Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B−/− (Wilson disease) mice
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health (1K08DK083774 [to J.P.H.], P30DK89502 [to J.P.H.], and PO1 GM067166 [to S.L.]).
Abstract
Wilson disease (WD) is a hepatoneurological disorder caused by mutations in the copper-transporter, ATP7B. Copper accumulation in the liver is a hallmark of WD. Current therapy is based on copper chelation, which decreases the manifestations of liver disease, but often worsens neurological symptoms. We demonstrate that in Atp7b−/− mice, an animal model of WD, liver function can be significantly improved without copper chelation. Analysis of transcriptional and metabolic changes in samples from WD patients and Atp7b−/− mice identified dysregulation of nuclear receptors (NRs), especially the liver X receptor (LXR)/retinoid X receptor heterodimer, as an important event in WD pathogenesis. Treating Atp7b−/− mice with the LXR agonist, T0901317, ameliorated disease manifestations despite significant copper overload. Genetic markers of liver fibrosis and inflammatory cytokines were significantly decreased, lipid profiles normalized, and liver function and histology were improved. Conclusions: The results demonstrate the major role of an altered NR function in the pathogenesis of WD and suggest that modulation of NR activity should be explored as a supplementary approach to improving liver function in WD. (Hepatology 2016;63:1828-1841)
Abbreviations
-
- ABC1
-
- ATP-binding cassette 1
-
- ALT
-
- alanine aminotransferase
-
- ASMase
-
- acid sphingomeylinase
-
- AST
-
- aspartite aminotransferase
-
- ATP
-
- adenosine triphosphate
-
- ATP7B
-
- ATPase Cu++ transporting beta polypeptide
-
- BCS
-
- bathocuproine disulfonic acid
-
- CAR
-
- constitutive androsterone receptor
-
- cDNA
-
- complementary DNA
-
- COL1a
-
- collagen 1 alpha
-
- cRNA
-
- complementary RNA
-
- CYP7a1
-
- cytochrome p450 family 7 subfamily A polypeptide 1
-
- DMSO
-
- dimethyl sulfoxide
-
- EC
-
- epoxycholesterol
-
- ERK
-
- extracellular signal-related kinase
-
- Fasn
-
- fatty acid synthase
-
- FXR
-
- farnesoid X receptor
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- HDL
-
- high-density lipoprotein
-
- HET
-
- heterozygote
-
- HMG-CoA
-
- 3-hydroxy-3-methylgultaryl-coenzyme A
-
- HSCs
-
- hepatic stellate cells
-
- iNOs
-
- inducible nitric oxide synthase
-
- JHUSOM
-
- Johns Hopkins University School of Medicine
-
- KO
-
- knockout
-
- IL-1β
-
- interleukin-1 beta
-
- LEC
-
- Long-Evans Cinnamon rat
-
- LDL
-
- low-density lipoprotein
-
- mRNA
-
- messenger RNA
-
- NR
-
- nuclear receptor
-
- SREBP1
-
- sterol regulatory element-binding transcription factor 1
-
- LXR
-
- liver X receptor
-
- MS
-
- mass spectrometry
-
- OCT
-
- optimal cutting temperature compound
-
- OD
-
- optical density
-
- OHC
-
- hydroxysterol
-
- P
-
- phosphylated
-
- p38-MAPK
-
- p38 mitogen-activated kinase
-
- RXR
-
- retinoid X receptor
-
- SAPK/JNK
-
- stress-activated kinase/c-Jun N-terminal kinase
-
- TG
-
- triglyceride
-
- Timp-1
-
- tissue inhibitor of metalloproteinase 1
-
- TNFα
-
- tumor necrosis factor alpha
-
- UV-vis
-
- ultraviolet-visible
-
- WD
-
- Wilson disease
Wilson disease (WD) is a potentially fatal disease caused by mutations in the adenosine triphosphate (ATP)-dependent copper transport protein, ATPase Cu++ transporting beta polypeptide (ATP7B). In hepatocytes, ATP7B facilitates copper delivery to the copper-dependent ferroxidase, ceruloplasmin, which undergoes functional maturation in a secretory pathway. ATP7B also maintains cytosolic copper at a nontoxic level by sequestering excess copper in vesicles for subsequent export into bile.1 WD-causing mutations disrupt ATP7B function, resulting in accumulation of copper in tissues, especially the liver. The disease manifestations are highly variable, indicative of modifying factors, and pose significant challenges for diagnosis and treatment.1, 2 Copper chelation is the major and largely successful therapy for WD. However, frequent side effects,3 poor compliance, neurological decompensation, and cost complicate therapy.2, 4 Development of additional or supplementary approaches requires a better understanding of the pathogenesis of WD. Atp7b−/− mice are established model for studies of WD.5 These animals recapitulate the major manifestations of WD, including hepatic copper overload, loss of ceruloplasmin activity, elevated urine copper, and liver disease. In both mice and humans, the severity of pathology is not proportional to the amount of hepatic copper, further indicating a modifying influence of metabolic and/or environmental factors. A significant role for metabolism is also suggested by discordant clinical disease in monozygotic WD twins with different nutritional histories.6
Our previous studies identified lipid metabolism, especially cholesterol biosynthesis, as the major metabolic pathway inhibited in response to hepatic copper accumulation in Atp7b−/− mice before onset of hepatitis.8 Similar findings in Long-Evans Cinnamon (LEC) rats7 prompted human studies that described lower total cholesterol in WD patients compared to patients with comparable liver disease.8 Diminished levels/activity of 3-hydroxy-3-methylgultaryl-coenzyme A (HMG-CoA) reductase is observed in all three species.7, 9 Here, we expand upon this work to identify the molecular basis of lipid dysregulation in WD. Our study provides direct evidence that abnormally reduced function of nuclear receptors (NRs) involved in the reciprocal regulation of lipid metabolism and inflammatory response is the major event that triggers the onset and progression of liver pathology in WD. Furthermore, we demonstrate that up-regulation of NR function can ameliorate the disease even in the presence of high copper. These findings provide a foundation for future efforts to develop novel treatments to supplement chelation in WD.
