Association between activity in the ventral premotor cortex and spinal cord activation during force generation—A combined cortico-spinal fMRI study
Robert Schulz and Christian Gerloff contributed equally to this study.
Abstract
Force generation is a crucial element of dexterity and a highly relevant skill of the human motor system. How cerebral and spinal components interact and how spinal activation is associated with the activity in the cerebral primary motor and premotor areas is poorly understood. Here, we conducted combined cortico-spinal functional magnetic resonance imaging during a simple visually guided isometric force generation task in 20 healthy young subjects. Activation was localized in the right cervical spinal cord and left primary motor and premotor areas. The main finding is that spinal activation was negatively correlated with ventral premotor cortex activation. Spinal activation was furthermore significantly correlated with primary motor cortex activation, while increasing target forces led to an increase in the amount of activation. These data indicate that human premotor areas such as the ventral premotor cortex might be functionally connected to the lower cervical spinal cord contributing to distal upper limb functions, a finding that extends our understanding of human motor function beyond the animal literature.
1 INTRODUCTION
Force generation and modulation are highly relevant prerequisites of human hand dexterity and important for mastering activities of daily living. Seminal neuroimaging studies (Cramer et al., 2002; Dai et al., 2001; Ehrsson et al., 2000; Keisker et al., 2009; Keisker et al., 2010; Mima et al., 1999; Spraker et al., 2009), studies in patient cohorts (Duque et al., 2003; Nowak et al., 2002; Nowak et al., 2003) and systematic reviews (Castiello & Begliomini, 2008; Olivier et al., 2007) have significantly contributed to our current understanding of cortical and subcortical representations of force generation and control. Key nodes of this network comprise the primary motor cortex (M1) and multiple secondary motor cortices of the frontal (Dafotakis et al., 2008; Evarts, 1968; Evarts et al., 1983; Hepp-Reymond et al., 1999) and parietal lobe (Davare et al., 2007), basal ganglia (Spraker et al., 2007), and the cerebellum (Spraker et al., 2012). Key pathways primarily include the cortico-spinal tract (CST), as well as cortico-cortical tracts, and various cortico-fugal pathways between the cortex, basal ganglia, and the cerebellum.
Technical advantages in neuroimaging sequences (Alonso-Ortiz et al., 2023; Finsterbusch et al., 2012; Finsterbusch et al., 2013; Islam et al., 2019), denoising (Eippert et al., 2017), and spinal analysis (De Leener et al., 2017) have recently paved the way for combined cortico-spinal functional magnetic resonance imaging (MRI). It has been applied to explore motor network dynamics during rest (Vahdat et al., 2020) or complex hand movements (Takasawa et al., 2022). These studies have extended the prior knowledge derived from a variety of earlier spinal functional MRI studies (Landelle et al., 2021). They have added spinal data with a high spatial resolution to previous electrophysiological data of cortico-spinal information throughput, such as those derived from cortico-muscular coherence analyses (Belardinelli et al., 2017; Gerloff et al., 2006; Mima & Hallett, 1999; Rossiter et al., 2013).
So far, combined cortico-spinal functional imaging during force generation has not been studied systematically. It would provide novel insights into multi-site cortico-spinal interactions during force generation, particularly in the context that not only M1, but also multiple secondary motor areas contribute to the CST and send trajectories to the spinal cord (Lemon, 2008; Lemon, 2021). In detail, studies of non-human primates have provided converging evidence that regions such as the dorsal and ventral premotor cortex (PMV) on the lateral surface of the hemisphere and the supplementary motor area (SMA) on the medial wall show cortico-spinal structural connectivity and have the potential to act in parallel to generate output to the spinal cord (Biber et al., 1978; Borra et al., 2010; Catsman-Berrevoets & Kuypers, 1976; Dum & Strick, 1991; Dum & Strick, 1996; He et al., 1993; He et al., 1995; Innocenti et al., 2019; Maier et al., 2002; Murray & Coulter, 1981). However, even in primates, there are still many open questions regarding potential relay nodes, and the precise craniocaudal extent of mono- or, more likely indirect poly-synaptic connectivity (Isa et al., 2013) along spinal inter-neural routes including the proprio-spinal system (Alstermark et al., 1984; Isa et al., 2006; Isa et al., 2007). For instance, tracing data in macaques have shown that PMV contributes about 5% of the total cortico-spinal projection with the majority terminating already in the upper segments of the cervical spinal cord, with only few terminating more caudally (He et al., 1993; He et al., 1995; Wise, 2006). Data obtained in rhesus monkeys did not show PMV projections below cervical level 2, but prominent terminal medial spinal densities for dorsal premotor cortex between C5 and T1 (Morecraft et al., 2019). Data in humans are strikingly limited. Transcranial magnetic stimulation (TMS) and multi-regional analysis of motor-evoked potentials have suggested the existence of fast and direct functional cortico-spinal connectivity between dorsal premotor areas and the hand (Fleischmann et al., 2021; Teitti et al., 2008). Using TMS, peripheral nerve stimulation and monosynaptic reflex analysis, another study argued for the existence of descending influences on proprio-spinal inter-neurons not only from M1 but also from PMV (Giboin et al., 2017). Recent tractography data have suggested potential direct connections between premotor areas including PMV and SMA and even lower cervical segments. However, these should be considered with caution due to reduced spatial resolution at the spinal levels (Usuda et al., 2022). Finally, studies in stroke patients have related the extent of damage to CSTs originating from premotor areas to deficits and recovery processes (Ito et al., 2022; Newton et al., 2006; Schulz et al., 2012).
