Questions and (some) answers on reactive astrocytes
Funding information: Agence Nationale de la Recherche, Grant/Award Numbers: 2010-JCJC-1402-1, 2011-BSV4-021-03, ANR-16-TERC-00; Association Huntington France; Centre National de la Recherche Scientifique; Commissariat à l'Énergie Atomique et aux Énergies Alternatives; Fondation pour la Recherche sur le Cerveau; Fondation Vaincre Alzheimer/LECMA, Grant/Award Number: FR-15015
Abstract
Astrocytes are key cellular partners for neurons in the central nervous system. Astrocytes react to virtually all types of pathological alterations in brain homeostasis by significant morphological and molecular changes. This response was classically viewed as stereotypical and is called astrogliosis or astrocyte reactivity. It was long considered as a nonspecific, secondary reaction to pathological conditions, offering no clues on disease-causing mechanisms and with little therapeutic value. However, many studies over the last 30 years have underlined the crucial and active roles played by astrocytes in physiology, ranging from metabolic support, synapse maturation, and pruning to fine regulation of synaptic transmission. This prompted researchers to explore how these new astrocyte functions were changed in disease, and they reported alterations in many of them (sometimes beneficial, mostly deleterious). More recently, cell-specific transcriptomics revealed that astrocytes undergo massive changes in gene expression when they become reactive. This observation further stressed that reactive astrocytes may be very different from normal, nonreactive astrocytes and could influence disease outcomes. To make the picture even more complex, both normal and reactive astrocytes were shown to be molecularly and functionally heterogeneous. Very little is known about the specific roles that each subtype of reactive astrocytes may play in different disease contexts. In this review, we have interrogated researchers in the field to identify and discuss points of consensus and controversies about reactive astrocytes, starting with their very name. We then present the emerging knowledge on these cells and future challenges in this field.
1 INTRODUCTION
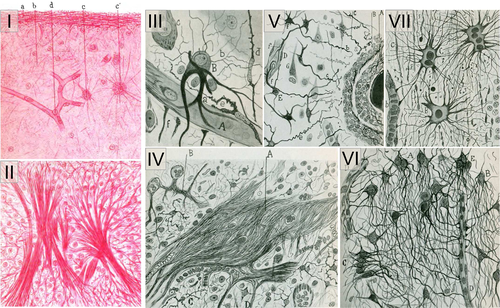
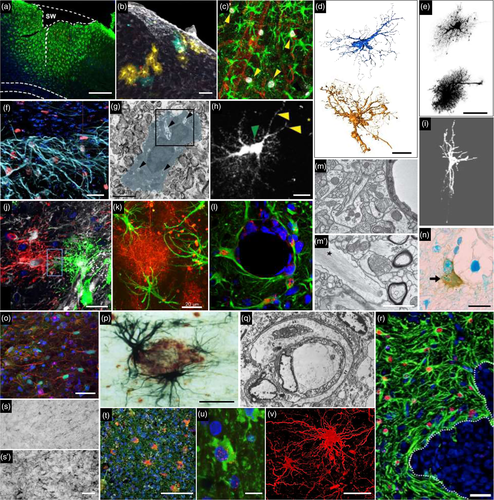
Astrocytes have key functions in the brain (Verkhratsky & Nedergaard, 2018). New discoveries are made regularly on their active roles in ion homeostasis, vascular coupling, synaptic plasticity, circuit building, synapse turnover, waste clearance or higher functions like sleep–wake cycle, food intake or memory (Verkhratsky & Nedergaard, 2018). One peculiar feature of these glial cells, first observed by 19th century anatomopathologists, is that they look different in the diseased brain [Figure 1, (Chaslin, 1891; Ramon y Cajal, 1925)]. The original observations reported morphological and histological changes, with increased fibrillary structures and more visible cellular elements in scarring brain tissue from patients [Figure 1, see also (Liddelow & Barres, 2017)]. Such changes in astrocyte appearance are observed in the brain of patients suffering from a broad range of brain pathologies (e.g., epilepsy, neurodegenerative disease, brain cancer, ischemia, infection, axotomy, invasive injury, and toxin exposure), as well as in animal models thereof (Figure 2, and references in legend). Astrocyte reactivity, Astrogliosis, Astrocyte activation, Reactive gliosis, or Astrocytosis are among the terms used to describe such molecular, morphological, and functional changes in astrocytes. Even if definitions of astrocyte reactivity were proposed by Prof. M. Sofroniew (Sofroniew, 2009, 2014a; Sofroniew & Vinters, 2010) and others (Pekny et al., 2016), a review of the literature shows that the use and meaning of these different terms are quite broad and inconsistent. Indeed, we surveyed ~40 researchers in the field (see complete list in Supplemental Table 1), and found that they have quite different visions of what reactive astrocytes are, or are not (Table 1) and they do not agree on the nomenclature (Table 2).
| Feature/concept | Agree | Agree and refine | Disagree | |
|---|---|---|---|---|
| What is it? | ||||
| A phenotypic change, alteration | AA AM ASP AP AVo CC CS EG FP GC HK HS LP MF MS NA PH SHRO SL SR VG | GeP: short-term changes also (Ca2+, Glu/GABA/K+ buffering, coupling.) | 
|
|
| KM: dynamic state | ||||
| IW: changes of viable, not wounded/dying astrocytes | ||||
| A response | AL AM ASP EG FP GC HS IW JOC MF MG MS NA SL SHRO SO SS VG | 
|
||
| A reprogramming, transformation, conversion | AVe KM | ASP: genes & functions down-regulated as well | ||
| AVo: go back to more immature/plastic state | ||||
| DB: may come from latent progenitors | ||||
| SK: become a different type of cells | ||||
| An ability, propensity | SS ASP SO | CC: they are now more reactive to a future stimulation | ||
| LP: capacity to become | ||||
| What does it change in astrocytes? | ||||
| Molecular (mRNA & proteins), morphological, functional changes | AA AM AVo ASP CS EG FP GC HK HS JOC LB MS NA SK SR SS VG | AP: reactive astrocytes integrate & regulate information differently | 
|
|
| CN: initial response biochemical, then transcriptional | ||||
| SHRO: biochemical as well | ||||
| GaP IW KM: Activation of specific signaling pathways | ||||
| Both gain & loss of functions | ASP CN MS | GC: importance of Ca2+ signaling | ||
| HS: global loss of proteins involved in ion/neurotransmitter homeostasis & change in extracellular matrix production | ||||
| SHRO: gain/loss of function can occur without reactive phenotype | ||||
| Proliferation not prevalent | ASP KM LP | EG: may refer to GFAP+ NSC converting into astrocytes | ||
| MS: newborn astrocytes form scars | ||||
| What induces it? | ||||
| Non-physiological conditions (injury, disease, stress, environment alterations) | ASP CN CS EG FP GC JOC HK HS IW MS NA SL SO SR VG | AL GeP MF SHRO: not exclusively pathological stimulus | 
|
|
| AM: exclude any physiological changes | ||||
| External/environmental stimulus or signal | AL ASP GaP MF SHRO | JOC: in response to glia injury as well = internal signal | ||
| Aging also | ASP SR | CS: Aging is not necessarily pathological | 
|
|
| Is it a homogeneous response? | ||||
| Heterogeneous: | AL AM ASP AVe AVo CC CJL CS CN DB DR EG GaP GC HK JOC KM LB MF MG MS NA PH RS SL SS | 
|
||
| - continuum | GaP MF PH | AVe MS SS: tuned to the nature & strength of the insult | AVo AM GL MG SL: Discrete states/phenotypes rather than continuum | 
|
| AVe CS DB: more than A1/A2 | ||||
| CS: worsens with disease progression | ||||
| DB: worsens with age | ||||
| - dictated by type of insult/disease … | AM ASP EG GC HK MF MG NA SL | |||
| … and by previous heterogeneity, brain regions, age | AVe AVo CS CN KM SS | DB MS: also by signaling | ||
| GFAP & morphological changes are core hallmarks/defining features | AA DR EG GeP JOC KM MF NA PH RS | LP: also associated with increased cytokines | ASP: Morphological changes not systematic | 
|
| AVo: Hypertrophy may be an artifact of increased GFAP staining | ||||
| AVe GL RS: Misleading | ||||
| CJL: GABA/proBDNF-based classification instead | ||||
| GC: Characterize only astrogliosis | ||||
| What are the consequences? | ||||
| Can have good or bad effects | DR GC LP MS SK | EG: adaptive response in acute injury to recover homeostasis by sealing off injured tissue. In chronic injury, maladaptive with dysfunctional astrocytes | AVe: Always good, evolutionary driven. Even neurotoxic astrocytes may have defensive roles | 
|
| AVe HS IW SO: Objective = restore homeostasis | ||||
| IW: Form a scar to limit lesion expansion | ||||
| GaP: not only secondary, also key contributor | CS: Reactive astrocytes are pathologically altered | |||
| CJL: Always bad in ND through GABA/H2O2 release | ||||
| Reversible & transient | CN GC MS SO | GeP SS: astrogliosis is not | ||
- Glu, glutamate; ND, neurodegenerative diseases; NSC, neural stem cells.
