The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease
Potential conflict of interest: Nothing to report.
Supported by grants from PRIN 2010–2011 (Prot. N. 2010C4JJWB).
Abstract
In nonalcoholic fatty liver disease, the influence of severity of steatosis on liver stiffness measurement (LSM) is poorly studied and still debated. We assessed the impact of steatosis severity and its ultrasonographic (US) sign, severe bright liver echo pattern, on LSM values and on transient elastography accuracy for the diagnosis of liver fibrosis in a cohort of consecutive patients with nonalcoholic fatty liver disease. Patients (n = 253) were assessed by clinical, US, and histological (Kleiner score) features. Transient elastography was performed using the M probe. Among patients with low amounts of fibrosis (F0-F1 and F0-F2), median LSM values, expressed in kilopascals, were significantly higher in subjects with severe steatosis (≥66% at liver biopsy) compared to those without (F0-F1 6.9 versus 5.8, P = 0.04; F0-F2 7.4 versus 6.0, P = 0.001) as well as in patients with severe bright liver echo pattern on US compared to their counterparts (F0-F1 7.3 versus 5.6, P = 0.001; F0-F2 7.6 versus 6.0, P < 0.001). In subjects without significant fibrosis (F0-F1) and without severe fibrosis (F0-F2), a higher rate of false-positive LSM results was observed in patients with steatosis ≥66% compared to those without (F0-F1 23.6% versus 14.9%, F0-F2 33.3% versus 13.2%) and in patients with severe bright liver echo pattern on US (F0-F1 22.2% versus 15.4%, F0-F2 28.8% versus 15.6%) compared to their counterparts. Conclusions: In patients with nonalcoholic fatty liver disease, the presence of severe steatosis, detected by histology or by US, should always be taken into account in order to avoid overestimations of liver fibrosis assessed by transient elastography. (Hepatology 2015;62:1101-1110)
Abbreviations
-
- BLEP
-
- bright liver echo pattern
-
- CAP
-
- controlled attenuation parameter
-
- CHC
-
- chronic hepatitis C
-
- IQR/M
-
- interquartile range/median
-
- LSM
-
- liver stiffness measurement
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- TE
-
- transient elastography
-
- US
-
- ultrasonography
The outcome of patients with chronic liver diseases is finally decided by the severity of liver fibrosis, this finding being the strongest predictor of liver morbidity and mortality.1, 2
Biopsy, even if invasive, painful, and with potentially life-threatening complications, remains the gold standard for the evaluation of liver fibrosis, even if not applicable to the entire population at risk of liver damage.3, 4 In addition, other factors like inter- and/or intraobserver discrepancies,5 width of biopsy sample, sampling errors, and inconsistency in defining histological features due to the variety of the several available scoring systems6, 7 are able to affect its diagnostic performance.
In the past few years many noninvasive biochemical and instrumental tools have been used to reduce the number of liver biopsies, obtaining contrasting results.8 In this complex setting liver stiffness measurement (LSM) using transient elastography (TE) emerged as a rapid, accurate, and noninvasive test to estimate the stage of fibrosis in patients with chronic liver disease of different etiologies.9 However, factors like alanine aminotransferase flares and severe liver necroinflammation,10-12 recent food intake,13 hepatic congestion,14 steatosis,15 extrahepatic cholestasis,16 high body mass index,17, 18 and insulin resistance—perhaps through steatosis induction19—were found to affect LSM diagnostic performance, chronic hepatitis C (CHC) being the best-validated clinical setting.
Considering nonalcoholic fatty liver disease (NAFLD), a growing and spreading liver disease wordwide,20 some studies have investigated the diagnostic accuracy of LSM in this clinical setting.18, 21-26 Of note, these studies reported overall a good performance in staging fibrosis, also identifying insufficient length of the liver biopsy23 and obesity18 as the factors that mostly limited the diagnostic accuracy of LSM in this clinical setting, while reporting few and contrasting data on the potential effect of severity of steatosis.12-14
The aim of the present study was to assess the impact of severe liver steatosis, detected by histology and ultrasonography (US), on LSM values and on the accuracy of TE for fibrosis diagnosis in a cohort of consecutive biopsy-proven patients with NAFLD.
