 7 | 0.240 |
| Alkaline phosphatase (IU/L) | 72 (61-92) | 75 (64-111) | 69 (60-90) | 0.038 |
| Alanine aminotransferase (IU/L) | 54 (36-83) | 53 (32-76) | 54 (37-84) | 0.395 |
| Aspartate aminotransferase (IU/L)c | 28 (19-44) | 25 (18-31) | 32 (21-47) | 0.012 |
| Fasting glucose (mmol/L) | 6.0 (5.2-7.3) | 5.8 (5.1-6.8) | 6.0 (5.3-7.5) | 0.061 |
| Hemoglobin A1c (%)b | 6.7 ± 1.3 | 6.4 ± 1.3 | 6.7 ± 1.3 | 0.042 |
| Total cholesterol (mmol/L) | 3.7 ± 1.9 | 3.7 ± 2.0 | 3.7 ± 1.8 | 0.871 |
| HDL-cholesterol (mmol/L) | 1.5 (1.1-4.6) | 1.5 (1.2-5.1) | 1.5 (1.1-4.5) | 0.252 |
| LDL-cholesterol (mmol/L) | 2.8 (2.1-3.4) | 2.8 (2.3-3.4) | 2.8 (2.0-3.4) | 0.654 |
| Triglycerides (mmol/L) | 1.4 (1.0-2.0) | 1.4 (1.0-2.3) | 1.4 (1.0-1.9) | 0.392 |
| Hemoglobin (g/dL) | 14.1(13.1-15.0) | 13.7 (13.0-14.6) | 14.2 (13.2-15.1) | 0.031 |
| Diabetes, n (%) | 170 (55.4) | 38 (52.8) | 132 (56.2) | 0.612 |
| Hypertension, n (%) | 170 (55.4) | 31 (43.1) | 139 (59.1) | 0.016 |
| MetS, n (%)a | 193 (62.9) | 31 (43.1) | 162 (69.8) | <0.001 |
| PNPLA3 rs738409d | ||||
| CC, n (%) | 89 (30.1) | 18 (25.7) | 71 (31.4) | 0.363 |
| CG or GG, n (%) | 207 (69.9) | 52 (74.3) | 155 (68.6) | |
| TM6SF2 rs58542926d | ||||
| CC, n (%) | 243 (82.1) | 58 (82.9) | 185 (81.9) | 0.849 |
| CT or TT, n (%) | 53 (17.9) | 12 (17.1) | 41 (18.1) |
- Continuous variables were expressed as mean ± SD or median (IQR), as appropriate.
- a Defined according to the International Diabetes Federation criteria.
- b Missing in 7 patients.
- c Missing in 121 patients.
- d Missing in 11 patients.
- Abbreviation: LDL, low-density lipoprotein.
CLINICAL CHARACTERISTICS OF NONOBESE AND OBESE PATIENTS
Compared to obese patients, nonobese patients had smaller waist circumference and waist-to-hip ratios, and lower diastolic blood pressure, alkaline phosphatase, and hemoglobin levels. Nonobese patients were less likely to have hypertension and MetS. Between nonobese and obese patients, there was no significant difference in the proportion of patients carrying the PNPLA3 G allele or the TM6SF2 T allele (Table 1).
Among nonobese patients, 29 were classified as lean (BMI < 23kg/m2) and 43 were classified as overweight (≥23 but < 25kg/m2). Their clinical characteristics were similar apart from the overweight patients having higher waist circumference (85 ± 6 vs. 79 ± 7 cm; P = 0.001) and BMI (median [IQR] 24.1 [23.7-24.5] vs. 22.1 [21.3-22.6]; P < 0.001; Supporting Table S2A).