- In December 2018 and January 2019, we surveyed 61 researchers actively working in the field of (reactive) astrocytes with different approaches, on different diseases and models or in physiology. We asked them the following two questions:
- 1—What is your definition of astrocyte reactivity, in a few sentences? (Responses listed in Table 1).
- 2—Do you consider “astrogliosis,” “astrocyte activation,” and “astrocyte reactivity” as synonymous terms? (Responses listed in Table 2).
- We collected 38 replies (see Supplemental Text for full replies) from junior and more senior researchers, from both genders and 12 countries (see list in Supplemental Table 1). It is not a perfect or unbiased sampling of the community, but it represents the first attempt to survey different researchers working and publishing on astrocytes. It shows that some points are consensual while others are more controversial.
- Replies were analyzed and grouped under different features or concepts (first column), addressing different questions listed in boldface above. Respondents, identified by their initials, either agreed to these definitions (second column), agreed and provided further explanations (third column) or disagreed (fourth column). Pie charts represent the percentages of responses (green = agree; red = disagree; gray = not mentioned) for features mentioned by more than 18 respondents or for controversial features.
- The definitions given in response to question 1 are linked to the nomenclature discussed in response to question 2, since several researchers provide specific definitions for each term. For the sake of simplicity, we considered that responses to question 1 may apply to any of the three terms (astrogliosis, astrocyte activation, and astrocyte reactivity). Responses to question 2 help better pin down further differences linked to terminology.
| Agree | Agree & Refine | Disagree | ||
|---|---|---|---|---|
| These three terms are ill-defined… | AM CC CS DB DR FP MG PH SO | EG RS: meaningless terms | MS: Still valid | 
|
| …need new terminology or at least precise definition | ASP AVe AVo CJL DB GaP LB MG | EG: based on molecular & functional profiles | SL: Better not add new names | |
| Use a simple, broad, inclusive, collective definition | MF MG MS SR | AL CC DB: but then need to precise categories/functions/specification | 
|
|
| GaP: umbrella definition | ||||
| These three terms have different meanings | ASP AVo CC CN GC GaP LB MG PH SS | CJL: GABA/proBDNF based classification for hippocampal/cortical astrocytes.* | AVe DB HK IW JOC RS SL: All 3 equivalent AVe: Also “Astrocytosis” |
|
| … refer to different grades/stages | CN: reactivity = biochemical response, maintained as activation (transcriptional) | |||
| GaP MG SS: increase severity: activation < reactivity < astrogliosis | ||||
| GeP: activation = response to normal neuronal activity, reactivity = response to abnormal neuronal activity | ||||
| LB: Astrocyte activation = functional/phenotypic changes. Astrocyte reactivity = morphological changes | ||||
| LP VG: Activation = transition to reactivity | ||||
| Astrocyte activation refers to physiology and should not be used for pathology | AA AM CS EG FP GC HS NA SR SS | Not appropriate, because: | AL AP DR MF SK SO: Activation = reactivity AL: Both involve response to stimulus (either physiological or pathological) |
|
| ASP: suggests independent of a trigger. May involve loss of function | ||||
| KM: better suited for microglia | ||||
| MS SL: suggests inactive otherwise | ||||
| Astrogliosis is different: | FP | ASP: astrogliosis should be abandoned [−osis = pathological state, proliferation/increased number or invasion/infiltration/spreading] | AA CS EG HS MS NA SR: Astrogliosis = reactivity | 
|
| - involves proliferation or increased numbers | AL ASP CC CN LP LB MF SK VG | |||
| - involves scar | GaP KM SO | CC: property of a population | ||
| - involves inflammation (=immune cell recruitment) | AL MG | DR: clinical term = injury | ||
| - involves hypertrophy | LB | AP: increase in 3D domain | ||
| GC: with GFAP expression | ||||
| - ultimate stage | AL AP | GeP SS: Irreversible |
- *see Supplemental Text.
Beyond the discrepant terminology, some common features of reactive astrocytes emerge from this survey. A broad definition could be summarized as “astrocytes sense and respond to an abnormal situation in the brain. They change at the morphological, biochemical, transcriptional, and functional levels. These changes are maintained while the pathological stimulus is present but some aspects may resolve. Reactive astrocytes are heterogeneous and may have various effects on disease progression.” Indeed, reactive astrocyte heterogeneity is underlined by most researchers and contested by none of them (Table 1). This opens new areas of research to understand its origin and consequences (Section 5).
| Levels of observed heterogeneity | Disease modeled | Experimental model | CNS region | Species | Examples of heterogeneity | Additional levels of heterogeneity | Hypertrophy observed? Method used | ↗ GFAP observed? Method used | Others markers of reactive astro | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Neuroinfla. | Primary astro exposed to LPS, IL1, Poly IC | H, Ms | Only Ms astro are dependent on TLR4 or CD14. Ms & H reactive astro produce different cytokines | Signaling | n.m. | n.m. (↗ cytokine) | Tarassishin, Suh, and Lee (2014) | ||
| PD | MPTP [Mc], 6-OHDA [R] | Str | Mc, R | Mc: ↘ Cx30 IR; R: ↗ Cx30 IR | Visual | IHC/IF | Charron et al. (2014) | |||
| Gender | Stroke | MCAO | Cx | Ms | ↗ GFAP IR F > M | Visual | IHC/IF | Cordeau (2008) | ||
| Trauma | SWI | Cx | Ms | Nbr CcI2+ astro around lesion M > F | Visual | IHC/IF | Vimentin | Acaz-Fonseca, Duran, Carrero, Garcia-Segura, and Arevalo (2015) | ||
| Disease | AD, epilepsy | Tg2576, KA | Cx | Ms | Loss of astro domain organization in epilepsy but not in AD | Morphological | Diolistic labeling | IHC/IF | Oberheim et al. (2008) | |
| Trauma, stroke, AD | SWI, MCAO, APP/PS1 | Cx | Ms | Shh promotes astro proliferation & stem cell potential in open lesion only (SWI & MCAO) | Signaling | Visual | IHC/IF | Sirko et al. (2013) | ||
| Neuroinfla., stroke | LPS, MCAO | Cx | Ms | 50% regulated genes are disease-specific | Molecular | Visual | IHC/IF | Serpina3n, Lcn2 | Zamanian et al. (2012) | |
| Trauma, AD | SWI, APP23 | Cx | Ms | Different GFAP mRNA ↗ (TBI: 82-fold, denervation: 30-fold, AD: 18-fold increase) | Visual | IHC/IF, qPCR | Burbach, Dehn, Del Turco, Staufenbiel, and Deller (2004) | |||
| Temporal | ALS | SOD1G93A | SC | Ms | Presymptomatic stage: astro NF-KB activation delays disease progression. Symptomatic: accelerates disease progression | Visual | IHC/IF | Ouali Alami et al. (2018) | ||
| MS | EAE | Cx, Hip, Cb, SC | Ms | Early stage: ↗↗ MHC-II mRNA, =cholesterol synthesis pathway. Late stage: ↗ MHC-II mRNA, ↘ cholesterol synthesis pathway. Gene expression changes differ between the four regions | Regional, molecular | n.m. | qPCR | Itoh et al. (2018) | ||
| Regional | AD | Astro cultures exposed to βAPP | Cx, Hip, Cb, SC | R | Reaction induced only in Cx & hip | Visual | IHC/IF | CSPG | Hoke, Canning, Malemud, and Silver (1994) | |
| Epilepsy | Pilocarpine | Hip, Cx | Ms | Different transcriptional changes between Hip & Cx astro | Disease stage, molecular | Visual | IHC/IF, qPCR | Clasadonte et al. (2016) | ||
| Topographical | AD | / | Cx | H | ↗ Cx43 IR around plaques | Visual | IHC/IF, EM | Nagy, Li, Hertzberg, and Marotta (1996) | ||
| AD | APPSWE/PS1dE9 | Cx | Ms | ↗ P2Y1R mediated Ca2+ signaling in reactive astro near plaques | Signaling | Visual | IHC/IF | Delekate et al. (2014) | ||
| Trauma | SWI | Cx | Ms | Proliferation of juxta-vascular astro only | Functional | Visual | IHC/IF | Bardehle et al. (2013) | ||