Materials and Methods
Patients
The study assessed consecutive patients with biopsy-proven NAFLD, recruited at the Gastrointestinal Liver Unit of the University Hospital in Palermo, Italy, and fulfilled all inclusion and exclusion criteria detailed below. The diagnosis of NAFLD was based on chronically elevated alanine aminotransferase for at least 6 months, alcohol consumption <20 g/day in the last year, and steatosis (≥5% of hepatocytes) at histology with or without necroinflammation and/or fibrosis. Exclusion criteria were as follows: (1) advanced cirrhosis (Child-Turcotte-Pugh B and C); (2) hepatocellular carcinoma; (3) other causes of liver disease or mixed etiologies (alcohol abuse, hepatitis C, hepatitis B, autoimmune liver disease, Wilson's disease, hemochromatosis, or α1-antitrypsin deficiency); (4) human immunodeficiency viral infection; (5) previous treatment with immunosuppressive drugs and/or regular use of steatosis-inducing drugs, evaluated by a questionnaire (for example, corticosteroid, valproic acid, tamoxifen, amiodarone); or (6) active intravenous drug addiction or use of cannabis.
The study was performed in accordance with the principles of the Declaration of Helsinki and its appendices and with local and national laws. Approval was obtained from the hospital's Internal Review Board and Ethics Committee, and written informed consent was obtained from all patients.
Clinical and Laboratory Assessment
Demographic, clinical, and anthropometric data were collected at the time of liver biopsy. The diagnosis of arterial hypertension was based on a systolic blood pressure ≥135 mm Hg and/or a diastolic blood pressure ≥85 mm Hg (measured three times within 30 minutes using a brachial sphygmomanometer) or on use of blood pressure–lowering agents. The diagnosis of type 2 diabetes was based on the revised criteria of the American Diabetes Association, using a fasting blood glucose value of ≥126 mg/dL on at least two occasions.27 A current therapy with insulin or oral hypoglycemic agents was documented in patients with a previous diagnosis of type 2 diabetes.
A 12-hour overnight fasting blood sample was drawn at the time of biopsy to determine serum levels of alanine aminotransferase, platelet count, total cholesterol, high-density lipoprotein cholesterol, triglycerides, blood glucose, and insulin. Insulin resistance was assessed with the homeostasis model assessment method using the following equation28: Insulin resistance = [Fasting insulin (μU/mL) × Fasting glucose (mmol/L)]/22.5.
Liver Stiffness Measurement
Transient elastography was performed with the FibroScan (Echosens, Paris, France) medical device, using the M probe (also named as the standard probe). On the same day of liver biopsy, LSM was assessed, before the procedure and after an overnight fast, by trained operators who had previously performed at least 300 determinations in patients with chronic liver disease. As recently reported in the literature,29 we classified all LSM examinations into three reliability categories: “very reliable” (interquartile range/median [IQR/M] ≤0.10), “reliable” (0.10 < IQR/M ≤ 0.30 or IQR/M > 0.30 with LSM median <7.1 kPa), and “poorly reliable” (IQR/M >0.30 with LSM median ≥7.1 kPa). “Poorly reliable” results were excluded from the analysis.
US Assessment
The US assessment was performed in the morning, on the same day of liver biopsy, by one operator trained for US techniques and particularly dedicated to liver examination, using a real-time Hitachi H21 apparatus with a 2-5 MHz, convex, multifrequency probe. Presence of hepatic steatosis was defined through detection of bright liver echo pattern (BLEP), that is, fine, packed, and high-amplitude echoes, with consequent brightness of liver, increase in liver-kidney contrast, and possible evidence of vascular blurring and deep attenuation signs.30 Mild steatosis was thus recognized by a slight increase in liver echogenicity, a slight exaggeration of liver and kidney echo discrepancy, and relative preservation of echoes from the walls of the portal vein. Moderate steatosis was accompanied by loss of echoes from the walls of the portal veins, particularly from the peripheral branches, resulting in a featureless appearance of the liver. In addition, greater posterior beam attenuation was found as well as a greater discrepancy between hepatic and renal echoes. Severe steatosis was recognized by a greater reduction in beam penetration, loss of echoes from most of the portal vein wall including the main branches, and a large discrepancy between hepatic and renal echoes.31
Histological Assessment
Histological slides were coded and read by one expert pathologist, who was unaware of the patient's identity and history. A minimum length of 15 mm of the biopsy specimen or the presence of at least 10 complete portal tracts was required.32 Steatosis was assessed as the percentage of hepatocytes containing fat droplets (minimum 5%) and evaluated as a categorical variable. The Kleiner classification33 was used to compute steatosis and lobular inflammation and to stage fibrosis from 0 to 4.
