The case for immune-based approaches in biliary tract carcinoma
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Potential conflict of interest: Nothing to report.
Abstract
Biliary tract cancers (BTC) comprise a group of uncommon malignancies in which the standard therapies are minimally effective and evolve slowly. Like the majority of gastrointestinal cancers, with some notable exceptions, the impact of immune-based approaches has yet to be seen. However, the etiological background of BTC—overlapping in almost every known causative or associated factor with inflammation—provides a strong clue that these approaches may have an impact in this group of diseases. This review covers what we currently know about the role of the immune system in the etiology of BTC, highlighting differences by subtype, and pointing to the therapeutic opportunities currently entering the clinic or about to do so. (Hepatology 2016;64:1785-1791)
Abbreviations
-
- BTC
-
- biliary tract carcinoma
-
- CCA
-
- cholangiocarcinoma
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- DC
-
- dendritic cell
-
- ECC
-
- extrahepatic cholangiocarcinoma
-
- GI
-
- gastrointestinal
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- ICC
-
- intrahepatic cholangiocarcinoma
-
- IgG4
-
- immunoglobulin G4
-
- IL-10
-
- interleukin-10
-
- MHC
-
- major histocompatibility complex
-
- MUC1
-
- mucin 1
-
- NK
-
- natural killer
-
- NKTs
-
- natural killer T cells
-
- PBC
-
- primary biliary cirrhosis
-
- PD1
-
- programmed death 1
-
- PD-L1
-
- programmed death ligand 1
-
- PSC
-
- primary sclerosing cholangitis
-
- pTNM
-
- pathological tumor node metastasis
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- Tregs
-
- regulatory T cells
-
- WT1
-
- Wilms' tumor protein 1
The term biliary tract carcinoma (BTC) refers to cancers that develop in the gallbladder and intra- and extrahepatic biliary ductal system. BTC is a relatively uncommon diagnosis, and randomized studies are few and far between. As is the case for most solid tumors, surgical resection is the only curative approach, although recurrence rates are high. For metastatic disease, the modest standard of care comprises gemcitabine-based chemotherapy, based on the ABC-02 trial.1 There is no outright standard second-line option. Clearly, there is an unmet need here to improve the treatment options at every stage for patients with this uncommon cancer.
The past number of years have seen much progress for immune-based approaches in solid tumor malignancies, with U.S. Food and Drug Administration approvals for various strategies, including dendritic cell (DC) vaccination as well as so-called immune checkpoint inhibition.2-4 The next frontier is for these treatments to prove themselves in diseases which have thus far proved refractory. So far, with notable exceptions, this has included cancers of the gastrointestinal (GI) tract, including BTC. One of the first studies evaluating programmed death 1/programmed death ligand 1 (PD1/PD-L1)-directed therapy was disappointing from a gastrointestinal (GI) cancer viewpoint.5 Similarly negative results for GI cancers were observed in other studies of checkpoint inhibition.6-8 The notable exceptions to this preliminary experience have been in mismatch repair-deficient colorectal cancer and also hepatocellular carcinoma (HCC).9, 10 The former situation results in a marked increase in the mutagenic burden within tumors, thereby—presumably—increasing the likelihood that a tumor-specific neoantigen is generated.11 The findings of activity of anti-PD1 therapy in HCC are apt for BTC, given their overlapping etiology in inflammation.