In summary, there is converging data to hypothesize that spinal cord activation during force generation might be significantly linked to M1 activation. In addition, and importantly, it can also be hypothesized that the extent of spinal activation might be modulated by premotor regions such as PMV and SMA. The present study was designed to explore these hypotheses in detail. Cortico-spinal functional MRI data were acquired during a simple visually guided isometric force generation task in a group of healthy young subjects. Brain activation was localized in the left M1, SMA, PMV, and right cervical spinal cord. Mixed-effects linear regression analyses were used to compare cortical and spinal activations between different force levels and to relate cortical activations in M1, SMA, and PMV to spinal activations.
2 MATERIALS AND METHODS
2.1 Subjects
Twenty-one young volunteers participated in the study. One subject was excluded from further analysis because of an accidental MRI finding. The subjects finally included (10 females and 10 males, mean age 27 years, range 19–34) were right-handed according to the Edinburgh handedness inventory (Oldfield, 1971), had normal or corrected to normal vision and reported no neurological or musculoskeletal diseases or contraindications to MRI. The study was conducted following the Declaration of Helsinki and approved by the local ethics committee of the Medical Association of Hamburg (PV6026). All subjects gave written informed consent and received monetary compensation.
2.2 Motor task
We employed an fMRI block design with three experimental conditions (block length 15 s) and interleaved resting baselines (Figure 1a,b) using Psychtoolbox version 3.0.16, ran in Octave version 4.0.3. In the experimental conditions, the subjects were asked to perform visually cued whole hand grips with their right hand with three different predefined force levels low, medium, and high corresponding to 30%, 50%, and 70% of the maximum output measurement (linear force measurement) covered by an MRI compatible grip force response device (Grip Force Bimanual, Current Design, Inc, Philadelphia, PA). The absolute force levels were the same for all subjects. All three experimental conditions had the same time course. First, the subjects were informed via a video screen, visible through a mirror attached to the head coil of the MR scanner, which force level they had to reach in the upcoming activation block. After 1.5 s, the instruction text was replaced by an empty circle. After a variable delay of 1.5, 2.0, or 2.5 s, a white cross blinked under the empty circle at 0.8 Hz (the cross appeared for 0.625 s). The subjects then performed almost isometric hand grips with the right hand synchronized with the white cross. When they reached 90% of the correct force level, the empty white circle changed to a solid white circle. The instruction was to maintain this strength for the duration of the appearance of the white cross. After 15 s, the blinking cross and circle were replaced by a black screen, which told the subjects to stop the hand movements and rest until the next instruction appeared.
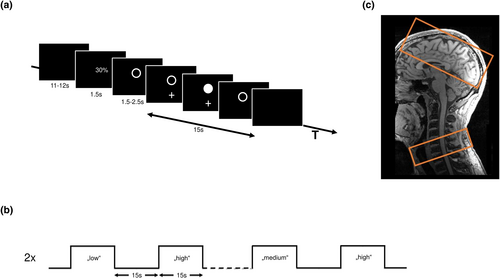
The duration of this resting (= baseline) condition depended on a variable delay between the end of the instruction text and the start of the cue lasting between 11 and 12 s, resulting in an inter-block interval of 15 s. The sequence of the three different force levels was pseudorandomized. Each fMRI session consisted of 490 images preceded by five dummy images allowing the MR scanner to reach a steady state in T2* contrast. After acquiring the dummy images, the experiment started with a baseline condition. The whole session consisted of 36 blocks (12 for each force level, lasting 15 s) and 36 baseline blocks + instruction conditions (lasting 15 s). The session was repeated two times. Each session lasted approximately 18.4 min. The subjects were trained outside the scanner to familiarize themselves with the task. During this training session, they were trained to perform the hand movements at the defined frequency and reach the correct force levels without overshooting. After the training session, the subjects received feedback on their performance to achieve a performance improvement. Inside the MR scanner, the subject's hand and arm were placed in a comfortable position on the belly of the subject. If necessary, medical tape fixed the force response device on the right hand. During the whole session, the force production was recorded for later analysis.