| SCI, trauma | Laminectomy L1/L2, Cryo-injury | SC, Cx | Ms | Scar border: elongation & proliferation (↗ radial glia markers). Distal: No proliferation & hypertrophy (↗ GFAP) | Morphological, functional | Morphometry | IHC/IF | Wanner et al. (2013), Kim et al. (2012) | ||
| Trauma/excitotoxicity | SWI/KA | Ms | NFATc3+ astro are found only around lesions | Topographical | Visual | IHC/IF | NFATc3 | Serrano-Pérez (2011) | ||
| Morphological | AD | 3xTg-AD | Hip | Ms | Astro atrophy away from plaques (↘ GFAP surface & volume) | Topographical | Morphometry | IHC/IF | Olabarria et al. (2010) | |
| Trauma | Needle-induced lesion | Cx | Ms | At injury site: Mesh like morphology & small cell bodies. At the border: Enlarged soma & thick processes | Topographical, functional | Visual | IHC/IF | Martin-Lopez, Garcia-Marques, Nunez-Llaves, and Lopez-Mascaraque (2013) | ||
| TBI | Lateral fluid percussion | WM, Hip, Cx, Th | R | Intertwined processes in Hip & Cx; thick shortened processes in Th. No vimentin induction in Th | Regional, disease stage | Visual | Cajal gold sublimate stain, dot-blot | S100β, vimentin | Hill, Barbarese, and McIntosh (1996) | |
| Signaling | Peripheral nerve injury | Purified astro treated with EphB1 or IL6 | Cx | Ms | EphB1 (but not IL6) induces protective transcriptional profile in astro via STAT3 signaling | Molecular, functional | Visual | IHC/IF, WB | EphB1/STAT3 | Tyzack et al. (2017) |
| Stroke | MCAO | Cx | Ms | Only Notch1-STAT3-ETBR+ reactive astro proliferate | Functional | Visual | IHC/IF, WB | Jagged1 | LeComte, Shimada, Sherwin, and Spees (2015) | |
| Stroke | Phototrombosis | Cx | Ms | Only Thbs4+ reactive astro proliferate in SVZ via Notch1-Nfia signaling | Functional | Visual | IHC/IF | Thbs4 | Benner et al. (2013) | |
| Trauma | SWI | Cx | Ms | Only galectin 1 & 3+ reactive astro proliferate | Functional | Visual | IHC/IF | Galectins | Sirko et al. (2015) | |
| SCI | Contusion | SC | Ms | Type I collagen+ astro induce scar formation via N-cadherin pathway. Not active in hypertrophic reactive astrocytes | Topographical, molecular, functional | Visual | IHC/IF, WB | Cdh2, Ctnnb1… | Hara et al. (2017) | |
| Molecular | Neuroinfla. | Immunopanned astro LPS-treated mice | Ms | “A1” neurotoxic reactive astro induced by microglia. “A2” reactive astro express different genes, function uncharacterized | Functional | Visual | IHC/IF, qPCR | A1 & A2 panels + pan markers | Liddelow et al. (2017) | |
| Functional | NWMI | Hypoxic–ischemic encephalopathy | Cx | H | “A2” reactive astro induce OPC maturation arrest via COX2-PGE2 signaling | Visual | IHC/IF, qPCR | COX2 | Shiow et al. (2017) | |
| Brain metastasis | Tumor resection | Cx | H | pSTAT3+ astro reduce immune cell recruitment to metastases | Signaling | Visual | IHC/IF | pSTAT3 | Priego et al. (2018) | |
| MS, multifocal encephalopathy | / | WM | H | Some reactive astro uptake myelin & produce more cytokines | Visual | IHC/IF | Ponath et al. (2017) |
- This table presents a selection of relevant studies, performed mostly in vivo, to illustrate heterogeneity of astrocyte reactions. Different levels of observed heterogeneity can be listed as originally proposed by Anderson, Ao, and Sofroniew (2014). In addition, differences in responses are observed depending on disease stage or age (temporal), species, and gender. Interestingly, several levels of heterogeneity may co-exist (see Additional levels of heterogeneity). Most of the time, the reactive state of astrocytes was established by GFAP overexpression and morphological changes. Morphological changes were estimated qualitatively (visual), unless mentioned in the table. Some studies identified additional markers of reactive astrocytes (or subtypes).
- Abbreviations (by lexical field): CNS regions: Cb, cerebellum; Cx, cortex; Hip, hippocampus; SC, spinal cord; Str, striatum; Th, thalamus; WM, white matter. Diseases: AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis; NWMI, neonatal white matter injury; Neuroinfla., neuroinflammation; PD, Parkinson disease; SCI, spinal cord injury; TBI, traumatic brain injury. Experimental models: EAE, experimental autoimmune encephalomyelitis; KA, kainate; LPS, lipopolysaccharide; MCAO, middle cerebral artery occlusion; SWI, stab wound injury. Species: H, human; Mc, macaque; Mm, mammalian; Ms, mouse; R, rat. Other fields: Astro: astrocytes; CSPG: chondroitin sulfate proteoglycans; IHC/IF, immunohistochemistry or immunofluorescence; IR, immunoreactivity; n.m., not measured; WB, Western Blotting; =, unchanged; ↗, increase.
Other points that remain controversial will be covered later in this review: Are glial fibrillary acid protein (GFAP) and morphological changes appropriate markers of reactive astrocytes (Section 4)? Can we identify better markers (Section 6)? Do reactive astrocytes do good or bad things in disease (Section 8)? Do they die (Section 9)? Should aging astrocytes be considered as reactive (Section 12)? Other points were not necessarily raised by surveyed researchers, but are still actively studied: What are the exact triggers and downstream signaling pathways controlling this response (Section 7)? Where do reactive astrocytes come from and what do they become (Section 10)? How do reactive astrocytes interact with other cell types involved in innate and adaptive immunity (Section 11)? Addressing all these questions will help provide a refined vision of this complex brain response.
The aim of this review is not to provide an extensive overview of the now abundant literature on reactive astrocytes, but instead, discuss emerging research questions and unresolved issues on this topic.
2 HOW TO BEST NAME THEM?
But first, it is important to name our research subject and define what we are talking about. Our survey of researchers in the field of astrocytes showed that even three broadly used terms had different inferred meanings (Table 2). Many surveyed principal investigators (PI) agreed that the very definition of astrocyte reactivity was ambiguous and that a clear nomenclature would benefit the field.
The heterogeneity of this response and the variety of “reactive states” (see Section 5), call for a broad and inclusive definition that can further be refined in each context. Indeed, all recognized that drastic changes happen to astrocytes in pathological conditions, with some core and some disease-specific alterations (Table 1, Supplemental Text). We thus need words to describe this phenomenon. What are the most appropriate ones? There was some agreement that astrocyte activation is misleading because it can apply to physiological activation like transient Ca2+ signaling in response to normal neuronal activity (see Section 4-Timescale). It is thus better to avoid this term. Another point of partial convergence was that astrogliosis implied something different (e.g., stronger or irreversible; with scarring, proliferation or immune infiltration), which definitely does not apply to all disease conditions (Table 2). We are therefore left with the term astrocyte reactivity. The problem with this expression, as stressed by several PIs, is that it implies an “ability” to become (see Table 1). As discussed by Dr. A. Serrano-Pozo, reaction would be more correct to define the final state of astrocytes (see Supplemental Text). We thus propose to avoid the use of astrocyte activation and astrogliosis and instead use reactive astrocytes or astrocyte reaction and add to stroke/epilepsy/Alzheimer disease (AD) to further define it. Expressions like scar-forming astrocytes, proliferating reactive astrocytes, and phospho-STAT3 + reactive astrocytes are also very useful to functionally or molecularly define these cells (see details in Section 5). We will use this nomenclature in our review, but a more widespread agreement on terminology would be beneficial to the astrocyte community.