Statistics
The results are reported as frequencies and percentage for categorical variables, mean ± standard deviation for normally distributed continuous variables, and median and IQR for nonnormally distributed continuous variables. The Student t test, the Mann-Whitney, and the χ2 test were used to compare continuous or categorical variables. Multivariate analysis, including all of the significant baseline variables (P < 0.05), was also performed using linear regression to identify independent variables associated with LSM as continuous variables.
Receiver operating characteristic curves were applied to find the best cutoff values and to identify the area under the receiver operating characteristic curve of LSM that is able to discriminate the different stages of fibrosis. Furthermore, descriptions of the operating characteristics (sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio) of US for the detection of steatosis >66%, with the assumption that the gold standard for the diagnosis of steatosis was histologic examination. All analyses were performed using SPSS v. 20.0 statistical package for MacIntosh (SPSS, Inc., Chicago, IL).
Results
Patients
From January 2008 to October 2013, we included 306 consecutive patients with NAFLD who underwent TE, US, and liver biopsy. Fifty-three (17%) failed to obtain 10 valid LSM acquisitions due to obesity or to unreliable results according to the manufacturer's recommendations (see above). Hence, 253 patients with valid LSM acquisitions could be included in the analysis. Table 1 shows the comparison between NAFLD patients with reliable and those with unreliable LSM. As expected, the latter were more obese compared to their counterparts, even if the overall severity of the liver damage was not different between the two groups. About the baseline features of the 253 included patients, the mean age was 45.2 years, with a prevalence of males (70%). The greater proportion of patients was in the overweight or obese range, one-quarter was hypertensive, and diabetes was present in about 20% of subjects. Mean values for total, high-density lipoprotein cholesterol, and triglycerides were within the normal range, whereas mean homeostasis model assessment values were elevated (4.0 ± 3.3).
| Variable | NAFLD With Reliable LSM (n = 253) | NAFLD With Unreliable LSM (n = 53) | P |
|---|---|---|---|
| Age (years) (mean ± SD) | 45.2 ± 13.0 | 49.2 ± 12.8 | 0.04 |
| Male gender | 177 (70%) | 20 (37.7%) | <0.001 |
| Body weight (kg) | 82.0 (70.9 – 89.4) | 93.3 (80.0 – 107.0) | 0.004 |
| Height (cm) | 168.0 (159 – 174) | 162 (153 – 174) | 0.05 |
| Body mass index (kg/m2) (mean ± SD) | 29.1 ± 4.2 | 34.7 ± 6.0 | <0.001 |
| Body mass index (kg/m2) | |||
| <25 | 37 (14.6%) | 4 (7.5%) | <0.001 |
| 25-29.9 | 119 (47%) | 8 (15.1%) | |
| ≥30 | 97 (38.3%) | 41 (77.4%) | |
| Arterial hypertension | 60 (23.7%) | 25 (47.2%) | 0.001 |
| Type 2 diabetes | 50 (19.8%) | 20 (37.7%) | 0.005 |
| Alanine aminotransferase (IU/L) (median, IQR) | 67.0 (47.0 - 97.0) | 66.0 (40.0 - 90.0) | 0.3 |
| Platelet count (×103/mmc) (mean ± SD) | 227.6 ± 64.6 | 234.0 ± 81.1 | 0.5 |
| Cholesterol (mg/dL) (mean ± SD) | 203.9 ± 47.7 | 200.8 ± 50.3 | 0.6 |
| HDL cholesterol (mg/dL) (median, IQR) | 48.0 (41.0 - 57.0) | 52.0 (41.0 - 59.0) | 0.4 |
| Triglycerides (mg/dL) (median, IQR) | 132.5 (84.0 - 177.5) | 118.0 (92.0 - 154.0) | 0.02 |
| GGT (IU) (median, IQR) | 61.0 (33.0 - 126.0) | 63.0 (31.0 - 106.0) | 0.6 |
| Blood glucose (mg/dL) (median, IQR) | 92.0 (83.0 - 106.0) | 96.0 (86.0 - 113.0) | 0.3 |
| Insulin (μU/mL) (median, IQR) | 13.8 (8.8 - 18.8) | 18.4 (12.7 - 24.8) | 0.001 |
| HOMA (median, IQR) | 3.0 (2.0 - 4.8) | 4.1 (3.0 - 6.3) | 0.001 |