NAFLD ACTIVITY IN NONOBESE AND OBESE PATIENTS
Similar proportions of obese and nonobese patients had NASH (51.9% vs. 43.5%; P = 0.217; Table 2). Forty-two of 72 nonobese patients had ballooning, among whom 12 did not have lobular inflammation. Nonobese patients had lower NAS generally (mean score, 3.3 ± 1.3 vs. 3.8 ± 1.2; P = 0.019). The difference in the NAFLD activity score was mainly contributed by lesser steatosis (mean score, 1.7 ± 0.8 vs. 2.0 ± 0.8; P = 0.014) and a smaller proportion of nonobese patients having hepatocyte ballooning (60.9% vs. 73.4%; P = 0.045). The proportion of biopsies that had lobular inflammation was similar between obese and nonobese patients (76.0% vs. 71.0%; P = 0.405) with similar scores. Serum CK-18 fragments, a biomarker of hepatocyte apoptosis and NASH, were also lower in nonobese patients (median, 282.5 vs. 403.8 U/L; P < 0.001), with a smaller proportion of nonobese patients having levels exceeding 338 U/L (46.5% vs. 63.0%; P = 0.014).
| BMI <25 kg/m2 (N = 72) | BMI ≥25 kg/m2 (N = 235) | P Value | ||
|---|---|---|---|---|
| Histology | ||||
| Biopsy length (cm) | 1.8 (1.5-2.4) | 1.9 (1.5-2.5) | 0.582 | |
| Steatosis (%) | 35 (16-54) | 50 (30-70) | 0.003 | |
| NASH, n (%)a | 30 (43.5) | 121 (51.9) | 0.217 | |
| NAS (0-8) | Mean | 3.3 ± 1.3 | 3.8 ± 1.2 | 0.019 |
| Median | 3 (2-4) | 4 (3-5) | ||
| Steatosis (0-3) | Mean | 1.7 ± 0.8 | 2.0 ± 0.8 | 0.014 |
| Median | 2 (1-2) | 2 (1-3) | ||
| Lobular inflammation (0-3) | Mean | 0.9 ± 0.7 | 1.0 ± 0.7 | 0.305 |
| Median | 1 (0-1) | 1 (1-1) | ||
| Ballooning (0-2) | Mean | 0.7 ± 0.6 | 0.8 ± 0.5 | 0.068 |
| Median | 1 (0-1) | 1 (0-1) | ||
| Presence of lobular inflammation, n (%) | 49 (71.0) | 177 (76.0) | 0.405 | |
| Presence of ballooning, n (%) | 42 (60.9) | 171 (73.4) | 0.045 | |
| Stage of fibrosis (0-4) | Mean | 1.3 ± 1.5 | 1.7 ± 1.4 | 0.004 |
| Median | 1 (0-3) | 1 (1-3) | ||
| Presence of fibrosis, n (%) | 38 (55.1) | 185 (80.1) | <0.001 | |
| Advanced fibrosis, n (%)b | 18 (26.1) | 64 (27.7) | 0.791 | |
| Noninvasive tests | ||||
| CK-18 fragments (U/L) | 282.5 (175.3-444.1) | 403.8 (263.5-660.3) | <0.001 | |
| CK-18 fragments > 338 U/L, n (%) | 33 (46.5) | 143 (63.0) | 0.014 | |
| Liver stiffness (kPa)c | 6.3 (4.8-8.6) | 8.6 (6.4-12.7) | <0.001 | |
| Liver stiffness ≥ 9.6 kPa, n (%)c | 12 (20.3) | 83 (43.0) | 0.002 | |
- Continuous variables are expressed as mean ± SD or median (IQR), as appropriate. Histological scores are expressed in both formats because the median values were not fine enough to show the difference between groups; the Mann-Whitney U test was nevertheless used to compare between groups because the variable was ordinal in nature.
- a NASH is defined as scoring at least 1 in all three components of the NAS.
- b Includes patients with F3 or F4 disease.
- c Missing in 55 patients
Among patients with NASH, nonobese patients tend to have lesser degrees of steatosis and ballooning degeneration. Otherwise, there were no significant differences in the histological patterns between nonobese and obese patients.