2.3 MRI data acquisition
A 3 T Prisma MRI scanner (Siemens Healthineers, Erlangen, Germany) and a 64-channel combined head–neck coil were used to acquire cerebral and spinal imaging data. The magnet's iso-center was set on the lower part of the chin of the subject, approximately centered to vertebral C2/C3, but in some cases, it had to be further adjusted depending on the subject's height. The imaging modalities included high-resolution T1-weighted, T2*-weighted, and task-evoked fMRI images. For the T1-weighted sequence, a three-dimensional magnetization-prepared rapid gradient echo (3D-MPRAGE) sequence was used, which covered the head and neck (cervical spine and upper part of the thoracic spine) with the following parameters: repetition time (TR) = 2300 ms, echo time (TE) = 3.4 ms, flip angle 9°, 236 coronal slices, 320 axial slices, with a voxel size of 1.0 × 1.0 × 1.0 mm3. The T2*-weighted image (MEDIC sequence) covered the lower part of the cervical spine, centered on the cervical vertebra C6, with the following parameters: TR = 307 ms, TE = 21 ms, flip angle 20°, eight axial slices, with a voxel size of 0.5 × 0.5 × 5.0 mm3, the slices were positioned identical to the spinal slices of the functional acquisitions, see below. For fMRI, a combined cortico-spinal fMRI protocol based on echo-planar imaging (EPI) was used to record BOLD responses in the brain and spinal cord (Finsterbusch et al., 2013; Tinnermann et al., 2017), 32 slices, divided into two sub-volumes (Figure 1c), were acquired. These two sub-volumes have different geometry, timing parameter (Chu et al., 2023; Finsterbusch et al., 2013), and shim settings. The shim settings were determined using a field map acquisition and a dedicated shim algorithm (Chu et al., 2023; Fricke & Finsterbusch, 2020). The upper volume included 24 axial slices (voxel-size: 2.0 × 2.0 × 2.0 mm3, 1 mm gap between slices) in the brain, which was initially oriented along the anterior–posterior commissure axis and, if necessary, tilted to cover the primary and secondary motor areas and if possible, the visual cortex. The lower sub-volume consisted of eight axial slices (voxel-size: 1.0 × 1.0 × 5.0 mm3, no gap between slices), centered at the vertebral body of C6 and covered the vertebral bodies of C5, C6, and C7. The whole sequence was measured with the following parameters: TR = 2231 ms, TE = 30 ms (brain) and 31 ms (spinal), flip angle = 75°. Additionally, we measured one whole-brain EPI volume with the following parameters: TR = 2385 ms, TE = 30 ms, flip angle 75°, and 36 axial slices with a voxel size of 2.0 × 2.0 × s2.0 mm3 with 1 mm gap between slices. During the fMRI sessions, pulse, ECG, respiration, and the trigger signal were recorded (sampling rate = 400 Hz) using the Physlog-function (Ideacmdtool, provided by Siemens Healthineers, Erlangen, Germany) and respiratory, ECG, and pulse measurement devices provided by Siemens Healthineers, Erlangen, Germany).
2.4 Behavioral data
The individual maximum grip force (whole hand grip) of the right hand was measured with a JAMAR Hand Dynamometer (built by Patterson Medical, Warrenville, USA).
2.5 Image pre-processing
Brain and spinal cord images were pre-processed separately. The brain fMRI images were pre-processed using the Oxford Center for fMRI of the Brain's (FMRIB) Software Library (FSL) version 6.0.4 (Jenkinson et al., 2012). The whole-brain EPI-image of each subject was linear co-registered to the brain-extracted high-resolution T1-image of each subject, and the individual T1-image was linear co-registered to the MNI152-T1-2mm image provided by the FSL library. The transformation matrices were concatenated for further pre-processing steps. The first 5 dummy volumes of the task-related fMRI images were discarded. The mean fMRI-image was registered to the whole brain EPI, and then the concatenated transformation matrices were used for registration on the MNI152-T1-2mm image. The fMRI images were further pre-processed with motion correction using MCFLIRT (Jenkinson et al., 2002), and the images from both sessions were concatenated into one time series at the subject level.