| Liver stiffness (kPa) (median, IQR) | 6.8 (5.4 - 10.0) | NA | NA |
| Stiffness IQR (%) | 12.0 (9.0-14.0) | NA | NA |
| Biopsy specimen length (mm) | 16.4 (15.5-18.5) | 16.9 (15.8-19.1) | 0.4 |
| Histology at biopsy | |||
| Steatosis grade | |||
| 1 (5%-33%) | 99 (39.1%) | 18 (34.0%) | |
| 2 (>33%-66%) | 82 (32.4%) | 11 (20.8%) | 0.04 |
| 3 (>66%) | 72 (28.5%) | 24 (45.3%) | |
| Lobular inflammation | |||
| 0 | 15 (5.9%) | 4 (7.5%) | |
| 1 | 133 (52.6%) | 29 (54.7%) | |
| 2 | 93 (36.8%) | 16 (30.2%) | 0.7 |
| 3 | 12 (4.7%) | 4 (7.5%) | |
| Hepatocellular ballooning | |||
| 0 | 36 (14.2%) | 4 (7.5%) | |
| 1 | 115 (45.5%) | 23 (43.4%) | 0.3 |
| 2 | 102 (40.3%) | 26 (49.1%) | |
| Stage of fibrosis | |||
| 0 | 63 (24.9%) | 10 (18.9%) | |
| 1 | 77 (30.4%) | 15 (28.3%) | |
| 2 | 60 (23.7%) | 11 (20.8%) | 0.4 |
| 3 | 36 (14.2%) | 13 (24.5%) | |
| 4 | 17 (6.7%) | 4 (7.5%) |
- Data are given as mean ± standard deviation or as median and interquartile range, or as number of cases (%).
- Abbreviations: GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; IU, international unit; kPa, kilopascal; SD, standard deviation.
At liver biopsy, approximately one patient in two had fibrosis ≥2 by Kleiner score, whereas severe liver fibrosis (≥3) prevalence was 20%. About 28% of the cases had steatosis of severe grade (≥66%).
Performance of US on the Assessment of Severe Steatosis
All patients had BLEP at US examination, while a severe BLEP was observed in 65 patients (25.7%). Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of US for the detection of steatosis ≥66% were 48.6%, 83.1%, 53.8%, 80%, 2.88, and 0.62, respectively.
Factors Associated With LSM and Accuracy of TE for Fibrosis Assessment
Median LSM was 6.8 (IQR 5.4-10.0). Liver stiffness values significantly increased according to fibrosis stage: in F0 5.7 (IQR 4.8-7.4), in F1 6.1 (IQR 5.2-7.7), in F2 7.2 (IQR 6.0-10.0), in F3 10.7 (IQR 8.3-13.3), and in F4 18.4 (IQR 12.1-25.0, P < 0.001).
Table 2 shows the univariate and multivariate analyses of parameters associated with LSM as continuous variables. Notably, steatosis grade was an independent predictor of higher LSM (P = 0.03) even after correction for clinicometabolic and histological variables, as well as for quality criteria such as biopsy length and IQR/M value of LSM.
| Variable | Univariate Analysis P | Multivariate Analysis β Standard Error P | ||
|---|---|---|---|---|
| Age (years) | <0.001 | 0.087 | 0.027 | 0.001 |
| Female gender | 0.048 | 0.073 | 0.735 | 0.92 |
| Platelet count | 0.002 | −0.008 | 0.005 | 0.13 |
| Biopsy length (mm) | 0.7 | 0.086 | 0.101 | 0.4 |
| Liver stiffness IQR/M | 0.3 | −0.087 | 0.058 | 0.1 |
| Histology at biopsy | ||||
| Kleiner steatosis grade | 0.045 | 1.469 | 0.670 | 0.03 |
| Kleiner inflammation grade | <0.001 | 2.289 | 0.469 | <0.001 |
- Data are given as mean ± standard deviation or as number of cases.
Receiver operating characteristic curves identified the best cutoffs of LSM that are able to maximize the accuracy of TE at 6.9 kPa for the diagnosis of significant fibrosis (area under the curve = 0.784, sensitivity 70.8%, specificity 68.6%) and at 8.4 kPa for severe fibrosis (area under the curve = 0.866, sensitivity 77.4%, specificity 76%). At the LSM cutoff of 6.9 kPa for significant fibrosis, the false-positive rate was 31.4% (44/140) and the false-negative rate was 29.2% (33/113). Concerning the performance of LSM >8.4 kPa for severe fibrosis, the false-positive rate was 24% (48/200) and the false-negative rate was 22.6% (12/53). The accuracy of LSM in diagnosing significant and severe fibrosis in the entire NAFLD cohort is summarized (Fig. 1).