Among nonobese patients, there was a trend that overweight patients had higher degree of steatosis and concentration of CK-18 fragments than lean patients (Supporting Table S2B). There was no difference in the overall NASH severity or degree of fibrosis.
FACTORS ASSOCIATED WITH DISEASE ACTIVITY IN NONOBESE PATIENTS
By univariate analysis, lower total bilirubin levels, higher hemoglobin A1c, triglycerides, a history of diabetes, and the number of MetS criteria were associated with a higher NAS in nonobese patients (Table 3). After excluding the number of MetS criteria attributed to multicollinearity, multivariate analysis of the remaining variables showed that only triglyceride levels stood as an independent predictor of disease severity (odds ratio [OR], 1.644; 95% CI, 1.079-2.502; P = 0.021). Nonobese patients who had elevated triglycerides (i.e., greater than 1.7 mmol/L) had a significantly higher NAS (3.8 ± 1.2 vs. 3.1 ± 1.2; P = 0.031). NASH was found in 57.1% of nonobese patients with elevated triglycerides and 34.1% of those with normal triglycerides (P = 0.058).
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factors | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Age (years) | 0.989 (0.951-1.029) | 0.599 | ||
| Male sex | 0.836 (0.360-1.943) | 0.678 | ||
| BMI | 0.984 (0.751-1.288) | 0.906 | ||
| Waist circumference (cm) | 1.007 (0.947-1.070) | 0.827 | ||
| Waist-to-hip ratio increase by 0.1 | 1.720 (0.745-3.972) | 0.204 | ||
| Systolic blood pressure (mm Hg) | 1.003 (0.978-1.029) | 0.797 | ||
| Diastolic blood pressure (mm Hg) | 1.004 (0.966-1.044) | 0.838 | ||
| Creatinine (µmol) | 1.007 (0.988-1.026) | 0.480 | ||
| Total bilirubin (mmol/L) | 0.936 (0.867-1.011) | 0.094 | 0.942 (0.867-1.024) | 0.164 |
| Alkaline phosphatase (IU/L) | 0.994 (0.981-1.006) | 0.330 | ||
| Alanine aminotransferase (IU/L) | 1.004 (0.994-1.015) | 0.402 | ||
| Fasting plasma glucose (mmol/L) | 1.217 (0.936-1.579) | 0.142 | ||
| Hemoglobin A1c (%) | 1.394 (0.986-1.968) | 0.060 | 1.022 (0.655-1.597) | 0.922 |
| Total cholesterol (mmol/L) | 0.869 (0.685-1.104) | 0.251 | ||
| HDL-cholesterol (mmol/L) | 0.936 (0.759-1.155) | 0.541 | ||
| LDL-cholesterol (mmol/L) | 1.054 (0.944-1.179) | 0.347 | ||
| Triglycerides (mmol/L) | 1.639 (1.151-2.330) | 0.006 | 1.623 (1.115-2.361) | 0.011 |
| Ferritin (ng/mL) | 1.000 (0.999-1.000) | 0.338 | ||
| Hemoglobin (g/dL) | 0.974 (0.790-1.202) | 0.811 | ||
| PNPLA3 rs738409 G carrier | 0.749 (0.281-2.002) | 0.565 | ||
| TM6SF2 rs58542926 T carrier | 0.787 (0.249-2.487) | 0.684 | ||
| History of diabetes | 2.356 (0.995-5.579) | 0.051 | 1.626 (0.575-4.591) | 0.360 |
| History of hypertension | 1.474 (0.627-3.466) | 0.374 | ||
| MetS | 1.556 (0.665-3.636) | 0.309 | ||
| No. of metabolic syndrome criteria | 1.415 (1.037-1.929) | 0.029 | ||
- Histological severity is measured by the NAS. Factors are determined by ordinal logistic regression; factors that reach a significance level of P < 0.1 on univariate analysis are entered into multivariate analysis.
- Abbreviation: LDL, low-density lipoprotein.