3 WHY BOTHER WITH REACTIVE ASTROCYTES?
There are more than 17,500 articles recovered in PubMed with the query “Reactive astrocyte” OR “Astrocyte reactivity” OR “Astrogliosis,” with more than 400 articles published each year since 2000. Why does such a broad and somehow ill-defined concept trigger this significant interest? Indeed, if astrocytes become reactive in response to something already going wrong, why should we care? It may already be too late; the initial pathological event may have started well before and triggered an irreversible disease cascade.
It is striking that astrocyte reaction is such a widespread response, reported in virtually all brain diseases, brain regions, and multiple species including invertebrates, like Drosophila, many mammals, and Humans [(Kremer, Jung, Batelli, Rubin, & Gaul, 2017; Sofroniew & Vinters, 2010), see Figure 2]. It is thus very important to better understand what it means for an astrocyte to engage in this phenotype switch and how it can influence surrounding cells.
Astrocyte reaction is by definition a secondary event; yet, it may still impact disease progression, which lasts for months or years in the case of tumor, neurodegenerative disease, or epilepsy. More importantly, astrocytes are reactive at the clinically visible stages of the disease, when patients seek diagnosis and treatment. Turning reactive astrocytes into beneficial partners for vulnerable neurons exposed to a chronic disease or to the long-term consequences of an acute injury would be a valuable therapeutic strategy.
In addition, as astrocytes react to altered homeostasis in the brain, they are endogenous biomarkers for brain diseases. Development of non-invasive imaging techniques, like magnetic resonance imaging (MRI) or positron emission tomography (PET) to visualize when and where astrocytes become reactive would help disease diagnostic. Available imaging methods are not quite specific for reactive astrocytes, they rather detect neuroinflammation as a whole (i.e., reactive glial cells, sometimes infiltration of peripheral immune cells) or associated changes, for example, in brain metabolism (Aiello et al., 2018; Carrillo-de Sauvage et al., 2015; Lavisse et al., 2012). Indeed, astrocytes may significantly contribute to imaging signals, such as those measured with blood oxygenation level–dependent functional MRI or [18F]-fluorodeoxyglucose uptake by PET, and therefore their reactive state may impact these measurements (Carter et al., 2019; Mishra, 2017). Refined approaches that are more selective for reactive astrocytes are being developed (Carter et al., 2019; Ligneul et al., 2019; Rodriguez-Vieitez et al., 2016; Scholl et al., 2015). Such improvements will be facilitated by basic studies that establish the molecular and functional profile of reactive astrocytes (see Section 6) but also of their microglia or neuronal neighbors, allowing the identification of new and specific astrocyte targets.
Last, some astrocyte proteins like GFAP or their break-down products may also end up in the cerebrospinal fluid of patients subject to traumatic brain injury [TBI, (Halford et al., 2017)], but also in more progressive diseases like Creutzfeldt-Jakob disease or AD [for review, see (Carter et al., 2019; Perez-Nievas & Serrano-Pozo, 2018)].
Therefore, defining how astrocytes change during disease may in fine lead to new biomarkers, better diagnosis tools, and even original therapeutic strategies, which are long-sought goals for many diseases.
4 WHAT ARE THE DEFINING FEATURES OF REACTIVE ASTROCYTES?
Several common features are observed in very different cases of brain injuries and diseases. They can be used to define the core hallmarks of reactive astrocytes.
4.1 Morphological changes
In pathological conditions, astrocytes display morphological changes, including hypertrophy of soma and main processes, but not only (see Figure 2). This was already noted by earlier pathologists using impregnation techniques to achieve sparse labeling of astrocytes (Figure 1). Diolistic labeling, dye filling, or expression of a cytosolic or membrane-tagged fluorescent protein reveal the complex and highly ramified astrocyte morphology. These methods evidence subtle alterations in astrocyte morphology that could be missed by cytoskeleton labeling with GFAP antibodies (See Figure 2b,d,e,h,i,j). They show process polarization toward a lesion [(Bardehle et al., 2013), Figure 2h] or ramification changes while the overall 3D domain covered by a single astrocyte is not massively disrupted [(Wilhelmsson et al., 2006), Figure 2e]. In more severe diseases like epilepsy, astrocytes may also retract their fine peripheral processes, become asymmetric and overlap more with their neighbors [(Oberheim et al., 2008; Sun & Jakobs, 2012), Figure 2j]. An extreme case is the glial scar caused by mechanical lesions of brain or spinal parenchyma (Figure 2f), or severe focal lesions like tumors (Figure 2r). Astrocytes become elongated and assemble to form a compact and permanent scar. Other cell types, such as fibroblasts, oligodendrocyte progenitor cells (OPC), ependymal cells, or pericytes, may also contribute to this scar (Adams & Gallo, 2018; Sabelstrom, Stenudd, & Frisen, 2014). This question and the overall effect of the glial scar on axonal regrowth are still debated (Anderson et al., 2016; Silver, 2016). Readers are referred to excellent reviews on the topic (Adams & Gallo, 2018; Sofroniew, 2018).
4.2 Molecular changes
A defining feature of reactive astrocytes is their overexpression of intermediate filament proteins like GFAP or vimentin. The “increased number and size of fibrils in neuroglia of sclerotic brain tissue in epileptic patients” was noted by anatomopathologists already at the end of the 19th century [see Figure 1, (Chaslin, 1891)]. Electron microscopy also evidences large bundles of filaments in reactive astrocytes (Figure 2m′). In AD brains, GFAP accumulates to the extent that it forms protein aggregates resembling Rosenthal fibers in reactive astrocytes (Wegiel & Wisniewski, 1994). The surveyed researchers broadly acknowledged the validity of GFAP as a marker for reactive astrocytes (Table 1), but several of them also underlined its limits (see further discussion in Section 6).
Owing to refined methods to isolate astrocytes and investigate their transcriptome at the genome-wide level, recent studies revealed that astrocyte reaction involves massive transcriptional changes that go well beyond Gfap induction. Hundreds of genes are either up-regulated or down-regulated in astrocytes in AD models and patients (Ceyzériat et al., 2018; Orre et al., 2014; Sekar et al., 2015), in a mouse model of hyperammonia (Lichter-Konecki, Mangin, Gordish-Dressman, Hoffman, & Gallo, 2008), or multiple sclerosis (MS) (Itoh et al., 2018), following spinal cord injury [SCI, (Anderson et al., 2016)], cortical stab wound injury [SWI, (Sirko et al., 2015)], middle cerebral artery occlusion (MCAO), or lipopolysaccharide (LPS) injection (Zamanian et al., 2012). Several genes induced in reactive astrocytes both in the LPS and MCAO models were identified. Among them, Serpina3n and Lcn2 were further validated as strongly, but transiently induced following LPS injection or MCAO (Zamanian et al., 2012). They are also induced in other disease models (Itoh et al., 2018; Suk, 2016; Switonski, Szlachcic, Krzyzosiak, & Figiel, 2015). Change in transcriptome is a conserved feature of astrocyte reaction, similarly observed in Drosophila glia (Lu et al., 2017).
Interestingly, genes that are down regulated in reactive astrocytes may also hold the key to understanding the roles of these cells in disease. Some genes associated with important astrocyte functions like the potassium channel KIR4.1 [Kcnj10, (Nwaobi, Cuddapah, Patterson, Randolph, & Olsen, 2016)], the glutamate transporter GLT1 (Slc1a2), or glutamine synthase GS [GluI, (Sheldon & Robinson, 2007)] are repeatedly reported as down regulated in disease. Likewise, reduced expression of several homeostatic astrocyte genes is reported in mouse models of SWI (Sirko et al., 2015) and TBI (Shandra et al., 2019) (see also Section 10), but there is no established list of genes down-regulated in reactive astrocytes across multiple diseases. Overall, the significant phenotypic alterations observed in reactive astrocytes involve large-scale transcriptome modifications and functional changes (see Section 8).
4.3 Migration & proliferation
The very first observers of astrocytes noticed increased numbers of nuclei in sclerotic tissue of epileptic brains, and discussed the proliferative capacity of neuroglia [Figure 1, (Chaslin, 1891; Ramón y Cajal, 1925)]. But more recent studies based on two-photon imaging of astrocyte reaction, fate mapping and bromodeoxyuridine (BrdU) labeling in animal models, challenged this dogma [(Bardehle et al., 2013; Buffo et al., 2008), Figure 2c, h]. Immunostaining with Ki67 or Proliferating Cell Nuclear Antigen in patients confirmed that only a small percentage of reactive astrocytes undergoes proliferation (Perez-Nievas & Serrano-Pozo, 2018). These proliferative astrocytes appear to require a stimulus from outside the central nervous system (CNS): they are in direct contact with the lesion in SCI [(Wanner et al., 2013), Figure 2f], are exposed to blood-borne substrates following SWI (Sirko et al., 2013), or their cell bodies are in apposition to blood vessels (Bardehle et al., 2013). Notably, proliferative astrocytes perform only one or two rounds of division (Bardehle et al., 2013; Sirko et al., 2013). Two-photon microscopy also showed that reactive astrocytes do not migrate like microglia or macrophages do after a focal mechanical injury (Bardehle et al., 2013; Nimmerjahn, Kirchhoff, & Helmchen, 2005). Similarly, in the spinal cord, astrocytes remain in their allocated regional domain and do not migrate to a nearby SWI (Tsai et al., 2012).