Inflammation and Predisposition to BTC
The causative link between inflammation and cancer development stretches back to the 19th century observations of Rudolph Virchow. Chronic inflammation can greatly facilitate cancer development through a number of means, culminating in an immunosuppressed microenvironment.12 This broad and sometimes paradoxical relationship between cancer and inflammation is particularly relevant in BTC, a cancer whose main associated predisposing conditions have chronic inflammation as their common underlying pathological denominator (Fig. 1). The dominant known risk factor for gallbladder carcinoma is cholelithiasis-induced chronic inflammation, whereas—worldwide—infestation of the biliary ducts by parasites or persistent hepatolithiasis are important predisposing factors for intrahepatic cholangiocarcinoma (ICC).13 In the United States and Europe, primary biliary cirrhosis (PBC) and sclerosing cholangitis (PSC) are all established risk factors for BTC, as are causes of intrahepatic inflammation (e.g., obesity).14 Recent studies have linked liver disease associated with the increasing incidence of chronic hepatitis C virus (HCV) infection with ICC.15
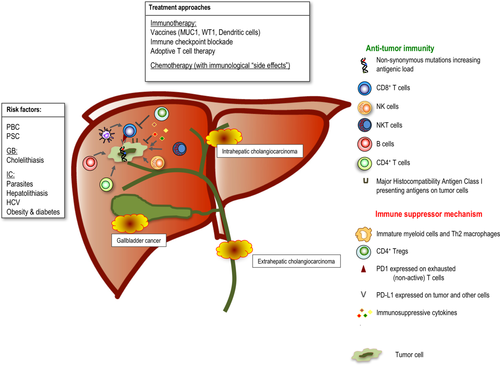
The finding of an immune role in the etiology of BTC is important because it raises the possibility of an immune-based remedy. This is not only relevant for established cancer, but also in the long antecedent period of inflammation where the opportunity for prevention exists. The engagement of biliary epithelium with elements of the immune system is not merely passive, but involves (or can involve) cholangiocytes actively presenting antigen. Major histocompatibility complex (MHC) class I molecules are constitutively expressed in the biliary tract, and Schrumpf et al. have shown that cholangiocytes can activate a subset of lymphocytes called natural killer (NK) T (NKT) cells.16 PSC and PBC are characterized by a marked hepatic mononuclear infiltration, but this is also a feature of other chronic liver diseases, such as alcoholic cirrhosis.17 In PSC, a marked CD4+ and CD8+ T-cell infiltration in portal tracts and around bile ducts is observed.18 Interestingly, it seems that the pattern of T-cell clonality may vary according to the underlying predisposing condition, with distinct T-cell receptor signatures reflective of different antigenic repertoires.17 Liaskou et al. performed high-throughput sequencing of liver-infiltrating T cells in samples derived from patients with PSC, PBC, and alcoholic cirrhosis.17 These analyses revealed the presence of disease-associated clonotypes, which differed by degree. For example, in alcohol-related disease, a pronounced oligoclonality was observed, in contrast to a higher diversity among PSC. It has also been shown by Harada et al. that a significant proportion of BTC is accompanied by a marked infiltration of immunoglobulin G4 (IgG4)-positive cells, which can effect the cytokine milieu by producing interleukin-10 (IL-10). Although the significance of this is uncertain, it does further reflect the interplay between inflammation and cancer, either as an etiological agent (IgG4-mediated cholangitis predisposing to malignancy) or secondary effect (from an IL10-induced immune-suppressed tumor microenvironment).
As is the case for other malignancies, such as pancreatic cancer, a major limitation in the past has been the lack of dependable, truly replicative orthotopic or autochthonous preclinical tumor models. This is arguably of even more importance in immunotherapy given the necessity of approximating the generally suppressive tumor microenvironment. This is changing in pancreaticobiliary malignancies, allowing better assessment of immune-based approaches.19, 20
Immunogenicity of BTC
The success of immune checkpoint inhibition is dependent upon the presence of an already existing endogenous antitumor immune response. Spontaneous immune responses do occur in established BTC. This was illustrated by Rosenberg et al., who performed whole-exomic sequencing on the resected tumor of a patient with metastatic chemorefractory cholangiocarcinoma (CCA).21 By generating tandem minigene constructs of each of the mutations and coculturing with expanded populations of isolated tumor-infiltrating lymphocytes (TILs), they identified a reactive population of CD4+ cells. Through adoptive transfer of enriched populations of these cells, the investigators were able to produce tumor regression. The wider relevance of this case to CCA is uncertain. It was notable in this patient that only 26 mutations were identified in the tumor, a relatively low number. CCA in general is not a highly mutated cancer. In 239 cases of BTC in whom whole-exome sequencing was performed, the median numbers of mutations across the ICC, extrahepatic cholangiocarcinoma (ECC), and gallbladder cancer subtypes were 39, 35, and 64, respectively.22 Interestingly, a small proportion (5.9%) of cases were identified as having a high number of mutations (median number = 641), 5 of whom had defects in the mismatch-repair apparatus.9 Also, of special interest in this analysis was that four molecular subgroups of BTC were elucidated, which could be divided into prognostic categories. The group that had the poorest prognosis demonstrated enrichment for genes involved in the immune system. The hypermutated tumors were part of this cluster, with higher expression of PD-L1.