Regarding individual histological features, nonobese patients had less-severe hepatocyte ballooning (mean score, 0.5 ± 0.6 vs. 0.9 ± 0.5; P = 0.006) and tended to have less-severe steatosis (mean score, 1.6 ± 0.7 vs. 2.0 ± 0.8; P = 0.06). More specifically, those with elevated triglycerides were more likely to have hepatocyte ballooning (83.3% vs. 48.9%; P = 0.045) and grade 2 steatosis or above (70.8% vs. 45.7%; P = 0.045). Nonobese patients with grade 2 steatosis or above also had a tendency to have higher triglyceride level (median [IQR] 1.5 [1.2-2.6] vs. 1.4 [1.0-1.7]; P = 0.072); this also applies to those with hepatocyte ballooning (1.7 [1.1-2.5] vs. 1.3 [1.0-1.6]; P = 0.055). In contrast, increased triglyceride level was not associated with lobular inflammation.
On the other hand, high alanine aminotransferase (adjusted OR, 1.034; P < 0.001), ferritin (adjusted OR, 1.001; P = 0.031), and diabetes (adjusted OR, 3.347; P = 0.006) were independent factors associated with a CK-18 fragment level over 338 U/L.
FIBROSIS IN NONOBESE AND OBESE PATIENTS
Nonobese patients had less-severe fibrosis overall (mean fibrosis stage 1.3 ± 1.5 vs. 1.7 ± 1.4; P = 0.004). Fewer nonobese patients had fibrosis (Table 2; Fig. 1). Similarly, nonobese patients had lower liver stiffness by TE than obese patients (median, 6.3 vs. 8.6 kPa; P < 0.001). On the other hand, the proportion of patients with advanced fibrosis was similar in both groups (26.1% vs. 27.7%; P = 0.791); however, significantly fewer nonobese patients had liver stiffness ≥9.6 kPa (20.3% vs. 43.0%; P = 0.002).
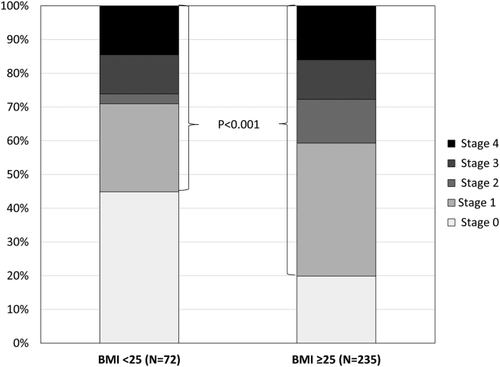
Percentage of patients with different histological fibrosis stages in the nonobese and obese groups.
FACTORS ASSOCIATED WITH ADVANCED FIBROSIS IN NONOBESE PATIENTS
Among nonobese patients, univariate analyses showed higher creatinine, fasting plasma glucose, hemoglobin A1c, triglyceride level, a history of diabetes, and presence of MetS to be associated with the presence of advanced fibrosis (Table 4). Multivariate analyses showed that only higher creatinine levels (OR, 1.059; 95% CI, 1.014-1.107; P = 0.010) was an independent predictor of advanced fibrosis in nonobese patients.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factors | OR (95% CI) | P Value | OR (95% CI) | P value |
| Age | 1.046 (0.987-1.108) | 0.129 | ||
| Male sex, n (%) | 0.900 (0.306-2.651) | 0.848 | ||
| BMI | 0.789 (0.567-1.098) | 0.160 | ||
| Waist circumference (cm) | 1.021 (0.944-1.104) | 0.605 | ||
| Waist-to-hip ratio | 1.152 (0.400-3.317) | 0.793 | ||
| Systolic blood pressure (mm Hg) | 1.012 (0.979-1.045) | 0.485 | ||
| Diastolic blood pressure | 0.993 (0.945-1.043) | 0.768 | ||
| Creatinine (µmol) | 1.033 (1.002-1.065) | 0.035 | 1.044 (1.005-1.083) | 0.025 |