The spinal fMRI images were pre-processed using the Spinal Cord Toolbox, version 5.2 (De Leener et al., 2017) and FSL version 6.0.4 (Jenkinson et al., 2012). The spinal fMRI images were cropped with the spinal cord at the center of the image. Motion correction was performed using two phases of movement correction. MCFLIRT (Jenkinson et al., 2002) was used for the first phase of motion correction with spline interpolation and a normalized correlation cost function. The images across the two runs were realigned to the first image of the first run with a three-dimensional rigid-body realignment. To correct for slice-independent motion due to the non-rigidity of the cervical spine and physiological motion from swallowing and the respiratory cycle, the second phase of motion correction was performed with two-dimensional rigid realignment independently for each axial slice (Cohen-Adad et al., 2009; Weber II et al., 2016). The images from both sessions were concatenated into one time series at the subject level. The spatial normalization from native to standard space was performed using tools from the open-source Spinal Cord Toolbox (De Leener et al., 2017; De Leener et al., 2018): The C5 and C7 vertebrae in the structural T2* images of the cervical spine were manually identified, and the spinal cord was automatically identified and segmented. The structural images were then normalized to the PAM50_T2s-template (resolution = 0.5 × 0.5 × 0.5 mm3) (De Leener et al., 2018). After motion correction, the mean functional image was segmented to identify the spinal cord. The resulting binary spinal cord mask and the reversed deformation fields of the structural normalization were used to register the PAM50_T2-template on the mean functional image. The inverted resulting deformation field was then used to normalize the functional images and other images (e.g., cope-images) to PAM50-space. The normalized images were visually inspected for quality control at each step.
2.6 Physiological noise modeling
Cardiac and respiratory cycles are significant noise sources in spinal cord fMRI and can confound signal detection (Weber II et al., 2016). To account for this noise, cardiac signals (pulse), respiratory signals, and MRI triggers were collected during scanning. The SPM (SPM12) based PhysIO Toolbox version 8.0.1 (Kasper et al., 2017), ran in MATLAB version R2018a was used to calculate the noise regressors. This toolbox uses a model-based physiological noise correction, which uses retrospective image correction (RETROICOR) of physiological motion effects (Glover et al., 2000), heart rate variability (Chang et al., 2009), and respiratory volume per time (Birn et al., 2006). Based on the physiological signals, 18 noise regressors were generated. A cerebrospinal fluid (CSF) regressor was also generated from the CSF signal surrounding the spinal cord using a subject-specific CSF mask, which was created from the PAM50_csf-template (De Leener et al., 2018; Weber II et al., 2016) and an individual spinal cord mask.
2.7 Data analysis: First- and second-level analyses
Two different first- and second-level analyses were performed. The analyses of the cerebral and spinal images were conducted separately. For both analyses, the same explanatory variables (EVs) were used in the design matrices of the general linear model (GLM) analyses. For the first-level analysis, the recorded force production, which was recorded during the MRI sessions, was further analyzed. For each volume (TR = 2.231 s), the mean of the maximum force produced was calculated and used as EV in the design matrix and hereinafter referred to as mean EV. In the second-level analysis, the force levels low, medium, and high were used as EVs. In both analyses, the temporally jittered instruction period was separately modeled as an additional EV but not further analyzed in the group analysis (Grefkes et al., 2008). For the group analysis, separate analyses for the brain and spinal cord data were performed.
For the brain images, the motion-corrected functional images were spatially smoothed with a Gaussian kernel of 5 mm full-width half-maximum (FWHM) and high-pass filtered (90 s) using the fMRI Expert Analysis TOOL (FEAT v6.00) (Woolrich et al., 2001; Woolrich et al., 2004). Statistical maps of the pre-processed time series were generated using FMRIB's improved Linear Model (FILM) with pre-whitening (Weber II et al., 2016; Woolrich et al., 2001). The design matrices included the hemodynamic response function (gamma convolution, phase 0 s, standard deviation 3 s, mean lag 6 s) convolved task vectors as EVs, motion parameters, and motion outliers, determined using fsl_motion_outliers (Analysis Group FOU, 2018) were entered as covariates to remove movement-related variance (Bönstrup et al., 2016; Rehme et al., 2011; Volz et al., 2015). For the second-level group analysis, spatial normalization of the statistical images from the subject-level analyses to the MNI template was performed. Group average activation maps for each contrast were generated with the demeaned individual grip force of the right hand as an additional covariate using FMRIB's Local Analysis of Mixed-effects (FLAME) stages 1 and 2 (Beckmann et al., 2003; Khatibi et al., 2022; Woolrich et al., 2004). The group average activation maps were thresholded using a Z-score > 3.5 with a cluster significance threshold of p < .05 to correct for multiple comparisons using Gaussian random field (GRF) theory, in line with previous recommendations (Khatibi et al., 2022; Woo et al., 2014).