Perhaps the best indicator of an already existing antitumor immunity is the presence of TILs. In HCC, the presence of TILs has been shown to be prognostic.23, 24 Recent evidence has highlighted the link between mutagenic burden and immune reactivity. However, Tran et al. have recently suggested that the relationship is more nuanced, with the finding of immunoreactive TILs even in the setting of low mutational burden.25
In BTC, the prevalence and prognostic relevance of TILs has also been documented. Sabbatino et al. evaluated lymphocyte infiltration in 27 cases of ICC.26 All tumors had TILs, and this correlated with human leukocyte antigen expression. Nakakubo et al. investigated the significance of TILs in 110 resected gallbladder specimens.27 CD4+ and CD8+ T-cell infiltration correlated with decreasing tumor invasion and survival. Goeppert et al. evaluated immune cell infiltration in 375 cases of BTC postresection.28 Approximately half the patients had some degree of TILs, and this correlated with survival. Interestingly, patients who had higher total regulatory T-lymphocyte counts had a better survival outcome. The prognostic value of regulatory T cells (Tregs) in other GI tumor types has been variable in terms of whether it was a favorable or adverse factor.29
It was also of note, in the large sample analyzed by Goeppert et al., that differences were observed in the composition of inflammatory infiltrate by anatomical subtype, with less CD8+ infiltration in intrahepatic relative to extrahepatic disease. Indeed, the prognostic significance of the presence of intraepithelial T and B lymphocytes pertained only to these subtypes and not to ICC. The potential difference in inflammatory infiltrate according to anatomical subtype noted by Goeppert et al. is intriguing, but perhaps not surprising given the differences in molecular derangement or gene expression, different cell of origin, as well as in incidence, risk factors, and indeed outcome.30, 31 Although clinically, the anatomical subtypes are treated the same in the advanced disease setting, this paradigm has evolved more through expediency rather than being driven by biological considerations. As we move into the immunotherapeutic era, we will need to pay more attention to this, perhaps by stratification.
One method of immune evasion is achieved by down-regulation of MHC I.32-34 Goeppert et al. analyzed the impact of MHC expression on survival in BTC (N = 334) as well as the relationship of this to TILs.35 MHC I expression was assessed semiquantitatively and divided into two groups of high and low expression. There was a trend for higher MHC I expression in lower disease stages and an association between MHC I expression and the number of TILs. Interestingly, the investigators found that associations differed by subtype, with the prognostic significance of MHC I expression, being by trend stronger in ECC compared with ICC, suggesting that mechanisms of immune escape may vary with anatomical subtype.
In addition to evasive mechanisms, tumors have the ability to suppress antitumor immunity in a variety of ways. Myeloid-derived suppressor cells, a heterogeneous cell population that suppress T-cell responses, have been shown to accumulate in the blood of patients with BTC.36 A separate mechanism results from expression by tumors of PD-L1, which can also inhibit T-cell reactivity. Ye et al. evaluated the expression of PD-L1 and its ligand, PD1, in N = 31 surgically resected cases in addition to the corresponding cancer adjacent tissues.37 Expression of PD-L1 was found to be up-regulated in CCA tissues compared with the cancer adjacent tissues. Tumor-related PD-L1 expression was significantly correlated with both tumor differentiation and pathological tumor node metastasis (pTNM) stage and was inversely correlated with CD8+ TILs. In the analysis by Sabbatino et al., 8 of 27 ICC cases expressed PD-L1.26 Table 1 summarizes the evidence for BTC immunogenicity.
| Immune Factor | Evidence | Comment |
|---|---|---|
| Tumor-associated antigen-provoked immune response | CD4+ T helper 1 (T(H)1) cells that were reactive to a mutation in erbb2 interacting protein (ERBB2IP) | Adoptive transfer of expanded population demonstrated tumor regression |
| Mutagenic burden | 5.9% of N = 239 BTC cases analyzed had a high number of mutations | Association with MMR defects in minority; may respond to aPD1 tx as single agent |
| TILs | Sabbatino et al. Present in all (N = 27) cases of ICC | |
| Nakakubo et al. N = 110 resected BTC: CD4+ T cell (51.1%), CD8+ T cell (37.8%), NK cell (33.3%), and DC (48.9%) infiltration | CD4+ and CD8+ T-cell infiltration correlated with decreasing tumor invasion. | |
| Goeppert et al. Approximately 50% some degree of intraepithelial CD8 TILs, decreased with increasing stage of disease | Less CD8+ infiltration in intrahepatic disease relative to extrahepatic disease and gallbladder cancer | |
| MHC 1 down-regulation | N = 334 BTC; trend to higher MHC I expression in lower UICC stages (P = 0.074) and, in particular, BilIN 3, where significantly higher MHC I expression levels were observed compared to invasive tumors (P = 0.004) | Association between high MHC I expression, low tumor grade, and number of TILs. Of note, differences observed by subtype |
| PD-L1 | Sabbatino et al.; 8 of 27 ICC cases expressed PD-L1 | |
| Ye et al. N = 31 surgically resected cases BTC | Expression of PD-L1 and PD1 was found to be up-regulated in CCA tissues compared with the cancer adjacent tissues. Tumor-related PD-L1 expression was significantly correlated with both tumor differentiation and pTNM stage and was inversely correlated with CD8+ TILs |
- Abbreviations: UICC, Union for International Cancer Control; BilIN 3, biliary intraepithelial neoplasia, grade 3; MMR, mismatch repair; aPD1, anti-PD1.