| Total bilirubin (mmol/L) | 1.049 (0.957-1.151) | 0.305 | ||
| Alkaline phosphatase (IU/L) | 1.007 (0.991-1.022) | 0.390 | ||
| Alanine aminotransferase (IU/L) | 1.006 (0.993-1.018) | 0.375 | ||
| Fasting plasma glucose (mmol/L) | 1.669 (1.165-2.390) | 0.005 | 1.721 (0.829-3.571) | 0.145 |
| Hemoglobin A1c (%) | 1.692 (1.062-2.697) | 0.027 | 0.660 (0.232-1.880) | 0.437 |
| Total cholesterol (mmol/L) | 1.058 (0.782-1.431) | 0.715 | ||
| HDL-cholesterol (mmol/L) | 0.914 (0.692-1.206) | 0.524 | ||
| LDL-cholesterol (mmol/L) | 1.007 (0.879-1.154) | 0.920 | ||
| Triglycerides (mmol/L) | 1.639 (1.059-2.537) | 0.027 | 1.370 (0.854-2.199) | 0.192 |
| Ferritin (ng/mL) | 1.000 (1.000-1.001) | 0.214 | ||
| Hemoglobin (g/dL) | 0.925 (0.720-1.189) | 0.542 | ||
| PNPLA3 rs738409 G carrier | 0.677 (0.196-2.338) | 0.537 | ||
| TM6SF2 rs58542926 T carrier | 0.667 (0.128-3.462) | 0.629 | ||
| History of diabetes | 3.170 (1.191-8.440) | 0.021 | 1.841 (0.329-10.302) | 0.487 |
| History of hypertension | 1.745 (0.642-4.749) | 0.275 | ||
| MetS | 3.368 (1.085-10.454) | 0.036 | 1.737 (0.391-7.721) | 0.468 |
- Advanced fibrosis is defined as having F3 or F4 disease. Factors are determined by binary logistic regression; factors that reach a significance level of P < 0.1 on univariate analysis are entered into multivariate analysis.
- Abbreviation: LDL, low-density lipoprotein.
DISEASE PROGRESSION IN NONOBESE AND OBESE PATIENTS
Eighty-two patients, 21 nonobese and 61 obese, had additional liver biopsies or LSM over the course of their follow-up (Supporting Table S3). The mean time intervals between the first and second liver biopsies and LSM were 30 ± 18 and 29 ± 16 months, respectively. Among these patients, 54 (16 nonobese and 38 obese) did not have advanced fibrosis at baseline according to histology and LSM. Thirteen of these patients progressed to advanced fibrosis according to subsequent liver biopsies or LSM; only 1 of these patients was nonobese. The relative rate of disease progression was lower among nonobese patients (6.3% vs. 31.6%; P = 0.079). In contrast, there was no significant difference in fibrosis regression in nonobese and obese patients (20.0% vs. 13.0%; P = 1.0).
PROGNOSIS OF NONOBESE AND OBESE PATIENTS
At a median follow-up of 49 months (IQR, 11-82), 34 of 307 patients (11.1%) had one or more event after the baseline biopsies; 28 of them were obese (Table 5). Patients with adverse events were older (56 ± 7.3 vs. 51 ± 12; P = 0.013), but otherwise their baseline clinical and histopathological characteristics (Supporting Table S4A,B) were similar to those without events. Patients with adverse events did not have higher disease severity or more-advanced fibrosis at baseline. Supporting Table S5 shows the detailed baseline characteristics of the 34 patients with events.