Materials and Methods
Human Studies
In accord with the Declaration of Helsinki and the institutional review board (U. Leipzig Reg. #236-2006; U. Heidelberg Reg. #346/2005) approved consent, WD patients underwent liver transplantation for acute or chronic liver failure. Control specimens were obtained from patients who underwent liver resections for other clinical reasons (Supporting Table 1). Liver sections were snap frozen in liquid nitrogen and stored. Total RNA was isolated using TRIzol reagent (Invitrogen, Grand Island, NY) followed by RNeasy clean-up procedure (Qiagen, Valencia, CA). Integrity of isolated RNA was verified by ethidium bromide staining and by optical densities (ODs) ratio (OD: 260 nm/280 nm >1.8). A total of 16 liver RNA samples (eight biological replicates for control liver and eight for WD patients) were examined for RNA integrity and concentration on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) using the RNA 6.000 LabChip Kit (Agilent Technologies) according to the manufacturer's instructions.
Affymetrix GeneChip analysis (Affymetrix, Santa Clara, CA) was conducted at the microarray core facility of the Interdisziplinäres Zentrum für klinische Forschung, Leipzig (Faculty of Medicine, University of Leipzig, Leipzig, Germany). Two micrograms of total RNA were used to prepare double-stranded complementary DNA (cDNA; Superscript II; Life Technologies, Gaithersburg, MD) primed with oligo-dT containing a T7 RNA polymerase promoter site (Genset SA, Paris, France). cDNA was purified by phenol-chloroform extraction before in vitro transcription using the IVT labeling kit (Affymetrix) to synthesize complementary RNA (cRNA). After the in vitro transcription, unincorporated nucleotides were removed using the RNeasy kit (Qiagen). The cRNA was fragmented and hybridized to two different (technical replicates) Human Genome U133 Plus 2.0 Arrays (Affymetrix). Washing and staining of the probe array was performed according to the manufacturer's instructions. The array was scanned with a third-generation Affymetrix GeneChipScanner 3000.
Image processing and analysis were performed using Affymetrix MAS 5.0 software. The resulting intensities and coordinate information were saved in a CEL file format and then subjected to global scaling with an average target intensity of 350 to allow for direct comparison of hybridization values from different targets. Scaled results for each sample were saved as CHP files, and these data were used to evaluate overall chip performance. The analysis indicated that the parameters describing the quality of RNA, hybridization, and detection were all within acceptable range.
The associations between altered genes or pathways were evaluated using the Ingenuity Pathways Analysis software (Ingenuity Systems, www.ingenuity.com). Affymetrix identifiers of the differentially expressed genes (the fold-change of 2.0 or higher) and their corresponding expression values were loaded into the software and mapped to its corresponding gene object (so-called focus genes) in the Ingenuity Pathways Knowledge Base. The significance of the associations between the data set and the canonical pathway and functional annotations was calculated in two ways. First, the number of genes from the data set mapping to a pathway was divided by the number of all known genes ascribed to the pathway. Second, the left-tailed Fischer's exact test was used to calculate related P values and distinguish those functional/pathway annotations that had more Focus Genes than expected by chance. The networks of the focus genes were algorithmically generated based on their connectivity.