4.4 Timescale
Increased GFAP and nestin protein expression is detected as soon as 90 min after mouse euthanasia and preparation of acute slices, showing that molecular changes may occur rapidly (and even ex vivo) (Takano et al., 2014). In addition, in different striatal injury models caused by acute neurotoxin injection, Gfap mRNA levels are induced 6 hr later, while GFAP protein levels increase significantly only after 12–72 hr, depending on the model (O'Callaghan, Kelly, VanGilder, Sofroniew, & Miller, 2014). Astrocyte reaction can be viewed as a change of state or a conversion (Table 1). By becoming reactive, astrocytes undergo a set of morphological, transcriptional, and functional changes that transform them into different cells, with acquired, lost or altered properties and functions (see Section 8). This is different from a rapid stimulation by neurotransmitters, for example, which will produce transient movements of perisynaptic processes (Bernardinelli et al., 2014), Ca2+ signals, or gliotransmitter release (Araque et al., 2014). This physiological response (which is better qualified as activation, see Table 2 and Section 2), will not necessarily be long lasting or associated with large-scale transcriptional changes that shift astrocyte phenotype. On the contrary, reactive astrocytes may persist over months and even years in chronic brain diseases, although they may evolve overtime.
4.5 Reversibility
Astrocyte reaction is reversible and may resolve. Manipulation of specific signaling cascades in vitro and in vivo can normalize the transcriptome of astrocytes. Whether such “de-activated” astrocytes are truly normal or are in a different state remains to be fully explored. Indeed, it is known that a given stimulus has a different effect on astrocytes, when they were previously exposed to a first injury, a process known as priming (Hennessy et al., 2015). A very elegant study recently showed that grafting reactive astrocytes in a normal mouse spinal cord is sufficient to revert them to a nonreactive state (Hara et al., 2017). Conversely (but less surprising), grafting normal astrocytes in an injured environment turns naïve astrocytes into reactive astrocytes based on both morphological and molecular criteria, showing that reactive astrocytes are plastic and regulated by environmental cues. Among the reported signals involved in maintaining a nonreactive state or resolving astrocyte reaction are fibroblast growth factor (Kang et al., 2014), β1 integrin (Hara et al., 2017; Robel et al., 2009), transforming growth factor α (Rothhammer et al., 2018), microbiome-derived tryptophan metabolites (Rothhammer et al., 2016), suppressor of cytokine signaling 3 [SOCS3, (Ceyzériat et al., 2018)], or Sonic Hedgehog [SHH, (Garcia, Petrova, Eng, & Joyner, 2010)]. Such signaling molecules offer unique opportunities to tune astrocyte reaction and evaluate the consequences on specific outcomes in different disease models (see Section 8).
5 DO ALL ASTROCYTES REACT THE SAME WAY?
As nearly all surveyed researchers noted (Table 1), the global expression “reactive astrocytes” falls short of describing the different responses that astrocytes may express in different disease conditions or even in response to the same stimulus (Table 3). As Prof. A. Messing puts it, citing Leo Tolstoy, “All happy families are alike; each unhappy family is unhappy in its own way,” suggesting that astrocytes react in a specific manner in each pathological situation. This was nicely illustrated by two-photon monitoring of astrocyte responses following focal SWI. All astrocytes proximal to the lesion became hypertrophic but only half of them polarized their processes toward the lesion, the other half remained static and only 10% of them underwent proliferation [(Bardehle et al., 2013), Figure 2h]. Likewise, a lineage tracing method was used to determine whether astrocytes derived from the same developmental clone display similar morphological changes following SWI or experimental autoimmune encephalomyelitis (EAE). In both cases, reactive astrocytes from the same clone tended to behave similarly, indicating that they are controlled by intrinsic cues established during development. However, some astrocytes reacted differently than other cells in the same clone, showing that environmental signals further diversify astrocyte response [(Bribian et al., 2018; Martin-Lopez et al., 2013), Figure 2b].
The heterogeneity of reactive astrocytes is also evidenced by spatial gradients in the intensity of response, which culminates at the core of ischemia injury, SCI, mechanical lesion, or epileptic focus (see Figure 2a for example). This is defined as topographical heterogeneity (Anderson et al., 2014) and is basically determined by the distance to the injury core. As the concentration of molecular stimuli decreases with distance to the injury core, so does astrocyte reaction, at least based on morphological changes or GFAP induction (see Table 3). As Prof. S. Sirko explains it “as in Newton's third law, the action and the reaction [of astrocytes] are equivalent in magnitude.” But, this apparent continuum in the intensity of astrocyte reaction may in fact hide different, discrete reactive states. For example, proliferative reactive astrocytes are only found proximal to the lesion [(Herrmann et al., 2008; Wanner et al., 2013), Figure 2f]. Nearly 10 years ago, Prof. M. Sofroniew described three categories of astrocyte reactivity: mild to moderate; severe diffuse, and severe with glial scar. It was a noticeable effort to better define this process and recognize its heterogeneity (Sofroniew & Vinters, 2010). This classification is based on measurable criteria (proliferation, process extension, maintenance or loss of exclusive 3D domains) but there is no absolute quantitative categories (e.g., to what extent astrocytes need to elongate their processes to fall into the mild or severe category?). This classification may thus be difficult to translate to any disease or model, and does not take advantage of objective molecular markers. Indeed, it was recently shown that reactive astrocytes that form the glial scar and lose their 3D domain have a different molecular profile than hypertrophic reactive astrocytes located farther away. “Scar-forming” astrocytes express specific transcripts such as chondroitin sulfate proteoglycans (e.g., Acan, Pcan) and N-Cadherin (Cdh2), while hypertrophic reactive astrocytes express several members of the β-catenin pathway (e.g., Ctnnb1, Plaur) (Hara et al., 2017). Interestingly, both types of reactive astrocytes overexpress Gfap and Vim compared to normal astrocytes (Hara et al., 2017). In addition, scar-forming astrocytes proliferate more than more distant hypertrophic reactive astrocytes [see Section 6, Table 3, (Wanner et al., 2013)]. Overall, these data suggest that the scar-forming astrocytes are a specific reactive astrocyte subtype and not a mere exacerbation of hypertrophic reactive astrocytes.
Other sources of heterogeneity have been described, such as regional differences, even more than 20 years ago (Table 3). Astrocytes from different CNS regions exposed to the same toxic stimulus (Amyloid β) respond differently in vitro (Hoke et al., 1994), showing that astrocyte heterogeneity is at least partially intrinsic, as it is maintained in a dish. Likewise, astrocytes from gray and white matter react differently to SWI (Mattugini et al., 2018). This could be due to preexisting differences between astrocytes from distinct brain regions (Boisvert, Erikson, Shokhirev, & Allen, 2018; Chai et al., 2017; Itoh et al., 2018; John Lin et al., 2017; Lanjakornsiripan et al., 2018; Morel et al., 2017; Torigoe, Yamauchi, Zhu, Kobayashi, & Murakami, 2015). Another demonstration of different molecular classes of reactive astrocytes came from the Barres laboratory. They reported that the molecular changes induced in astrocytes by LPS injection or MCAO only partially overlap (Zamanian et al., 2012). This led to the description of A1 and A2 reactive astrocytes, each characterized by a set of 10–13 genes, in addition to a panel of 10–13 common genes (i.e., pan reactive) including Gfap, Vimentin, and Serpina3n (Liddelow et al., 2017). The molecular profile of LPS-induced A1 reactive astrocytes was replicated quite faithfully by treating immunopanned rodent astrocytes with interleukin 1α (IL1α), complement 1q (C1q), and tumor necrosis factor (TNF) (Liddelow et al., 2017). A detailed functional characterization of A1 reactive astrocytes showed that they release factors that are toxic to neurons and oligodendrocytes, are less synaptogenic and have defective phagocytosis. But, this dual classification may be quite restrictive to define other potential types of reactive astrocytes existing in the complex world of brain diseases (Cunningham, Dunne, & Lopez-Rodriguez, 2018). Indeed, many intermediate molecular profiles were observed after treatment of immunopanned astrocytes with different molecules, not only A1 or A2 (Liddelow et al., 2017). In a complex in vivo multicellular environment with multiple stimuli, reactive astrocyte diversity may even be stronger. Both A1 and A2 genes are induced concomitantly in different models, such as mouse models of AD (Ceyzériat et al., 2018), Tauopathy (Litvinchuk et al., 2018), or ischemia (Liddelow et al., 2017).