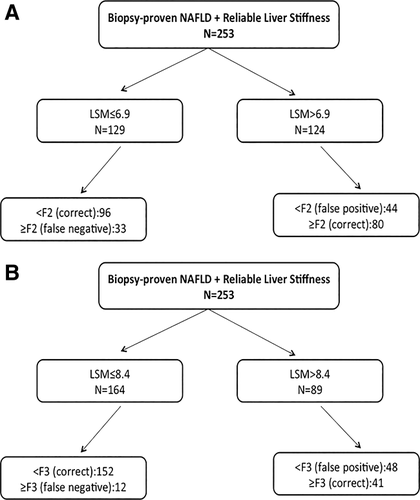
Accuracy of TE for the prediction of significant (A) and severe (B) fibrosis in patients with NAFLD and reliable TE.
Impact of Severe Steatosis Detected by Histology or by BLEP on LSM Values and on Accuracy of TE
Due to the independent association of liver stiffness with steatosis, we assessed the variations of LSM values and of TE accuracy within the same stages of liver fibrosis and according to the presence or absence of severe steatosis (≥66%) on histological examination or on severe BLEP detection on US, a widely used, if not optimal, surrogate of steatosis.
Supporting Figs. 1 and 2 show that when patients were considered at each fibrosis stage, for lower grades of fibrosis only, LSM values were significantly higher in patients with severe steatosis by histology or by US compared to those without. Along this line, similar results were observed when discriminating patients according to the presence or absence of significant (F2-F4) or severe (F3-F4) fibrosis. Specifically, Fig. 2 shows, in subgroups of patients with and without severe steatosis, median LSM values among patients with F0-F1 fibrosis stages (6.9 versus 5.8, P = 0.04; Fig. 2A) and F2-F4 fibrosis stages (9.0 versus 8.8, P = 0.70; Fig. 2B) and among patients with F0-F2 fibrosis stages (7.4 versus 6.0, P = 0.001; Fig. 2c) and F3-F4 fibrosis stages (11.9 versus 12.0, P = 0.70; Fig. 2d). As expected, similar results were obtained when the entire cohort was split according to the presence or absence of severe BLEP on US (Fig. 3).
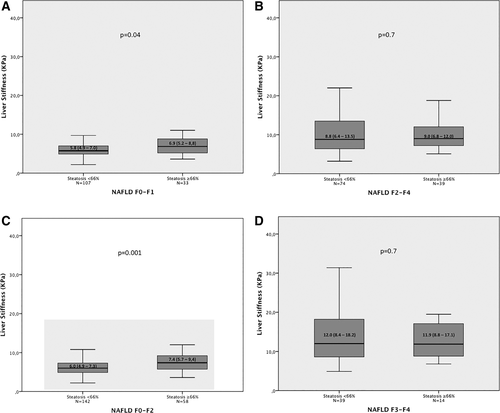
Liver stiffness value distribution among patients with F0-F1 fibrosis stages (A) and F2-F4 fibrosis stages (B) and patients with F0-F2 fibrosis stages (C) and F3-F4 fibrosis stages (D), according to the presence or absence of severe steatosis. The horizontal bar inside the box represents the median value.
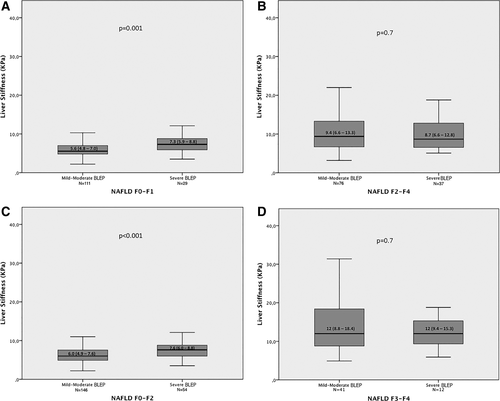
Liver stiffness value distribution among patients with F0-F1 fibrosis stages (A) and F2-F4 fibrosis stages (B) and patients with F0-F2 fibrosis stages (C) and F3-F4 fibrosis stages (D), according to the presence or absence of severe BLEP on US. The horizontal bar inside the box represents the median value.