Animal Studies
ATP7b knockout (KO; Atp7b−/−) and heterozygote (Atp7b+/−) mice were generated as previously prescribed.10 T0901317 (Cayman Chemical, Ann Arbor, MI) powder was mixed with Teklad 2018 powdered chow (Harlan, Madison, WI) and fed to animals ages 6-7 weeks old at a dose of 50 mg/kg/day, thrice-weekly. After 8 weeks, animals were sacrificed. Animals were housed at the Johns Hopkins University School of Medicine (JHUSOM; Baltimore, MD) Animal Care Facility and protocols designed according to National Institutes of Health (Bethesda, MD) guidelines. Normal pellet chow was fed to the mice on days they were not receiving the drug. Animals were weighed weekly. At the time of sacrifice, trunk blood and livers were harvested. The institutional animal care and use committee of JHUSOM approved the above experimental protocols.
Serum Biochemical Analysis
Animals were fasted overnight before sacrifice. Whole blood was collected from the aorta and vena cava in amber Microtainer tubes (Beckton Dickinson, Franklin Lakes, NJ), and serum was separated after centrifugation at 5,000 rpm for 10 minutes. Liver biochemistries and lipid panels were performed in the Molecular and Comparative Pathobiology Core Laboratory of JHUSOM.
Quantitative Reverse-Transcription Polymerase Chain Reaction
Sections of mouse liver were immersed in RNAlater, homogenized, and frozen at -80°C. RNA was isolated with TRIzol according to the manufacturer's instructions, then transcribed into cDNA using the SuperScript III first strand synthesis system (Invitrogen). RNA quantity was determined by an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). Samples were run in triplicate and performed on a 7900 HT machine (Applied Biosystems, Foster City, CA) and analyzed with the SDS 2.4.1 software. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the control to which gene of interest expression was normalized.
Quantitative reverse-transcriptase polymerase chain reaction for ATP-binding cassette (Abc1), HmGCoA1, cytochrome p450 family 7 subfamily A polypeptide 1 (CYP71a), fatty acid synthase (Fasn), liver X receptor (LXR)α (NR1H3), LXRβ (NR1H2), retinoid X receptor (RXR)α (NR2B2), sterol regulatory element-binding transcription factor 1 (SREBP1), farnesoid X receptor (FXR), tissue inhibitor of metalloproteinase 1 (TIMP1), collagen 1 alpha (COL1a), and GAPDH was performed with sequence-specific primers and probes using TaqMan gene expression assays (Applied Biosystems).
Immunoblotting
Antibodies were purchased from Santa Cruz Biotechnology, Inc (Dallas, TX), cell signaling (Beverly, MA), or Abcam (Cambridge, MA). Cytoplasmic and nuclear protein was extracted using the NE-PER kit (Thermo Fisher Scientific, Waltham, MA). Immunoblotting for LXRα (SC-1202), LXRβ (SC-1203), RXR (SC-831), p38 mitogen-activated kinase (p38-MAPK; CS-8690S), phosphorylated (P)-p38-MAPK (CS-4511S), stress-activated kinase/c-Jun N-terminal kinase (SAPK/JNK; CS-9252S), P-SAPK/JNK (CS-9251S), extracellular signal-regulated kinase (ERK)1/2 (CS-9102S), P-ERK1/2 (CS-9101S), and GAPDH (ab8245) was performed as previously described.11 Densitometry was performed using Image J software (National Institutes of Health).
Histology
Sections of mouse liver were covered in optimal cutting temperature compound (OCT) and snap frozen in liquid nitrogen. Sections were then cut and stained with hematoxylin and eosin. Deidentified samples were analyzed for the presence of inflammation by an expert in veterinary pathology (D.H.). Inflammation was scored as follows: none, 0; mild, 1; moderate, 2; and severe, 3. Severely inflamed livers had karyomegaly, cytomegaly, binucleate hepatocytes, intranuclear vacuoles, multiple nuclei, and inflammatory cell infiltrates. Sirius red staining and subsequent morphometric analysis was performed as previously described.12
Testing Copper Binding to T0901317
First, 0.5 mM of Cu(I) stock solution was prepared by diluting CuCl2 into 20 mM of HEPES (pH 7.4) buffer with 2 mM of tris(2-carboxyethyl)phosphine. T0901317 stock solution was prepared in dimethyl sulfoxide (DMSO). For the bathocuproine disulfonic acid (BCS) titration experiment, 0.5 mM of Cu(I) were gradually added to 10 mM of BCS in a 0.6-mL volume of 20-mM HEPES (pH 7.4) solution to a final concentration of 8.3 mM. Coordination of Cu(I) to BCS was examined by ultraviolet-visible (UV-vis) spectroscopy by monitoring the changes in absorption at 484 nm. For the T0901317 titration experiment, 0.5 mM of Cu(I) was gradually added to 10 mM of T0901317 in a 0.6-mL volume of DMSO solution, to a final concentration of 16.7 mM. Coordination of Cu(I) to T0901317 was examined by UV-vis spectroscopy by monitoring the absorption changes in the range from 300 to 800 nm.