Additionally, three binary masks were generated from the HMAT-Template (Human motor area template) (Mayka et al., 2006) covering left primary motor cortex (M1), left ventral premotor cortex (PMV), and left SMA. The masks were used to detect the peak voxels in the individual subject-specific activation maps for the mean EV. Each peak voxel location was manually checked for plausibility and, if necessary, manually corrected. The mean parameter estimates (PEs) of the three different force levels were extracted using spheres of a radius of 5 mm centered on the peak coordinates. For a more complete examination of the cerebral motor network, the activation of further left and right hemispherical cortical motor areas was calculated and supplemented in the Supporting Information (Figure S1).
For the spinal images, the motion-corrected functional images were spatially smoothed with a Gaussian kernel of 2 × 2 × 5 mm3 FWHM and the spinal cord was extracted from the data using a spinal cord mask, which was created from the PAM50_cord_template and spatial transformed in the subject-specific space. The data were further analyzed with FEAT from the FMRIB software library (Woolrich et al., 2001) and were high-pass filtered (90 s). The statistical maps of the pre-processed time series were generated using FILM with pre-whitening (Weber II et al., 2016; Woolrich et al., 2001). The design matrices included the hemodynamic response function (gamma convolution, phase 0 s, standard deviation 3 s, mean lag 6 s), convolved task vectors as EVs, the physiological noise regressors, the CSF time series, the motion parameters, and motion outliers, determined using fsl_motion_outliers (Analysis Group FOU, 2018). For the second-level group analysis, spatial normalization of the statistical images from the subject-level analyses to the PAM50-template was performed. Group average activation maps for each contrast were generated with the demeaned individual maximum force as an additional covariate using FLAME stages 1 and 2 [Beckmann et al., 2003; Khatibi et al., 2022; Weber II et al., 2016]. The group average activation maps were thresholded using a Z-score > 2.5 (lower Z-threshold than in the cerebral images, adapted to the lower detected activation in the spinal images, such as in Weber II et al. (2016)) with a cluster significance threshold of p < .05 to correct for multiple comparisons using GRF theory (Khatibi et al., 2022). Additionally, a binary mask was generated to analyze the individual activation maps, which covered the right hemi-cord between the vertebral-level C5 and C7. The mask was used to detect the peak voxel in the individual subject-specific activation maps for the mean EV. In-plane spheres with a radius of 1.5 mm centered on the peak voxel coordinates were created. The mean PEs of the three different force levels were extracted from these spheres and used for further statistical analysis.
2.8 Further statistical analysis
The statistical package R 4.0.4 (RStudio Team, 2021) was used for further statistical analysis. To analyze brain (M1, PMV, and SMA) or spinal cord activation under varying target forces, linear mixed-effects models with repeated measures (package lme4) were used, with brain activation values as the dependent variable, force levels as the independent factor of interest, individual maximum grip force as additional fixed factor and subject as a random factor. Post hoc tests with pairwise comparisons between force levels were carried out using emmeans. To analyze the relationship between cerebral and spinal activation, the following linear mixed-effects models were fitted with spinal activation as the dependent variable and activation values as independent, fixed factor of interest and subject and force levels as random factors. (1) Three separate models were computed to analyze the relationship between spinal activation and regional brain activation of the three regions of interest (M1, SMA, and PMV). (2) One model was additionally estimated combining significant cerebral activations from the univariate models. (3) The combined model was further explored by adding an interaction term for the relevant brain activations. Individual maximum grip force was added as additional fixed factor. Statistical significance was assumed at p < .05. Leave-one-out model analyses of the fitted models were applied to ensure the robustness of the findings with statistical significance remaining stable when iteratively excluding all single subjects.
3 RESULTS
3.1 Behavioral data and motor task
The average maximum grip force across the group was 42 ± 10 kg (mean ± standard deviation, range 31.3–71.3 kg). During the experiment, the exerted target forces were 37.1 ± 6.3% for low, 58.4 ± 7.6% for medium, and 76.5 ± 5.8% for high, respectively. On average, across all three target forces, the actual target force was overachieved by 7.36%. Paired two-sample t tests revealed no significant difference between exceeding the three force levels.
3.2 Spinal cord and cortical brain activation during force generation
Force generation across force levels led to a significant BOLD activity on group level primarily in the right spinal cord between the lower parts of the C5 and C7 vertebral level, with a maximum activity localized at C6 vertebral level. This level would correspond to the C7 spinal cord segment (Figure 2a). Force level-specific analyses resulted in an increase in the spatial extent of BOLD responses from rather focal activation at C6 vertebral level during low force generation towards more distributed spinal activations between C5 and C7 and a peak between C6 and C7 (vertebral level) during high force generation. This extent would correspond to lower C7 and upper C8 spinal cord segments (Figure 2b, see Table S1 for statistics on peak activations and force-dependent increase in spatial activation).