Overall, some markers may display graded changes, proportional to the intensity of the initial injury [GFAP, cytokines, (Sofroniew, 2014a)], while others may undergo an all-or-none response, being present only in some forms of reaction [e.g., A1 versus A2 reactive astrocytes (Liddelow et al., 2017); scar-forming versus hypertrophic reactive astrocytes (Hara et al., 2017), ephrin type-B receptor 1 (EphB1) versus interleukin (IL)6-induced reactive astrocytes (Tyzack et al., 2017), Table 3].
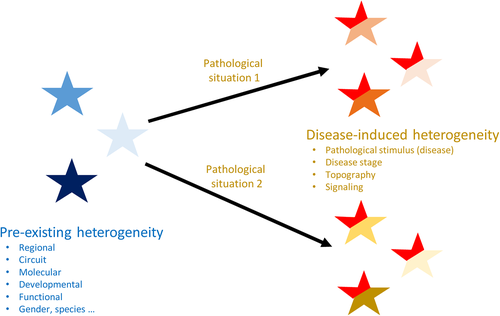
There are several origins and several manifestations of reactive astrocyte heterogeneity (Figure 3, Table 3). Indeed, as stated by Prof. A. Volterra “the combination of the intrinsic features of a given astrocytic population, of its microenvironment and the type of insult will concur to produce different types of reactivity and different functional outcomes.” More precisely, astrocyte reaction is determined by core changes (induction of Gfap expression, morphological plasticity), combined with disease-specific changes on a preexisting heterogeneous background (Figure 3), resulting in an extreme variety of possible reactive astrocyte subtypes. This leads us to the next question, very central to the field.
6 WHAT ARE THE APPROPRIATE MARKERS OF REACTIVE ASTROCYTES?
As discussed earlier, GFAP overexpression and morphological changes are the most commonly used reactive astrocyte markers (Liddelow & Barres, 2017). Both are easy to monitor on different types of samples (see Figure 2, Table 3). Gfap is one of the most consistently induced gene in transcriptomic datasets of reactive astrocytes, confirming its usefulness as a reactive marker [(Hol & Pekny, 2015; Liddelow et al., 2017; Orre, Kamphuis, Osborn, Jansen, et al., 2014; Zamanian et al., 2012), see also Table 3 and Section 4]. It is important to note, however, that the level of Gfap induction can be very different between conditions (see Table 3 and Section 5) or even between cells (Pekny, Wilhelmsson, Tatlisumak, & Pekna, 2019), but GFAP expression globally increases at the population level, in a wide range of brain diseases.
Are GFAP induction and morphological changes enough to identify reactive astrocytes? Several surveyed PIs think that these indexes can be misleading (Table 1). Indeed, additional markers would be helpful with some specific forms of reactive astrocytes that display unconventional morphological alterations or lower GFAP immunoreactivity (see Section 9). In addition, they would be useful to define specific types of reactive astrocytes. Genome-wide transcriptional profiling can identify potential common and class-specific genes (Hara et al., 2017; Liddelow et al., 2017). How many markers are needed to define a class? For example, of the ~10 genes forming the A1 and A2 panels, should they all be induced in a given condition to qualify a cell as an A1 reactive astrocyte or 2–3 are enough? In fact, the expression of only a few protein markers (Complement 3, Complement factor b, and MX dynamin-like GTPase 1) was tested in the brain of patients and these three genes were not in the original A1 panel [(Liddelow et al., 2017), Figure 2t,u]. When the full panel of A1 genes is tested, not every single gene of the A1 cassette is induced in several diseases, like in vitro and in vivo models of Parkinson disease (Yun et al., 2018) or AD mouse models (Ceyzériat et al., 2018). In addition, individual reactive astrocytes co-expressing A1 and A2 genes are observed in the MCAO rodent model and in the aging mouse brain (Clarke et al., 2018), suggesting that it may be difficult to define classes of reactive astrocytes with only a few genes.
To improve the panel of reactive astrocyte molecular markers, it would be useful to extend the transcriptomic analysis of reactive astrocytes performed by Zamanian et al. (2012) in LPS and MCAO models, to many different, genetic and sporadic, acute and chronic, degenerative and inflammatory diseases. It would help define with greater power the core sets of genes systematically induced or down regulated in reactive astrocytes, and identify disease-specific markers. Of course, such analysis would provide even more insight if performed on human samples. However, post mortem delays induce noise and artifacts and the physical isolation of astrocytes is quite difficult, although it may be possible to gain some insight into astrocyte-specific transcriptomic changes by co-expression analysis on bulk samples (Kelley, Nakao-Inoue, Molofsky, & Oldham, 2018). A method of choice to study cell diversity at the molecular level is single-cell RNAseq (scRNAseq) (Svensson, Vento-Tormo, & Teichmann, 2018) or single-nuclei RNAseq (Habib et al., 2017). These methods have gained significant momentum, and several landmark papers have reported brain cell heterogeneity, including glial cells, throughout development or between regions (Macosko et al., 2015; Pollen et al., 2014; Zeisel et al., 2018). There are only few articles on brain diseases, and to date, only on other glial cells like microglia (Hammond et al., 2018; Masuda et al., 2019). Such unsupervised approaches will help define populations of reactive astrocytes with associated gene markers in different conditions (see Section 13).
Another option to classify reactive astrocytes is to combine molecular with functional indexes. A good example is the variable capacity of reactive astrocytes to proliferate. As mentioned earlier, only a subset of reactive astrocytes undergoes cell division. These proliferative astrocytes may thus be considered as a different class, of particular interest because they may repopulate the damaged brain (see Section 10). But other specific functions (either gained, lost or altered, see Section 8) may also serve as functional markers of reactive astrocytes, like enhanced phagocytic capacities around plaques, as shown for “disease associated microglia” (Keren-Shaul et al., 2017). For example, only a fraction of reactive astrocytes phagocyte myelin debris in the brain of patients with MS or leukoencephalopathy [(Ponath et al., 2017), Figure 2n]. A classification based on production of γ-amino-butyric acid (GABA) and brain-derived neurotrophic factor by reactive astrocytes was proposed by Prof. C. J. Lee [see Table 1, and Supplemental Text, (Chun et al., 2018)], since reactive astrocytes produce more GABA in AD (Jo et al., 2014; Wu, Guo, Gearing, & Chen, 2014). This is a potentially interesting functional classification of reactive astrocytes, but it needs to be further explored, to evaluate whether this is translatable to multiple diseases, animal models, and of course patients. For example, contrary to AD models, astrocyte GABA production is reduced in a mouse model of Huntington disease [HD, (Jo et al., 2014; Wojtowicz, Dvorzhak, Semtner, & Grantyn, 2013)]. Importantly, a good marker needs to be easy to use and compatible with other exploratory techniques like electrophysiology or functional imaging. Therefore, mRNA and protein markers remain favored options.
A problem that may be very difficult to overcome is the dynamic, flexible, and context-dependent phenotype of astrocytes. It was nicely discussed for astrocytes in physiology (Poskanzer & Molofsky, 2018), and it may even be exacerbated in disease, when astrocytes express gene markers of other cell types and cell identities become blurrier (see Section 10). It may thus be impossible to establish fixed classes and we rather should try to define states, dependent on previous phenotype, activated signaling, and environment (see Figure 3). The ambitious Human Cell Atlas project that aims to define all cells in the human body with a range of single-cell approaches will probably provide the astrocyte field with new tools and concepts to better address the challenge of reactive astrocyte classification.