Hepatic Copper Measurements
Hepatic copper levels were measured by polarized atomic absorption spectroscopy as previously described.5 Briefly, 50-100 mg of liver was dissolved in 2 mL of HNO3 at 90°C. Copper levels were determined using a Hitachi Z-8279 spectrophotometer (Hitachi, Tokyo, Japan). Samples were compared to freshly prepared standards.
Hepatic Triglyceride Measurements
The triglyceride (TG) colorimetric assay kit (Cayman Chemical) was used for measuring hepatic TG content using the provided protocol. First, 120-160 mg of liver tissue were homogenized and samples were assayed in triplicate. Absorbance at 540 nm was detected on an Envision plate reader (Perkin-Elmer, Waltham, MA). TG concentrations were calculated using a standard curve, then normalized to the volume of homogenate and the mass of the liver tissue.
Hepatic Oxysterol Measurements
Oxysterols were extracted and measured using the protocol described by McDonald et al.13 First, 1 mg of liver tissue was macerated, solubilized with methylene chloride and methanol, saponified with 10 N of KOH, and purified with hexane and aminopropyl SPE cartridges (Biotage, Uppsula, Sweden). Mass spectrometry (MS) was carried out using liquid chromatography/MS on a triple-quadrupole mass spectrometer (AB SCIEX 5500, Framingham, MA) in multiple reaction monitoring mode. The high-performance liquid chromatography column used for these analyses was a 2 × 150 mm × 2.6 mm particle size Kinetix column (Phenomenex, Torrance, CA) at a flow rate of 2.5 mL/min. Quantitation was performed by establishing a linear relationship between measured amounts of the analytical standards combined with the internal standard cocktail. This regression line was applied to the measured peak areas for analytes in the extracted samples to determine measured quantities present in each sample. The numbers were normalized to the wet weight of the tissue extracted and reported as picograms of analyte/wet weight tissue.
Statistical Analysis
Data are presented as the mean and the standard error (SE) of the mean. Data were analyzed with the two-tailed Student t test, and P values less than 0.05 were considered significant.
Results
Lipid Metabolism Is Abnormal in WD Patients
Lipid metabolism in Atp7b−/− mice is significantly affected by copper overload, even before liver disease is detected.9 The messenger RNA (mRNA) profiling and pathway analysis suggests that down-regulation of cholesterol biosynthesis is caused by inhibition of signaling by NRs, especially the LXR/RXR heterodimer (Fig. 1A).9 To examine the relevance of these observations to human WD, we performed mRNA profiling of liver explants from 6 WD patients (two samples did not pass quality control standards) using microarrays and compared the profiles to those from 8 control individuals. All WD livers were at the advanced stage of disease (Supporting Table 1), which produced changes in a large number of biological processes and pathways (Supporting Tables 2 and 3). The most significant altered biological processes were “metabolism” (824 genes), “positive and negative regulation of biological process” (273 and 257 genes, respectively), “immune system process” (172 genes), “cell proliferation” (143 genes), and “biological adhesion” (108 genes). Within the category of metabolism, “lipid metabolism” was the most significantly changed (220 genes), with a highly significant Z-score of 15.54. The primary change was down-regulation (165 genes). The pathways most affected were LXR/RXR activation (down-regulated) and interleukin-1 beta (IL-1β) inactivation of RXR (up-regulated; Fig. 1B). Changes in lipid and carbohydrate metabolism represent the largest percentage of metabolic changes (32.76%), with lipid metabolism responsible for most of these changes (24%; Fig. 1C). The most significant change was down-regulation of signaling mediated by the FXR/RXR or LXR/RXR NRs (47 of 116 genes affected: P = 2.28 × 10-22; Fig 1B,C; Supporting Tables 2 and 3).
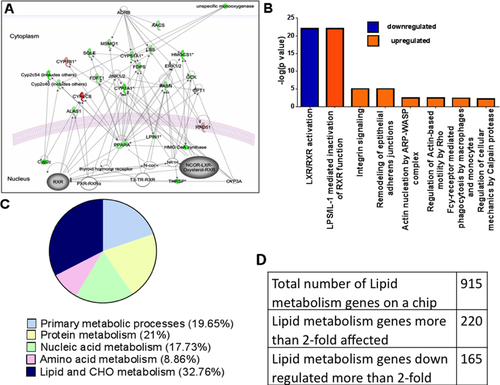
Taken together, the in silico analysis of gene expression indicated that LXR/RXR-mediated regulation of lipid metabolism was the most affected pathway in patients with WD and Atp7b−/− mice and therefore a promising target for further investigation.