| No. of Patients With Events | BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | Remarks |
|---|---|---|---|
| Any event (%) | 6 (8.3) | 28 (11.9) | |
| Death (%) | 0 | 6 (2.6) | Causes of death: Intracranial hemorrhage, Brain tumor, ruptured abdominal aortic aneurysm, chest infection, liver failure, and unknown |
| Cardiovascular events (%) | 3 (4.2) | 19 (8.1) | |
| Stroke | 1 (1.4) | 7 (3.0) | Nonobese: hemorrhagic stroke Obese: 3 × hemorrhagic stroke, 4 × ischemic stroke |
| Myocardial infarction | 2 (2.8) | 6 (2.6) | |
| Others | 0 | 7 (3.0) | Events: 3 × ischaemic heart disease, congestive heart failure, cerebral aneurysm, transient ischemic attack, cardiac arrest |
| Liver-related events (%) | 2 (2.8) | 3 (1.3) | |
| HCC | 0 | 2 (0.9) | |
| Others | 2 (2.8) | 1 (0.4) | Nonobese: portal vein thrombosis, liver abscess Obese: hepatic encephalopathy, hepatorenal syndrome |
| Other malignancies (%) | 1 (1.4) | 5 (2.1) | Nonobese: skin cancer Obese: 2 × colorectal cancer, 1 × brain tumor, 1 × uterine cancer, 1 × pancreatic cancer |
- One obese patient had two cardiovascular events. Five patients who died had multiple events.
Clinical events occurred in 11.9% of obese patients and 8.3% of nonobese patients (P = 0.190; Fig. 2). All 6 patients who died were obese. Cardiovascular events occurred in 19 (8.1%) obese patients and 3 (4.2%) nonobese patients. In addition, there were 2 cases of HCC and 1 case of liver failure; again, all were in the obese group.
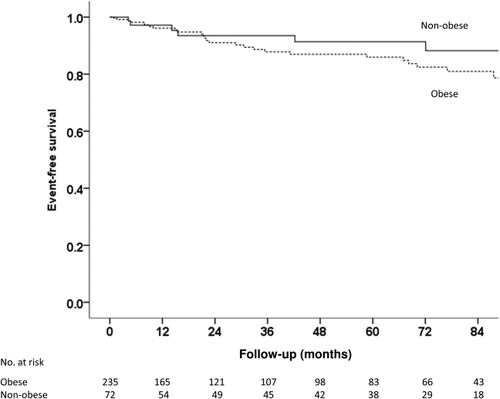
Event-free survival in nonobese and obese NAFLD patients (P = 0.190 by log-rank test). Events include cardiovascular events, liver-related events and malignancies (please refer to Table 5 for details).
Discussion
Despite similar prevalence of NASH in obese and nonobese NAFLD patients, nonobese patients had a lower NAS, contributed by a lower degree of steatosis and hepatocyte ballooning. Nonobese patients were also less likely to have liver fibrosis, but the proportions of patients with advanced fibrosis were similar between the obese and nonobese. Higher serum triglyceride level was associated with higher NAS in nonobese patients, and creatinine was the only independent predictor of advanced fibrosis.
Among the few published histological series on nonobese NAFLD, the results have been conflicting and inconclusive. 10-13 One study by Dela Cruz et al. showed that nonobese NAFLD patients were more likely to have histological lobular inflammation and had higher mortality.10 Whereas other studies have not confirmed this observation, only a small number of nonobese patients were included, precluding confident comparison between groups. 11-13 In our study, nonobese patients had slightly less-severe steatosis and fibrosis, but the overall proportion of patients with NASH and advanced fibrosis did not differ between nonobese and obese patients. This suggests that factors other than adiposity come into play as the disease advances further.