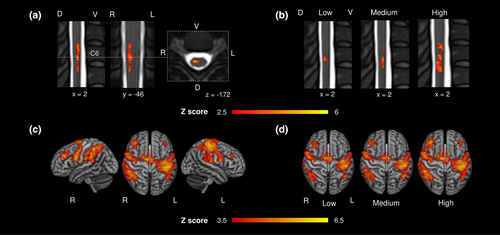
Cerebral BOLD activity on group level was detected across force levels primarily in the left primary sensorimotor cortex comprising M1 and the primary sensory cortex (Table S2). We also found activations in bilateral SMA, right dorsal premotor cortex, bilateral regions corresponding to PMV, and a widespread activation in posterior parietal cortices along the intraparietal sulcus (Figure 2c, Table S2). Increasing force levels resulted in more pronounced brain activation in left primary sensorimotor cortices and bilateral prefrontal and parietal brain regions (Figure 2d). The left dorsal premotor cortex did not show significant activations on group level.
In the following, spinal cord and cerebral peaks of activation were localized in each individual subject in the right spinal cord and left motor cortices M1, PMV, and SMA (see Table S3 for coordinates) using the mean effect contrast across the three force levels (mean EV). Subsequently, PEs of the BOLD response were extracted on these coordinates for the individual force levels, that is, three estimates were obtained for all regions in each subject for further statistical analyses.
Linear mixed-effects regressions with repeated measures were used to assess the evolution of spinal and cortical activations with increasing force levels. We found a significant increase (p < .001) in right spinal cord activation (estimated means: low 30.4, medium 60.9, high 76.5, Figure 3a), post hoc tests confirmed a significant difference between all three force levels (Table 1). Similarly, also left M1 exerted a force level dependent increase (low 215, medium 246, and high 286) in focal brain activation (p < .001) and post hoc tests also confirmed a significant difference between all three force levels (Table 1, Figure 3b). For SMA (low 130, medium 128, and high 139), the model also revealed a significant dependence on the force levels (p = .019), but the post hoc tests revealed only a significant difference between medium and high (p = .027, Table 1). For PMV (low 106, medium 108, and high 110), there were no significant differences between the force levels (p = .61, Figure 3b, Table 1).

| Comparisons | Spinal | M1 | SMA | PMV |
|---|---|---|---|---|
| Low—medium | .0001** | .0001** | .80 | .93 |
| Medium—high | .0479* | <.0001*** | .027* | .82 |
| Low—high | <.0001*** | <.0001*** | .11 | .60 |
- Note: p Values are given uncorrected for pairwise post hoc comparisons between force levels for spinal, M1, SMA, and PMV activations.
- Abbreviations: M1, primary motor cortex; PMV, ventral premotor cortex; SMA, supplementary motor area
- * p < .05,
- ** p < .001,
- *** p < .0001.
3.3 Relationships between spinal cord and cortical brain activation
Finally, linear mixed-effects regressions with repeated measures were used to address the relationship between cerebral activation in M1, PMV, and SMA and spinal cord activation. We found a significant and positive correlation between M1 activation and spinal cord activation (p = .00024, Figure 3c). In contrast, PMV was negatively related to spinal cord activation (p = .0077, Figure 3c). SMA did not exhibit any significant association with the amount of spinal activation (p = .84, Figure 3c). To further analyze the association of M1 and PMV activation with spinal activation, a linear mixed model with M1 and PMV BOLD responses as fixed factors was estimated. This model confirmed the negative association of PMV activation with spinal activation (p = .0025) independent from the association of M1 and spinal activation (p < .0001). A potential interaction of M1 and PMV onto spinal activation was not evident (p = .09). Also, M1 and PMV did not show any cross-correlation (p = .60).
4 DISCUSSION
The most remarkable novel finding of the present study is the significant association between PMV activity and spinal cord activation during force generation. This involvement was independent of the positive relationship between activation in the left primary motor cortex (M1) and the right spinal cord. More precisely, PMV activation was negatively linked with spinal cord activation. PMV and M1 activations were not correlated with each other. Although the present work did not assess inter-regional connectivity or interactions per se, these correlative findings could indicate that the PMV might be functionally connected to lower cervical spinal cord motoneurons in segments contributing to distal upper limb functions, most likely via indirect, poly-synaptic pathways. The SMA, an alternative secondary motor region, did not exhibit comparable associations with spinal cord activation during force generation. Supplemental analysis of the relationship between cerebral activation and spinal activation (Figure S1) also did not reveal similar associations between PMD and anterior interparietal sulcus (AIPS) on either side, right PMV, right SMA, right M1 and Cingulate Gyrus and spinal activation.