The Level of RXR NR and LXR/RXR Targets Are Decreased in Atp7b−/− Mice
The functional status of the LXR/RXR pair was evaluated by measuring expression levels of LXR/RXR targets—Fasn and HMG-CoA reductase. The mRNA levels for both genes were significantly down-regulated in Atp7b−/− livers, consistent with lower LXR/RXR activity (Fig. 2A). The apparently diminished LXR/RXR function could be either the result of decreased levels of endogenous activating ligands or lower expression of these receptors. Oxysterols (OHCs) are potent activators of LXR. MS analysis of OHC profiles demonstrated that the mean levels of LXR ligands 24S-OHC and 22R-OHC were lower in Atp7b−/− mice compared to control; however, the decrease was not statistically significant. Statistically significant decreases were observed for 7α,β-OHC (produced by the FXR target, Cyp7a1) and 5,6β-epoxycholesterol (5,6β-EC). By comparison, levels of 4β-hydroxycholesterol were higher in Atp7b−/− mice compared to control (Fig. 2B); this OHC is a marker of activation of CYP3A4/5, which is regulated primarily by constitutive androstane receptor (CAR)-mediated signaling, rather than LXR.14
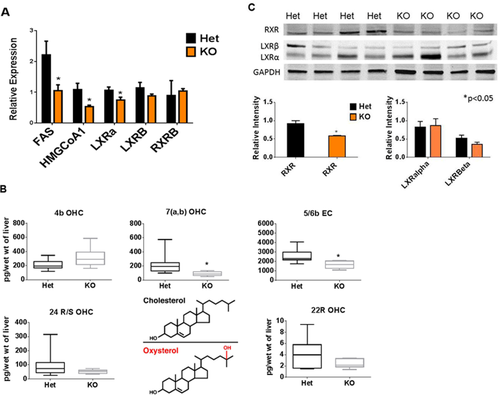
Because the decrease in LXR ligands was modest, we also examined abundance of LXRα, LXRβ, and RXR. LXRα mRNA (NR1H3) was decreased in Atp7b−/− liver, whereas protein levels were not changed significantly. There was no difference in LXRβ (NR1H2) or RXRβ (NR2B2) mRNA (Fig. 2A) and protein levels of LXRβ were only slightly decreased. In contrast, RXR was significantly less abundant in both the cytoplasmic and nuclear fractions from Atb7b−/− liver (Fig. 2C and Supporting Fig. 1). Thus, lower expression of LXR/RXR-regulated genes could be the result of decreased RXR and a reduced number of functional LXR/RXR dimers. Oxidative stress is known to stimulate phosphorylation of ERK1/2, p38-MAPK, and SAPK/JNK, which represses RXR expression and signaling.15, 16 However, we did not find evidence of increased phosphorylation of these proteins (Supporting Fig. 2A-F).
Treatment With LXR Agonist T0901317 Does Not Prevent Copper Accumulation in the Liver
Activation of LXR/RXR up-regulates lipid metabolism and inhibits inflammatory response.17 We hypothesized that reductions in LXR/RXR function may be responsible for inhibition of cholesterol biosynthesis and increased hepatic inflammation observed in WD patients and Atp7b−/− mice and play an important role in development of liver pathology in WD. If true, activation of the LXR/RXR pathway may prevent hepatitis and improve liver function. To test this hypothesis, we examined the effect of an LXR agonist (T0901317) on copper levels, lipid metabolism, liver morphology, and function in Atp7b−/− mice (KO). The histologically and biochemically normal heterozygote (HET) mice were used as controls.
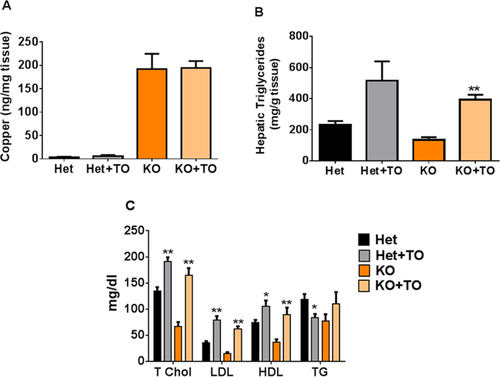
The HET animals that received the drug were significantly heavier than the age-matched controls (26.43 ± 1.35 vs. 22.53 ± 0.72 g; P < 0.05; Supporting Fig. 3A), whereas there was neither a difference in body weights (24.03 ± 0.91 vs. 24.67 ± 1.54 g) nor liver weights between treated and untreated KO animals (Supporting Fig. 3B). Copper overload is the initial metabolic trigger for development of liver pathology in WD, and it was important to determine that the drug acts downstream of copper by activating lipid metabolism and not by diminishing copper levels in tissue. At the end of the experiment, copper levels in KO mice were increased 38-fold compared to HET control. A similar increase was observed in drug-treated Atp7b−/− mice (Fig. 3A), indicating that T0901317 did not prevent copper accumulation. We then tested directly whether T0901317 can bind Cu(I) and observed no binding (Supporting Fig. 4), further arguing against a possibility that the LXR agonist acts as a copper chelator.