To further strengthen the independent effect of steatosis on LSM, Table 3 depicts the LSM value distribution among patients with F0-F1 fibrosis stages and F0-F2 fibrosis stages and the same inflammation grade and/or hepatocyte ballooning presence, according to the presence or absence of severe steatosis and of severe BLEP. Overall, the presence of severe steatosis and its US detection remained significantly associated with higher LSM values in almost all these subgroups.
| Liver Stiffness (Median + IQI) | P | Liver Stiffness (Median + IQI) | P | |||
|---|---|---|---|---|---|---|
| Steatosis <66% | Steatosis ≥66% | Mild-Moderate BLEP | Severe BLEP | |||
| F0-F1 | ||||||
| No hepatocyte ballooning | 5.6 (4.9-6.4) n = 30 | 4.8 (4.0-8.8) n = 2 | 0.7 | 5.5 (4.7-6.3) n = 31 | 7.9 (6.9-8.8) n = 2 | 0.04 |
| Hepatocyte ballooning | 5.9 (4.9-7.3) n = 77 | 7.1 (5.3-8.8) n = 31 | 0.05 | 5.7 (4.9-7.2) n = 80 | 7.3 (5.9-8.8) n = 27 | 0.009 |
| Grade 0-1 lobular inflammation | 5.4 (4.8-6.8) n = 86 | 7.2 (5.2-8.9) n = 24 | 0.01 | 5.4 (4.8-6.8) n = 84 | 7.1 (5.9-8.6) n = 26 | 0.003 |
| Grade 2-3 lobular inflammation | 6.2 (5.7-7.7) n = 21 | 5.7 (5.3-7.7) n = 9 | 0.5 | 6.0 (5.3-7.1) n = 27 | 8.8 (8.3-12.1) n = 3 | 0.01 |
| Grade 2-3 lobular inflammation + hepatocyte ballooning | 6.2 (5.5-8.7) n = 16 | 5.7 (5.3-7.7) n = 9 | 0.4 | 5.9 (5.3-7.2) n = 22 | 8.8 (8.3-12.1) n = 3 | 0.02 |
| Grade 0-1 lobular inflammation + no hepatocyte ballooning | 5.4 (4.6-6.2) n = 25 | 4.8 (4.0-8.8) n = 2 | 0.8 | 5.0 (4.4-6.1) n = 25 | 7.9 (6.9-8.8) n = 2 | 0.03 |
| F0-F2 | ||||||
| No hepatocyte ballooning | 5.6 (4.9-6.6) n = 33 | 6.8 (4.4-10.4) n = 3 | 0.7 | 5.5 (4.8-6.3) n = 36 | 8.8 (6.9-12.0) n = 3 | 0.01 |
| Hepatocyte ballooning | 6.1 (5.1-7.6) n = 109 | 7.4 (5.8-9.4) n = 55 | 0.003 | 6.1 (5.1-7.8) n = 111 | 7.4 (6.0-8.8) n = 50 | 0.003 |
| Grade 0-1 lobular inflammation | 5.6 (4.8-7.0) n = 99 | 6.6 (5.6-8.9) n = 38 | 0.005 | 5.6 (4.8-7.0) n = 100 | 7.1 (5.8-8.8) n = 38 | 0.002 |
| Grade 2-3 lobular inflammation | 6.4 (5.6-7.8) n = 43 | 8.4 (5.9-10) n = 20 | 0.04 | 6.2 (5.4-7.8) n = 47 | 8.5 (6.7-15.3) n = 15 | 0.002 |
| Grade 2-3 lobular inflammation + hepatocyte ballooning | 6.4 (5.6-8.1) n = 37 | 8.4 (5.9-10) n = 20 | 0.08 | 6.2 (5.3-8.8) n = 42 | 8.5 (6.7-15.3) n = 15 | 0.004 |
| Grade 0-1 lobular inflammation + no hepatocyte ballooning | 5.5 (4.7-6.3) n = 30 | 6.8 (4.4-10.4) n = 3 | 0.7 | 5.3 (4.4-6.1) n = 30 | 8.8 (6.9-12) n = 3 | 0.01 |
When considering the impact of steatosis on TE accuracy, a higher rate of false-positive LSM results for the diagnosis of significant fibrosis (F2-F4) was found among patients with steatosis ≥66% (17/72, 23.6%) compared to those without (27/181, 14.9%) (Fig. 4A) and in patients with detection of severe BLEP (16/72, 22.2%) compared to those without (28/181, 15.4%) (Fig. 4B). Similar differences in false-positive LSM results for the diagnosis of severe (F3-F4) fibrosis were observed (Fig. 4C,D). When comparing the false-positive rates among patients with F0-F1 or F0-F2 fibrosis, discriminated according to the presence or absence of steatosis ≥66%/severe BLEP, we obtained again higher false-positive rates in patients with severe steatosis/BLEP (Supporting Fig. 3).