The presented force generation task activated a bilateral and widespread cerebral network including left-sided sensorimotor cortices, and premotor areas such as the PMV, SMA, dorsolateral prefrontal cortices and areas of the posterior parietal lobe along the intraparietal sulcus. These areas are all well in line with previous imaging data, derived from power grip (Alahmadi et al., 2021; Dai et al., 2001; Keisker et al., 2009) and precision grip force tasks (Castiello & Begliomini, 2008; Olivier et al., 2007). Compared to this cortical brain network, a systematic analysis of cortico-spinal interactions during force generation was not available so far. The present study aimed at answering two main questions: Does activation in the left M1 show a significant association with right-sided spinal cord activation? Might activation in premotor cortices also show an association with spinal cord activation during force generation?
Both M1 and the spinal cord showed a force level-dependent increase in the spatial extent of activated voxels and in the strength of activation. Force level dependent dynamics in M1 activation are well in line with previous animal data (Evarts, 1968; Hepp-Reymond et al., 1989) and data from human power grip force experiments (Alahmadi et al., 2021; Cramer et al., 2002; Dai et al., 2001; Keisker et al., 2009; Saleh et al., 2021). On the spinal level, similar patterns were observed at increasing force levels: Not only did the peak activation increase with higher force levels, but also the extent of activated spinal tissue showed gradually increases suggesting that more and more motor units and muscles got active to generate the target force (Heckman & Enoka, 2004; Mendell, 2005; Purves et al., 2001). In fact, several muscle groups work in synergy during finger flexion for power grip. The primary finger flexors at lower force levels are the flexor digitorum superficialis and the flexor digitorum profundus muscle. They are innervated by motor neurons residing in C6 to T1 spinal cord segments. The palmaris longus muscle and the lumbrical muscles, innervated by C7 to T1 spinal cord segments, can contribute. Hence, finger flexion is primarily located in spinal cord segments C6-T1 (Ghez & Krakauer, 2000; Moore et al., 2014; Stifani, 2014). The observed BOLD signals, mainly located in the C6–C8 spinal cord segments (corresponding to the C5–C7 vertebral level), agree with these anatomical considerations. When comparing the evolution of M1 and spinal activation under increasing forces, the numerical increase in activation was more pronounced at the spinal level when compared to M1 suggesting that the spinal activation is more directly linked to the applied force than M1 activity.
In addition to the force level-dependent dynamics in M1 and the spinal cord, we found a significant positive correlation between M1 activation and spinal cord activation. This matches widely established concepts of M1 as the main origin of the CST. Similar results of such cortico-spinal co-activation in functional MRI have been recently obtained during complex finger movements (Takasawa et al., 2022).
In addition to M1, force generation also involved bilateral PMV and SMA. However, in contrast to M1, PMV showed no significant modulation while target forces increased. For SMA, post hoc tests only showed marginal effects between medium and high force levels. Stable activations in the dorsal premotor cortex were not detected at all. Similar results have already been reported by one previous study (Keisker et al., 2009). Therefore, the subsequent analyses for associations between cortical and spinal activations were limited to PMV and SMA.
We observed a significant negative association between PMV activation and spinal activation at lower C7 to upper C8 spinal cord segments. This finding is novel in two important aspects.
First, it provides empirical functional data in humans to support the view that PMV activation might be associated with spinal motor neuronal activity at lower cervical segments which are involved in distal upper limb functions. Animal tracing data in non-human primates have reported PMV analogs to primarily terminate in the upper segments of the spinal cord only (He et al., 1993; He et al., 1995; Morecraft et al., 2019; Wise, 2006). Poly-synaptic connections have been proposed to reach distal forelimb segments along different inter-neural indirect routes (Alstermark et al., 1984; Isa et al., 2006; Isa et al., 2007; Isa et al., 2013). Human data on the contribution of premotor cortices such as PMV to spinal processing are still limited. For instance, using TMS, peripheral nerve stimulation and monosynaptic reflex analysis, one study has addressed the input convergence between peripheral afferents and cortico-spinal inputs originating from PMV onto proprio-spinal neurons. This study has suggested the existence of descending influences on the proprio-spinal system not only for M1 but also for PMV (Giboin et al., 2017). Tractography data have recently argued—despite critical limitations in the spatial resolution—for the existence of direct connections between premotor areas including PMV and SMA and even lower cervical segments (Usuda et al., 2022).