T0901317 Normalizes Lipid Metabolism in Atp7b−/− Mice by Up-Regulating Expression of a Subset of the LXR Target Genes
LXR is a transcription factor for multiple genes that regulate cholesterol synthesis, transport, and metabolism. In untreated Atp7b−/− mice, in addition to Fasn and 3-hydroxyl-3-methylglutaryl-CoA synthase 1 (Hmgcs1), expression of Nr1h4 and cytochrome P450 family 7 subfamily A polypeptide 1 (Cyp7A1) was significantly lower compared to untreated controls, whereas transcripts for Abc1 and Srebp1 were not significantly different. Treatment with T0901317 significantly increased Fasn expression in both Atp7b+/− mice and Atp7b−/− mice. Srebp1 mRNA was increased in Atp7b+/− mice after treatment, but there was no change in Atp7b−/− mice. In both KO and HET mice, treatment did not change mRNA levels for Nr1h2, Nr1h3, Nr1h4, Abc1, Hmgcs1, or Cyp7a1 (Supporting Fig. 5).
The mixed mRNA response to T0901317 indicated that only a subset of predicted targets of the LXR/RXR pathway was stimulated by the drug. RXR dimerizes with other NRs (Fig. 1A), so the observed partial correction of transcriptional changes could be the result of contributions from other RXR-dependent pathways. Treatment with T0901317 did not change the levels of Lxrα, Lxrβ, or Rxr in Atp7b+/− mice or Atp7b−/− mice (Supporting Fig 1B). Given this partial response, we directly tested whether treatment with T0901317 reversed changes in lipid metabolism. Livers of both drug-treated HET and KO mice showed a statistically significant increase in TGs, compared to respective untreated controls (Fig. 3B), which was expected for treatment with T0901317.18 Plasma lipid analysis revealed that total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels were all significantly increased in sera of drug-treated Atp7b−/− mice compared to untreated Atp7b−/− mice (P < 0.001, P < 0.001, and P < 0.005, respectively). Importantly, although total cholesterol, LDL, and HDL more than doubled in treated Atp7b−/− mice compared to untreated mice, these resulting levels were similar to treated and untreated heterozygote mice (Fig. 3C). Collectively, the increases in hepatic TGs and serum lipids in treated mice are consistent with activation of LXR/RXR signaling.
Treatment With T0901317 Decreases Inflammation and Liver Fibrosis in Atp7b−/− Liver
Chronic inflammation underlies the development of liver fibrosis and, ultimately, cirrhosis. Inactivation of LXR/RXR is expected to relieve inhibition of expression of genes associated with the nuclear factor kappa B inflammatory cascade,19 whereas treatment with LXR/RXR agonist would facilitate inhibition. Indeed, untreated Atp7b−/− mice had a 10-fold increase in tumor necrosis factor alpha (TNFα), a 2-fold increase in IL-1β, and a 25-fold increase in inducible nitric oxide synthase (iNOs) expression when compared to controls. Treatment with T0901317 significantly reduced expression of these genes in Atp7b−/− mice (Fig. 4A). There was no effect of drug treatment in HET animals.
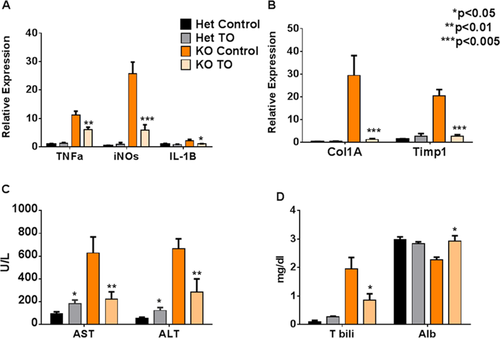
Inflammatory cytokines TNFα and IL-1β activate hepatic stellate cells (HSCs)20 to produce collagen 1a, leading to fibrosis. Atp7b−/− mice have a more than 25-fold increase of Col1a mRNA compared to control mice. This increase in expression of collagen was completely abolished by treatment with T0901317 (Fig. 4B). Similarly, Timp-1, a marker of inflammatory/fibrotic liver disease,20 was 20-fold higher in untreated Atp7b−/− mice and similar to control after treatment with the drug (Fig. 4B).
LXR Agonist Improves Liver Function
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are enzymes elevated in sera during liver inflammation and injury. In Atp7b−/− mice, AST levels were 626 ± 142 U/L and ALT was 666 ± 85 U/L. Treatment with T0901317 significantly reduced liver transaminases compared to untreated Atp7b−/− mice (P < 0.05). Mean AST was reduced to 222 ± 64 U/L and ALT to 285 ± 115 U/L. Liver enzymes (particularly AST) in drug-treated KO mice were similar to drug-treated heterozygote mice (Fig. 4C). Compared to nontreated Het mice, AST and ALT were higher in drug-treated Het mice, suggesting that prolonged administration of T0901317 may result in hepatotoxicity (Fig. 4C). Liver function was further assessed by serum bilirubin and albumin levels. In treated Atp7b−/− mice, total bilirubin was significantly lower than in the untreated Atb7b−/− mice (0.85 ± 0.22 vs. 1.96 ± 0.39 mg/dL; P < 0.05). Similarly, treatment improved albumin levels to 2.93 ± 0.19 vs. 2.27 ± 0.09 mg/dL in untreated, KO mice (P < 0.05; Fig. 4D).