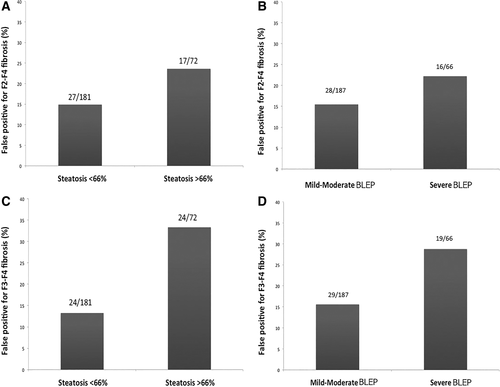
Distribution of false-positive LSM results for the diagnosis of F2-F4 fibrosis according to the presence or absence of severe steatosis (A) and to detection of severe BLEP on US (B) and of false-positive LSM results for the diagnosis of F3-F4 fibrosis according to the presence or absence of severe steatosis (C) and to detection of severe BLEP on US (D).
Conversely, the impact of severe steatosis and of severe BLEP on false-negative LSM results was negligible (data not shown).
Table 4 summarizes the best cutoffs of LSM and their operating characteristics at different grades of histological or US steatosis.
| NAFLD (n = 253) | Best LSM cutoff (kPa) | AUROC | Sensitivity | Specificity | FPR | FNR |
|---|---|---|---|---|---|---|
| Significant Fibrosis (F2-F4) | ||||||
| Steatosis <66% (n = 181) | 6.8 | 0.788 | 70.3% | 73.8% | 28/181 (15.5%) | 22/181 (12.1%) |
| Steatosis ≥66% (n = 72) | 7.9 | 0.746 | 71.8% | 66.7% | 11/72 (15.3%) | 11/72 (15.3%) |
| Mild-moderate BLEP (n = 187) | 6.7 | 0.813 | 74.7% | 70.9% | 32/187 (17.1%) | 19/187 (10.2%) |
| Severe BLEP (n = 66) | 7.9 | 0.679 | 63.9% | 65.5% | 10/66 (15.1%) | 13/66 (19.7%) |
| Severe Fibrosis (F3-F4) | ||||||
| Steatosis <66% (n = 181) | 7.8 | 0.884 | 84.6% | 81.7% | 26/181 (14.4%) | 6/181 (3.3%) |
| Steatosis ≥66% (n = 72) | 10.0 | 0.825 | 71.4% | 81.0% | 11/72 (15.3%) | 4/72 (5.5%) |
| Mild-moderate BLEP (n = 187) | 8.0 | 0.889 | 80.5% | 79.2% | 30/187 (16.0%) | 8/187 (4.3%) |
| Severe BLEP (n = 66) | 10.0 | 0.798 | 75.0% | 79.2% | 11/66 (16.7%) | 3/66 (4.5%) |
- Abbreviations: AUROC, area under the receiver operating characteristic curve; FPR, false-positive rate; FNR, false-negative rate.
Discussion
In our cohort of 253 consecutive biopsy-proven patients with NAFLD, we found that severe steatosis, diagnosed by histology or by US—detection of severe BLEP—is independently linked to increased LSM values. As a consequence, we reported higher rates of false-positive LSM results for the noninvasive assessment of both significant and severe fibrosis by TE in patients with severe BLEP on US examination compared to their counterparts.
Transient elastography is an easy-to-perform and reliable tool to diagnose liver fibrosis and cirrhosis, especially in patients with CHC,9 even if few studies have also suggested it as a useful noninvasive instrument in NAFLD patients.18, 21-26 In our cohort of patients with chronic liver disease due to fatty liver accumulation, the accuracy of TE for the assessment of liver fibrosis was good at diagnosing significant and severe fibrosis, as expressed by their areas under the receiver operating characteristic curve. These findings agree with previously reported published data.18, 21-26 Besides, we identified LSM values of 6.9 kPa and 8.4 kPa as the best cutoffs for discriminating significant (F2-F4) and severe (F3-F4) fibrosis, respectively. Once again, these LSM cutoff values were comparable with those identified in other studies.18, 21-26 Some variability may be related to the different prevalence of fibrosis and of several factors affecting liver stiffness independently of the amount of liver fibrosis in the various examined populations.