7 WHAT ARE THE MOLECULAR CASCADES TRIGGERED IN REACTIVE ASTROCYTES?
First, astrocytes sense a pathological signal. This signal can be extracellular (e.g., cytokines, purines, aggregated proteins, and myelin debris), trans-cellular (e.g., transmembrane adhesion molecules like ephrins or integrins), membrane bound [e.g., phosphatidylserine (Chung et al., 2013)], as well as intracellular (e.g., aggregated proteins, nucleic acids from infecting pathogens, ions like Ca2+), [see (Buffo, Rolando, & Ceruti, 2010; Cunningham et al., 2018; Kang & Hebert, 2011; Sofroniew, 2014a) for review]. Interestingly, a recent study reported that astrocytes are also very sensitive to environmental pollutants (Wheeler et al., 2019). To sense all these molecular triggers, astrocytes are equipped with a wide range of membrane or intracellular receptors, including G Protein-coupled receptors (like metabotropic glutamate or P2Y purinergic receptors), ionotropic receptors (e.g., P2X purinergic receptors), multimeric cytokine receptors (e.g., IL6 family receptors), Toll like receptors [although their expression by astrocytes is quite disputed (Cunningham et al., 2018)], or tyrosine kinase receptors (e.g., Epidermal growth factor receptor), [see (Kang & Hebert, 2011; Verkhratsky & Nedergaard, 2018) for review]. It is important to note, however, that there is rarely a direct and formal demonstration that each of these stimuli can trigger a full reactive program (i.e., resulting in morphological as well as complex transcriptional changes), without the involvement of other cell types like microglia. Indeed, this is typically tested by applying high doses of these compounds in vitro or through injection or overexpression in vivo. It is therefore difficult to know whether they activate astrocytes directly or through microglial cells (see Section 11). Interestingly, astrocytes are also mechano-sensitive. They can detect changes in their environment mechanical properties, discriminate soft from stiff material, and adjust by changing their own stiffness (Moeendarbary et al., 2017; Moshayedi et al., 2014).
After stimulus sensing, a step of signal transduction takes place and converge to the nucleus. This can occur through direct shuffling of activated down-stream transcription factors to the nucleus after phosphorylation, dephosphorylation, or release from inhibitors. Signal transducer and activator of transcription 3 (STAT3), nuclear factor κB (NF-κB), and nuclear factor of activated T-cells (NFAT), the downstream effectors of the Janus kinase (JAK)-STAT3, NF-κB, and calcineurin-NFAT pathways respectively, are regulated by such a mechanism (Ceyzériat, Abjean, Carrillo-de Sauvage, Ben Haim, & Escartin, 2016; Sompol & Norris, 2018; Zhang, Lenardo, & Baltimore, 2017). Many signaling cascades are associated with astrocyte reaction (Ben Haim, Carrillo-de Sauvage, Ceyzeriat, & Escartin, 2015; Buffo et al., 2010; Kang & Hebert, 2011), but the STAT3 pathway seems to play a prominent role in different disease conditions, acting as a master regulator of reactive astrocytes [(Ben Haim, Ceyzeriat, et al., 2015; Ceyzériat et al., 2016; Herrmann et al., 2008), Figure. 2o]. Ca2+ signaling may also be involved in astrocyte reaction. Ca2+ can activate or inhibit many downstream signaling intermediates like the phosphatase calcineurin (Sompol & Norris, 2018) or the transcriptional repressor Pumilio 2 (Kanemaru et al., 2013), which will activate other downstream pathways like the NFAT or N-cadherin pathways, respectively, and trigger important transcriptional changes.
Activation of specific transcription factors will induce expression of target genes such as Gfap or cytokines but also of transcription factors or retro-inhibitors of other pathways (e.g., SOCS3 for the JAK-STAT3 pathway or IκB for the NF-κB pathway), which will further shape the transcriptome of reactive astrocytes. This step may involve successive waves of transcriptional regulation, starting with induction of immediate early genes (Jenab & Quinones-Jenab, 2002; Priller, Reddington, Haas, & Kreutzberg, 1998; Wu, Pan, Zuo, Li, & Hong, 2017), followed by subsequent waves of gene induction. The transcription of some genes may be repressed. Indeed, the number of down-regulated genes may even be higher than those over-expressed, as observed AD mouse astrocytes (Orre, Kamphuis, Osborn, Jansen, et al., 2014). Importantly, the precise time course of transcriptional induction is not very well known. It was established for specific transcripts or proteins (mostly GFAP and cytokines) in mice exposed to neurotoxins (O'Callaghan et al., 2014) or LPS (Biesmans et al., 2015; Norden, Trojanowski, Villanueva, Navarro, & Godbout, 2016). Transcriptional profiling of astrocytes was performed in a mouse model of epilepsy, in two brains regions and three time points following pilocarpine injection (Clasadonte et al., 2016). This extensive analysis showed subtle differences between disease stages or brain regions (see Table 3). Such genome-wide, longitudinal analysis of relevant in vivo models of other brain diseases would provide key insight into the temporal regulation of astrocyte reaction.
Some changes observed in reactive astrocytes do not require transcriptional induction, such as morphological plasticity. The molecular mechanisms underlying astrocyte morphological changes and process motility are mostly studied in vitro, where these cells are grown in 2D and usually have a much simpler morphology (sometimes even no processes) [see (Schiweck, Eickholt, & Murk, 2018), for review]. But in vivo, reactive astrocytes do not migrate [(Bardehle et al., 2013), Section 2] and show little motility of main processes compared to microglia (Nimmerjahn et al., 2005), calling for in vivo validation of the signaling described in vitro. For example, knockout of the Rho GTPase cdc42 reduces astrocyte polarization in vitro in the scratch wound assay, while it exacerbates it after SWI in vivo (Robel, Bardehle, Lepier, Brakebusch, & Gotz, 2011). As mentioned earlier (Section 3), reactive astrocytes not only present hypertrophy, but also subtle morphological changes. For example, reactive astrocytes retract their processes following stimulation of Slit-Robo signaling by neuroblasts migrating from the subventricular zone after stroke. This cdc42-dependent remodeling of astrocyte cytoskeleton facilitates neuroblast migration toward lesion sites (Kaneko et al., 2018).
Less is known about mechanisms of epigenetic regulation in reactive astrocytes in disease. Based on what happens during astrocyte development, it is suspected that epigenetic processes (chromatin remodeling through DNA methylation or histone posttranslational modifications, as well as expression of regulatory microRNA) could influence astrocyte conversion into reactive cells (Neal & Richardson, 2018).
The profound transcriptional changes occurring in reactive astrocytes translate into functional alterations that may impact virtually all astrocyte functions.
8 DO REACTIVE ASTROCYTES DO GOOD THINGS?
Reactive astrocytes are the usual suspects in many diseases, but they are now considered by many, as beneficial partners for neurons, as illustrated in Table 1. An overview of the literature shows that depending on the strategy employed to modulate reactive astrocytes (e.g., pharmacological or genetic approaches, targeting of astrocyte proteins or signaling pathways, ablation of astrocytes), the outcomes vary (Ben Haim, Carrillo-de Sauvage, et al., 2015; Cunningham et al., 2018). Of course, the disease studied and its model itself (e.g., transgenic versus knock-in, in vivo versus in vitro, species) will influence how astrocyte reaction will play out. More importantly, some models do not reproduce astrocyte reaction well. For example, most HD mouse models do not display reactive astrocytes, while GFAP upregulation is robustly detected in the caudate-putamen of HD patients (Faideau et al., 2010; Selkoe, Salazar, Abraham, & Kosik, 1982; Vonsattel et al., 1985). This could be due to low GFAP expression in the mouse striatum compared to other brain regions (Chai et al., 2017), significant species differences (Oberheim et al., 2009; Zhang et al., 2016) or a simple failure of mouse models to recapitulate all HD features.
How do reactive astrocytes functionally impact the diseased brain? Virtually all astrocyte functions are reported to be altered in disease: neurotransmitter uptake (Escartin et al., 2006; Sheldon & Robinson, 2007), gliotransmitter release (Jo et al., 2014; Wu et al., 2014), metabolic activity (Escartin et al., 2007; Gavillet, Allaman, & Magistretti, 2008; Valenza et al., 2010), ion buffering (Tong et al., 2014), release of cytokines, complement factors or trophic factors (Chou et al., 2008; Lian et al., 2015; Sofroniew, 2014b), phagocytosis (Gomez-Arboledas et al., 2018; Liddelow et al., 2017; Morizawa et al., 2017), production, and detoxification of reactive oxygen species [ROS, (Allaman et al., 2010; Cassina et al., 2008; Ye et al., 2015)]. These functions may be enhanced or reduced by the reactive state. For example, reactive astrocytes may display enhanced or reduced connectivity through gap junctions, depending on the disease (Escartin & Rouach, 2013; Pannasch & Rouach, 2013). They may display deficits in glutamate uptake (Sheldon & Robinson, 2007), or show enhanced uptake capacity when reaction is induced by the cytokine ciliary neurotrophic factor (CNTF) (Escartin et al., 2006). Reactive astrocytes may release more GABA in AD mice (Jo et al., 2014; Wu et al., 2014) or do the opposite in HD mice (Wojtowicz et al., 2013).