Liver histology was examined by light microscopy and confirmed significant improvements in drug-treated KO mice. Untreated KO mice livers have ballooning hepatocytes, intracytoplasmic vacuoles, karyomegaly, multiple nuclei, and inflammatory cell infiltrates. These pathological features disappeared in 5 of 7 treated KO mice (Fig. 5A,B). Although aminotransferases were increased in Het mice compared to control, treatment had no effect on liver histology in 7 of 8 heterozygote mice (Fig. 5B and Supporting Fig. 6).
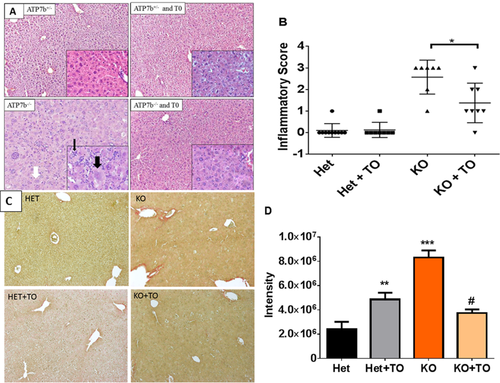
Morphometric analysis of Sirius red-stained liver sections revealed a more than 50% (P < 0.001) decrease in liver fibrosis in treated KO mice, compared to control KO mice. There was a small, yet significant, increase in Sirius red staining in treated Het mice when compared to control Het mice. Untreated KO mice had a 4-fold increase in liver fibrosis compared to untreated Het mice (Fig. 5C,D).
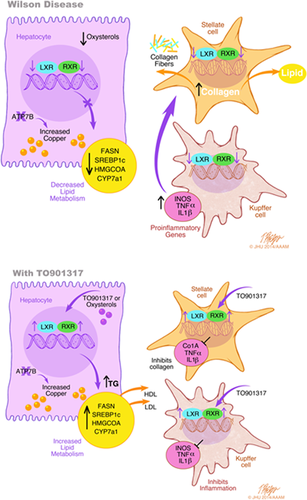
Altogether, our findings are summarized in Fig. 6. The results suggest that copper accumulation caused by inactivation of ATP7b is associated with inhibition of NR signaling, especially, but not exclusively, the LXR/RXR heterodimer. We propose that the combined effects of reduced LXR/RXR activity in hepatocytes, stellate cells, and Kupffer cells all contribute to the pathogenesis of WD. Activation of this pathway with an endogenous agonist improved liver inflammation, histology, and liver function, but did not alter hepatic copper.
Discussion
We demonstrate that the genetic program governing lipid metabolism and cholesterol biosynthesis is down-regulated in patients with WD and in ATP7b KO mice. The change in transcriptional profiles is associated with decreased levels of cholesterol and TGs in serum and increased liver inflammation. In silico analysis linking gene expression to signaling pathways and metabolic processes pointed to inhibition of NRs in both mice and humans as a primary cause of the observed changes. Consistent with this prediction, stimulation of NR activity using the LXR agonist, T0901317, abrogated the major negative consequences of copper overload and improved liver morphology and function. Significantly, in the presence of the drug, liver function was maintained (or had less decompensation) for a prolonged period of time despite elevated hepatic copper. We propose that in WD, accumulating copper inhibits NR signaling, which is a critical mediator of liver injury in WD. This represents a novel mechanism in the pathogenesis of WD.
Copper chelation is an established and by and large successful approach to decreasing copper toxicity in WD. Lifelong treatment using copper chelators improves liver function, but also has a wide range of side effects. Reaching a delicate copper balance in different tissues is difficult, and prolonged chelation frequently produces neurological decompensation and has other unintended consequences. Targeting pathways downstream of copper offers an alternative strategy for improving liver function in WD, especially under circumstances when copper chelators are ineffective or poorly tolerated. At the current stage, our data represent a proof of concept that stimulating NR function can greatly diminish pathological changes in WD liver. In this study, we initiated treatment with the drug when copper was high, lipid profile was altered, and AST/ALT were elevated, but the structural morphology of liver was still preserved and we obtained very promising results. Whether similar treatment will be equally effective if started at a more advanced stage of the disease needs to be determined. It is likely that treatment combining mild copper chelation and activation of NR signaling could be most effective.