An interesting issue arising from our data is that the presence of severe liver steatosis is not only independently associated with higher LSM values, especially in patients with low grades of fibrosis but also able to interfere with the overall diagnostic performance of TE in patients with NAFLD, leading to potential overestimations of liver fibrosis. In this line, literature data have reported a significant reduction of TE reproducibility in patients with chronic liver diseases due to different etiologies and with steatosis.34 Furthermore, higher LSM values in the presence of liver steatosis have been reported in patients with CHC,15, 35-37 while in NAFLD these data were not confimed23 or an inverse relation was highlighted.25 In any case, the presence of fat droplets in the hepatocytes influences the architectural structure of the liver, thus potentially changing the propagation time of the vibratory wave through the liver, which is the key principle of TE.
We also reported that the rates of false-positive LSM results for the identification of both significant and severe fibrosis were strongly influenced by the presence of severe steatosis. These data are new concerning the issue of LSM in NAFLD patients, and they absolutely agree with what we recently reported in the clinical setting of CHC.15
However, all data we reported raised the paradoxical problem that the diagnostic accuracy of a noninvasive technique, TE, is affected by a variable, severe steatosis, arising from an invasive instrument, liver biopsy. To overcome this problem, we evaluated the impact of a noninvasive assessment of severe hepatic steatosis, US detection of severe BLEP, on TE performance. Along this line, another relevant finding arising from our analysis is the association of severe BLEP detection with both higher LSM values and false-positive results. Of note, when patients were grouped according to the presence or absence of severe BLEP on US, we observed that the variations of LSM values and of TE accuracy were comparable with those observed when our cohort was divided according to the presence or absence of severe steatosis. Considering that the accuracy of US for severe steatosis detection is not good, especially because of poor positive predictive values for moderate to severe degrees of steatosis, as our group recently reported38 and as shown in our NAFLD patients, we could speculate that BLEP may be the US expression not only of histological steatosis but also of other factors able to increase LSM independently from the amount of fibrosis.
From a clinical point of view, our study suggests careful interpretation of TE in NAFLD patients with severe steatosis, where LSM might overestimate liver fibrosis unless different thresholds are used, thus improving diagnostic accuracy. Anyway, it should be acknowledged that the accuracy of US for the detection of severe steatosis in this clinical setting is a limitation when using US paired with LSM because we risk losing a considerable number of patients with severe steatosis and thus with potential overestimation of fibrosis assessed by LSM. Computed tomography and magnetic resonance imaging are able to grade liver fatty infiltration39, 40 and may be useful in this setting, but their high costs and, for computed tomography, the risk of radiation exposure limits their applicability in routine clinical practice. The controlled attenuation parameter (CAP) might be a good alternative,41 although it was not evaluated in this study; and future studies should be conducted with the aim of correcting the LSM by CAP values, both parameters recorded using two different types of software in the same machine. Thus, while waiting for a wide availability of techniques to accurately estimate fatty liver infiltration, US, despite the above-noted limits, could help in the identification of NAFLD patients at higher risk of overestimation of liver fibrosis using TE.
This study has some other limitations. First, inter- and intraobserver agreement of LSM and US examinations were not assessed. Second, a comparison between histological/US findings and CAP data was not performed, as mentioned; and the analysis of LSM was carried out using a conventional M probe only, while the XL probe was not used. However, CAP is not widely available, and the combined use of M and XL probes can raise concerns in the interpretation of data due to different LSM values generated by the two probes. Another methodological issue could reside in the accuracy of liver biopsy examination related to sampling errors and intraobserver and interobserver variability; in addition, the assessment of hepatic steatosis may be affected by the frequently uneven distribution of fat throughout the liver, and the quantification of hepatic steatosis as a percentage of steatotic hepatocytes may not represent a good marker of liver fat content because it does not take into account the number and size of lipid droplets in single hepatocytes. A further methodological question is the potentially limited external validity of the results for different populations and settings. Our study included a cohort of Italian NAFLD subjects, largely overweight or obese, who were enrolled in a tertiary referral center for liver disease, limiting the broad application of the results. Finally, lack of follow-up data may limit the power of our results.
In conclusion, although LSM is a useful and noninvasive tool for estimating the severity of fibrosis in NAFLD, the presence of severe steatosis per se or evaluated by US should always be taken into account in order to avoid overestimations of liver fibrosis.
References
Author names in bold designate shared co-first authorship.