Second, the present results with correlated PMV activations and spinal activations and uncorrelated PMV and M1 activations significantly extend previously published studies that have addressed connectivity and interactions during force generation more directly. One study has used effective connectivity analyses to characterize information flow between M1 and premotor cortices. Premotor-M1 coupling has increased linearly from lower to higher grip forces. It has been speculated that premotor cortices might be involved in force generation while primarily modulating the output of M1 to the spinal motor neurons (Saleh et al., 2021). Another study has discussed widespread human cortical and subcortical brain networks involved in force generation. The actual data indicate now that the PMV might not only contribute to networks at the cortical level, but it may also exert additional, more direct influences on the spinal cord. Importantly, as the present work did not cover measures of connectivity or undirected or directed coupling estimates, but remained associative in nature, the term influence has to be taken speculative. Nevertheless, we would argue that our data could indicate that brain activity involved in force generation could converge towards the spinal cord not only via M1 and its CST as the main outflow tract, but cortico-fugal information outflow might be also mediated via premotor cortices. This would further strengthen the CST bypass concepts that have emerged over the last years in stroke recovery research. Studies have consistently evidenced that the integrity of secondary CSTs originating from premotor cortices might influence residual motor output and recovery after stroke (Koch et al., 2016).
How can we interpret the negative correlation between PMV activation and spinal cord activation? Data in non-human primates have suggested the existence of cortico-spinal connections targeting the upper cervical spinal cord. Results are variable regarding the precise topography, potential relay nodes, and involved secondary inter-neuronal networks (Zholudeva et al., 2021). For instance, non-human primate concepts have been developed proposing a poly-synaptic course via segmental interneurons, proprio-spinal neurons, reticulo-spinal and rubro-spinal neurons (Isa et al., 2013). Evidence suggesting proprio-spinal inter-neurons as important inhibitory target networks for top-down PMV influence in humans comes from a recent spatial facilitation study combining TMS and peripheral nerve stimulation on the upper limb (Giboin et al., 2017). Finally, the absence of retrograde cortical degeneration in PMV in patients after spinal cord injury and absent of motor responses after PMV electrical stimulation has argued against direct mono-synaptic connections between PMV and the spinal cord (Usuda et al., 2022). Therefore, we speculate that poly-synaptic trajectories originating from PMV with long-range excitatory pathways converging onto inhibitory spinal networks which modulate spinal motor neurons, are most likely to transmit cortico-spinal influences from PMV during force generation.
The fact, that similar associations between cerebral and spinal activation were not found for PMD and AIPS on either side, right PMV, right SMA, right M1, and Cingulate Gyrus supports the hypothesis that the presented associations are area-specific for left M1 and PMV.
There are several important limitations to note. First, they include possible artifacts in the spinal images related to movement and physiological signals (Fratini et al., 2014). The analysis pipeline was adapted according to established protocols for spinal fMRI artifact minimization (Brooks et al., 2008; Cohen-Adad et al., 2009; De Leener et al., 2017; Eippert et al., 2017; Kasper et al., 2017). Second, smoothing of the fMRI data might complicate the precise in-plane localization and slight shifts in the single-subject activation maps during normalization might explain why activation peaks at group level are distributed between the anterior and posterior horn. Third, the cerebral and spinal field of view was limited due to technical restrictions. Therefore, it was not possible to investigate the influence of other brain regions, for example, basal ganglia or the cerebellum on spinal activation. Furthermore, the dorsal premotor cortex (PMD) was not included in the main analysis of the mixed model although PMD belongs to the premotor areas. This was done because no clear activation in the left PMD could be detected in the second level analysis (Figure 2), but it was added in a supplementary analysis in the Supporting Information. Fourth, the force levels were equal for all subjects and not adjusted to the individual maximum grip force; it is possible that this has led to a greater variance in the individual BOLD data. In the future, further studies should be conducted to examine the relationship between very low and very high force levels especially in the spinal cord and the dependence of the individual maximum grip force. Finally, as stated above, the present analyses were limited to associations between cortical and spinal strength of activations. Future studies are also warranted to add further cortico-spinal functional connectivity or effective connectivity data to shed further light on coupling and interactions between primary motor and premotor areas and the spinal cord.
AUTHOR CONTRIBUTIONS
Hanna Braaß: Conceptualization; investigation; methodology; formal analysis; writing—original draft preparation; writing—review and editing. Jan Feldheim: Investigation; methodology. Ying Chu: Methodology; writing—review and editing. Alexandra Tinnermann: Methodology; writing—review and editing. Jürgen Finsterbusch: Methodology; writing—review and editing. Christian Büchel: Methodology; writing—review and editing. Robert Schulz: Formal analysis; writing—original draft preparation; writing—review and editing. Christian Gerloff: Conceptualization; writing—review and editing.
ACKNOWLEDGMENTS
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) SFB 936-178316478—C1 (Christian Gerloff) & A6 (Christian Büchel) and Exzellenzstipendium from the Else Kröner-Fresenius-Stiftung (2020_EKES.16) (Robert Schulz). The funding sources were not involved in the study design, the collection, analysis and interpretation of the data, writing the manuscript, and the decision to submit the article for publication. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supporting Information. Processed brain and spinal cord activation data and demographic data to reproduce the findings are available from the corresponding author on reasonable request.