Inflammation, lipid dishomeostasis eventually leading to steatosis, and fibrosis are commonly observed in WD. Many of the genes regulating these processes are under the control of NRs, including the LXR/RXR heterodimer. LXRα and LXRβ levels were unchanged in KO mice, whereas RXR levels were lower in KO mice (see also Muchenditsi et al.). A lower abundance of RXR is likely to decrease transcriptional activity of the LXR/RXR heterodimer, although this remains to be formally demonstrated. Interestingly, IL-1ß expression transiently stimulates SAPK/JNK, resulting in RXR export from the nucleus, followed by proteosomal degradation.21 IL-1ß is elevated in WD mice, suggesting a possible explanation for diminished protein levels of RXR. Another factor potentially contributing to lower abandance/activity of RXR are levels of endogenous ligands. Oxysterols regulate activity of the LXR/RXR dimer, and the oxysterol profile differed significantly between control and knockout animals. In particular, 7α,β-OHC and 5,6β-EC levels were significantly lower in KO compared to healthy controls, whereas 4β-OHC was increased in ATP7b-deficient mice. Treatment with the drug did not reverse changes in oxysterol levels (not shown), indicating that that the cause for oxysterol misbalance was upstream (or produced independently) of LXR/RXR signaling. Such causes may involve copper dependent, nonenzymatic oxidation, and/or inhibition of activity of enzymes involved in oxysterol metabolism.
The LXR agonist used in this study had an uneven effect on gene expression. Genes commonly associated with liver fibrosis (Col1a and Timp1) and liver inflammation (Tnfα, IL-1β, and iNOS) were dramatically down-regulated by treatment in KO animals, suggesting primary control by LXR. At the same time, only a subset of genes involved in lipid metabolism responded to treatment with the drug. This result indicates that the decrease in RXR may have a negative effect on the function of several receptors involved in lipid metabolism with which RXR dimerizes, such as FXR, peroxisome proliferator-activated receptor, and retinoic acid receptor. Despite partial recovery of mRNA levels, serum lipids in treated mice increased to levels similar to those found in control mice. In addition, hepatic TG content was increased in treated mice, strongly suggesting the recovery of LXR signaling in treated Atb7b−/− mice.
Our findings of reduced Col1A and TIMP-1 in treated Atb7b−/− mice corroborate previous studies that indicate that LXR activation in HSCs is a critical determinant of liver inflammation and fibrosis (Fig. 6). LXRα/ß−/− mice have an exaggerated inflammatory and fibrotic response to classic experimental hepatic insults. Furthermore, bone marrow transplants between wild-type and LXRα/ß−/− mice had no effect on severity of fibrosis, suggesting that LXR signaling in HSCs and/or hepatocytes is critical for development of hepatic fibrosis.28 Future studies are needed to test the effects of ATP7b deletion, copper overload, and NR function in specific cell types in the liver.
Treatment of hepatoblastoma cells (HepG2) with copper induces apoptosis that is mediated by an increase in acid sphingomeylinase (ASMase) activity and production of ceramide.29 ASMase activity is increased by TNFα and ASMase deficiency renders cultured hepatocytes resistant to TNFα-mediated apoptosis.30 We observed high levels of TNFα mRNA in liver extracts from Atp7b KO mice and the LXR agonist dramatically reduced TNFα. Analysis of sphingolipids in 1 KO animal and age-matched control showed increased levels of ceramide, although a separate study is needed to determine significance of this pathway in mice and humans. Elevated levels of serum TNFα31 and IL-1β32 are found in patients with WD (as in our mouse model).
The beneficial effects of T0901317 in WD mice are tempered by the increase in liver enzymes and fibrosis in treated, control mice. Review of the histology indicates that increased hepatic steatosis is the most likely cause of liver injury. In our study, we treated the mice for 2 months, and although we found increased levels of AST and ALT, there was no significant increase in bilirubin or decrease in albumin. Moreover, there were no increases or changes in inflammatory genes (TNFα, Il-1β, and NOS). Newer-generation LXR agonists in development do not cause hepatic lipogenesis.35, 36 In addition, there are several concomitant treatments that can suppress T0901317-induced steatosis, such as resveratrol,37 ursodeoxycholic acid,38 and n-3 fatty acids.39 Thus, future modifications or adjuvant therapies may diminish the observed toxic effects.
In conclusion, treatment with an LXR agonist improved liver histology, liver enzymes, and liver function in a mouse model of WD. The improvements were associated with a decrease in genetic markers of liver fibrosis and inflammatory cytokines. There was no change in hepatic copper. These findings suggest that alterations in NR-mediated lipid metabolism and inflammation may contribute to the pathogenesis of liver disease in WD, and that alternative therapies targeting these receptors are possible.
REFERENCES
Author names in bold designate shared co-first authorship.