We found serum triglyceride level to be independently associated with higher NAS among nonobese subjects. Other studies have found dyslipidemia, including higher triglyceride levels, to be associated with NAFLD among nonobese patients.20, 21 In particular, the study by Kim et al. demonstrated that triglyceride levels were significantly associated with both the development and regression of NAFLD among nonobese Koreans.21 Increased intracellular concentration of fat in the liver can increase oxidative stress to hepatocytes.22 However, the exact relationship behind triglycerides and the progression from NAFLD to NASH is unclear. In our study, we found triglyceride levels to be associated only with more-severe steatosis and hepatocyte ballooning in nonobese patients. It did not seem to affect lobular inflammation. Other studies suggest that increased free fatty acids can induce hepatic lipoapoptosis, a major step in the pathogenesis in NASH, through c-Jun N-terminal kinase (JNK) activation.23 JNK activation can stimulate migration of monocytes into the liver, further causing hepatic inflammation and NASH.24 To extend further, the role of triglycerides as a treatment target has not been fully explored. A pilot study with 16 patients found that fenofibrate was able to decrease the degree of hepatocyte ballooning and serum liver enzymes.25
Interestingly, high serum creatinine level was the only factor associated with advanced fibrosis in nonobese patients. The association between NAFLD and chronic kidney disease has been shown in multiple observational studies.26 Whereas this can be explained by shared risk factors, a causal relationship attributed to systemic inflammation and activation of profibrotic cascades remains a possibility.27 In diabetic patients, even albuminuria, the early manifestation of diabetic nephropathy, is associated with NAFLD and advanced fibrosis.28
In our study, there was a notable difference between the prevalence of advanced fibrosis detected by liver biopsy and LSM, particularly among the obese patients. This could be explained by the modest positive predictive value of LSM in diagnosing advanced fibrosis. Previous studies have consistently shown that whereas a cutoff of 9.6 kPa has excellent negative predictive value in excluding advanced fibrosis, the positive predictive value is limited at a range of 60%-70%.19, 29, 30 This phenomenon is reflected in the obese patients (27.7/43 = 64.4%); a previous study also showed that LSM results in obese people tend to be higher despite lower fibrosis stages.31
Recent genomic studies and observational studies have identified PNPLA3 and TM6SF2 polymorphisms as genetic factors associated with NAFLD and/or its severity.32-35 The impact of PNPLA3 on hepatic steatosis is even more pronounced in patients without MetS.36 A previous meta-analysis showed that the according PNPLA3 polymorphism is associated with disease severity and progression.37 Previous studies also suggested a significant association between the TM6SF2 polymorphism and disease severity or progression.35, 38 However, we did not find any significant associations between presence of the above alleles and histological severity in the nonobese patients in this study by univariate analyses. Furthermore, we could not find any relationship between the genetic polymorphisms and disease progression among the patients that had multiple biopsies or LSM. This may be attributed to the small number of nonobese patients with advanced disease.
In this study, we found that more clinical events occurred among obese patients during the follow-up period. In particular, obese patients had significantly more cardiovascular events and deaths. This suggests that obese patients may have a worse prognosis and a higher mortality. The link between NAFLD and cardiovascular complications is well established.4 Whereas obesity itself is also a risk factor of cardiovascular diseases, weight loss is the mainstay of treatment of obese NAFLD and also prevention of its cardiovascular complications. Although our study shows that nonobese patients have a better prognosis and lower mortality, the study by Dela Cruz et al., which has a longer follow-up period, shows a higher mortality in nonobese patients.10 Further studies should be done on the long-term prognosis of NAFLD in nonobese patients.
Our study has the strengths of a prospective design, systematic follow-up, and the inclusion of histological and outcome data. But it also has several limitations. First, although this represents one of the largest histological cohorts of nonobese NAFLD patients, the number of patients with NASH or advanced fibrosis remains relatively small. This precludes extensive analysis of factors associated with advanced disease. Second, whereas liver biopsy was considered the gold standard to assess disease severity, it only represents 1/50,000th of the entire liver and may suffer from sampling bias. However, our biopsy results were largely verified by noninvasive assessments. Our previous population study also suggests that nonobese NAFLD patients have less-severe liver disease.8 Third, the follow-up duration was relatively short. We will continue to follow this cohort and study the natural history of NAFLD.
In conclusion, nonobese patients with NAFLD tend to have less-severe disease and may have a better prognosis than obese patients. Hypertriglyceridemia and higher creatinine are the factors associated with advanced liver disease in nonobese patients. Further research on the relationships between these risk factors and advanced liver disease is needed.
REFERENCES
Author names in bold designate shared co-first authorship