Translational Efforts
There is a paucity of randomized, clinical trials for BTC, no doubt because of the relative rarity of this tumor type. In addition, the CCA-specific phase 2 studies that are available to patients tend toward the evaluation of chemotherapeutics, which have already shown efficacy in other indications. Progress in BTC seems therefore to lag behind that of other, more common solid tumors, with a dearth of studies evaluating new and innovative drugs. Traditionally, BTC patients could, for the most part, only access clinical trials in the phase 1 setting. With the advent of immune-based approaches, this paradigm shows signs of change, with less emphasis on the initial dose-escalation phase of drug development in favor of studies allowing multihistology cohorts. Table 2 shows the currently open and accruing studies employing an immune-based approach currently available on clinicaltrials.gov and which are specific to BTC or have BTC explicitly stated as an eligible histology.
| ID | Title | MOA |
|---|---|---|
| NCT01868490 | The Adoptive Immunotherapy for Solid Tumors Using Modified Autologous Cytokine-induced Killer Cells | Adoptive cell transfer |
| NCT01174121 | Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients With Metastatic Cancer | Adoptive cell transfer |
| NCT02628067 | Study of Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-3475-158/KEYNOTE-158) | Anti-PD1 |
| NCT01853618 | A Pilot Study of Tremelimumab - A Monoclonal Antibody Against CTLA-4 in Combination With Trans-Arterial Catheter Chemoembolization (TACE), Radiofrequency Ablation (RFA), Stereotactic Body Radiation Therapy (SBRT) or Cryoablation in Subjects With Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinomas (BTC) | Anti-CTLA-4 |
| NCT02632019 | Dendritic Cell-precision T Cell for Neo-antigen in the Treatment of Advanced Biliary Tract Malignant Tumor | DC-based vaccine |
- a Accessed from clinicaltrials.gov on December 21, 2015.
- Abbreviation: MOA, mechanism of action.
In terms of published clinical data for immune approaches in BTC the experience is dominated by peptide vaccines. These small studies have been well tolerated without showing a strong signal of efficacy.38, 39 For example, Aruga et al. conducted a small phase I clinical trial evaluating multiple-peptide vaccination for patients with advanced BTC.40 Peptide-specific T-cell immune responses and disease stability were observed in some patients. DC-based vaccines have also been evaluated, most commonly in combination with standard modalities, which complicates assessment of efficacy.41, 42
The experience with checkpoint inhibitors is very preliminary. Documented response to anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) therapy has been observed, manifesting as a delayed response after initial disease progression.43 The early data for anti-PD1 inhibition has been recently presented, showing encouraging evidence of efficacy, and which seems consistent with use of this agent in other solid tumor studies.44
One of the advantages of immune-based approaches is the potential role for their combination with standard therapies, either cytotoxic agents, radiation, or interventional radiological procedures.45 Certain chemotherapeutics can activate, rather than suppress, the immune system, and a robust immune response is a necessary component determining tumor response.46 Gemcitabine is a nucleoside analogue that is part of the standard treatment, and probably the most common chemotherapeutic agent used in BTC.1, 47 The immune effects of gemcitabine have been studied perhaps more than for any other drug used in GI cancer. Its effects on the immune system are diverse.45 With regard to BTC, Koido et al. demonstrated that, in ICC cells isolated from a patient with malignant ascites, immunogenic modulation of cells could be induced by gemcitabine with up-regulation of MHC class I and II, calreticulin—a modulator or immunogenic cell death—mucin 1, cell surface associated (MUC1) and Wilms' tumor protein 1 (WT1) messenger RNA.48 Interestingly, the investigators found that gemcitabine also induced up-regulation of immunosuppressive PD-L1, suggesting a potential rationale for combination therapy. Ablative therapies are occasionally employed in the management of BTC, with studies documenting an increase in peripheral immunity.49 We are currently conducting a study evaluating subtotal radiofrequency ablation in combination with anti-CTLA-4 therapy (NCT01853618) in patients with BTC.
Conclusions
The advantages of immune-based treatments are clear, such that the initial results of the checkpoint inhibitors in particular have captured the imaginations of physicians and the general public alike. Although the benefit observed thus far is real, BTC has not been among the group of malignancies in which these agents have so far shown efficacy. No doubt this is partly attributed to the rarity of this illness and the relatively small number of patients with BTC who have been able to partake in clinical trials. The close etiological relationship between BTC and inflammation speaks to an important role for the immune system in this disease, as do the data discussed showing by almost any measure—TIL, MHC down-regulation, and PD-L1 expression—evidence of a reactive surveillance that can be potentially manipulated by the new drugs and technologies that are making their way in the clinic.