It is beyond the scope of this review to list all their described functional changes and contributions to specific diseases. Readers are referred to recent reviews on astrocytes in HD (Khakh et al., 2017), AD (Chun & Lee, 2018; Osborn, Kamphuis, Wadman, & Hol, 2016; Perez-Nievas & Serrano-Pozo, 2018), MS (Wheeler & Quintana, 2019), SCI (Adams & Gallo, 2018; Sofroniew, 2018), TBI (Burda, Bernstein, & Sofroniew, 2016), stroke (Pekny et al., 2019), and epilepsy (Coulter & Steinhauser, 2015; Robel & Sontheimer, 2016). In general, deficits in normal astrocyte functions are reported and the existence of “killer astrocytes” releasing toxic molecule(s) was even proposed in amyotrophic lateral sclerosis [ALS, (Haidet-Phillips et al., 2011; Nagai et al., 2007)] and other neurodegenerative diseases [(Liddelow et al., 2017), see Section 5]. But, there is also strong evidence for beneficial reactive astrocytes in acute or chronic diseases. For example, over the years, the Sofroniew laboratory has convincingly demonstrated that scar-forming reactive astrocytes demarcate damaged tissue (Bush et al., 1999; Faulkner et al., 2004) and even promote axonal regrowth through the lesion (Anderson et al., 2016). Likewise, STAT3-dependent reactive astrocytes reduce degeneration of axotomized motoneurons in the facial motor nucleus, and promote synapse maintenance through thrombospondin 1 release (Tyzack et al., 2014). CNTF-induced reactive astrocytes display enhanced glutamate uptake, promote metabolic resilience, and protect neurons against excitotoxicity (Beurrier et al., 2010; Escartin et al., 2006; Escartin et al., 2007). Even A1 neurotoxic reactive astrocytes may have some beneficial roles in specific diseases, for example by killing damaged, dysfunctional, or infected neurons (Liddelow et al., 2017).
A way to study common functional features of reactive astrocytes is to activate (or inhibit) a specific signaling pathway to trigger reactive conversion (see Section 8). For example, JAK-STAT3 pathway activation in astrocytes induces many reactive markers and is sufficient to alter synaptic transmission and plasticity in the hippocampus (Ceyzériat et al., 2018). Activation of the NF-κB pathway by expression of a constitutive form of the upstream IκB kinase in adult astrocytes also induces a reactive profile and Purkinje cell loss in the cerebellum (Lattke et al., 2017; Oeckl, Lattke, Wirth, Baumann, & Ferger, 2012). Such approaches are useful to isolate functional changes in reactive astrocytes, independently of a specific disease condition. Alternatively, blocking astrocyte reaction may help uncover the net effect of reactive astrocytes in each disease context [see (Ben Haim, Carrillo-de Sauvage, et al., 2015)]. This requires an efficient strategy that (a) only impacts the reactive state and not normal astrocyte functions, (b) does not affect other cell types, and (c) inhibits all (or only the targeted) classes of reactive astrocytes. With new and maybe more selective molecular markers, it will be important to extensively validate the efficiency and universality of such methods and determine how these “de-activated” astrocytes are different from nonreactive astrocytes.
Overall, the range of functional changes displayed by reactive astrocytes and their effects on disease are extremely large. They are governed by the preexisting astrocyte molecular and functional profile, in addition to the pathological trigger itself (Figure 3, Section 5). Indeed, even reactive astrocytes activated by the same pathway can have different effects on specific disease outcomes. STAT3 signaling in reactive astrocytes has detrimental effects in mouse models of AD (Ceyzériat et al., 2018; Reichenbach et al., 2019), or hypoxia (Hristova et al., 2016), no significant effect in the context of acute mitochondria toxicity (O'Callaghan et al., 2014) but has beneficial effects in neonatal white matter injury (Nobuta et al., 2012), after SCI, by promoting glial scar formation (Anderson et al., 2016; Herrmann et al., 2008; Okada et al., 2006), and in HD models, by reducing mutant Huntingtin aggregation (Ben Haim, Ceyzeriat, et al., 2015). Intriguingly, a recent study by Tyzack et al. (2017) suggested that the upstream activator (IL6 or EphB1-ephrine-B1 signaling) controls the final molecular profile induced by STAT3 in reactive astrocytes (Table 3).
It is now important to define what reactive astrocytes do in each situation and the underlying signaling cascades, before targeting these cells for therapy. There are new opportunities and yet many challenges to use reactive astrocytes for therapeutic purposes.
9 DO REACTIVE ASTROCYTES EVENTUALLY DIE?
There may be extreme forms of responses leading to the loss of key astrocyte proteins and functions or even their death. Astrocytes are considered more resilient than neurons, but they may degenerate as well. As seen with TUNEL or caspase stainings, astrocytes may undergo apoptotic cell death in ischemia (Giffard & Swanson, 2005), in ALS mice (Rossi et al., 2008), or in the brain of AD patients (Perez-Nievas & Serrano-Pozo, 2018). Alternatively, mouse astrocytes may die by necrosis at the infarct core after stroke (Lukaszevicz et al., 2002).
Major alterations of astrocyte morphology, including severe process atrophy or fragmentation, swollen or vacuolated cell bodies are described in brain samples from patients with AD, stroke or infection, for example, and it is called clasmatodendrosis [see references in (Perez-Nievas & Serrano-Pozo, 2018; Tachibana et al., 2019)]. It is also reproduced in animal models of these diseases (Olabarria et al., 2010; Sullivan, Bjorkman, Miller, Colditz, & Pow, 2010). In addition, astrocytes may be directly or primarily targeted by the disease process, like in Alexander disease caused by Gfap mutation (Messing, 2018) or neuromyelitis optica caused by autoantibodies against the astrocyte protein aquaporin 4 [see (Verkhratsky, Zorec, & Parpura, 2017) for a complete review].
Interestingly, in a mouse model of tauopathy (Bussian et al., 2018) and in AD patients (Bhat et al., 2012), astrocytes express markers of cellular senescence (p16INK4a and β-galactosidase). Genetic ablation of senescent cells prevents tau pathology and cognitive decline (Bussian et al., 2018), suggesting that they have deleterious effects. It is not possible, however, to know whether senescent astrocytes are responsible alone or if senescent microglia also play a role. Interestingly, interleukins IL6 and IL1β are classic senescence markers (Salminen et al., 2011) and senescent astrocytes in AD brains express high levels of GFAP (Bhat et al., 2012), suggesting that senescence could be yet another specific reactive state.
Overall, such atypical astrocyte responses characterized by major loss of key homeostatic astrocyte proteins, senescence and/or altered morphology will need to be further characterized by genome wide and refined temporal analysis to really qualify as a form of astrocyte reaction. Do astrocytes first become reactive and then adopt this dysfunctional, senescent phenotype and die, or these are distinct nonoverlapping processes?
10 WHERE DO REACTIVE ASTROCYTES COME FROM OR WHAT DO THEY BECOME?
Reactive astrocytes are in a very distinct state from astrocytes in physiological conditions. Thus, one may wonder whether reactive astrocytes (a) can further transform into other cell types and (b) come only from the conversion of a nonreactive astrocyte. Both questions have important therapeutic implications, especially if reactive astrocytes are able to generate new neurons.
As mentioned earlier, only a subset of reactive astrocytes proliferates (e.g., juxta-vascular or scar-forming astrocytes), they only undergo one round of division and invariably generate two sister astrocyte cells (see Section 4). Strikingly, the in vitro situation is quite different. In a culture medium promoting cell proliferation, acutely isolated reactive astrocytes are able to generate different cell types, including neurons (Gotz, Sirko, Beckers, & Irmler, 2015). It shows that some permissive signals are lacking in the brain environment [like SHH signaling, (Sirko et al., 2013)] or conversely, that restrictive factors prevent astrocytes from returning to a multipotent state and differentiating into neurons [like Notch signaling, (Magnusson et al., 2014)]. Significant efforts are made to identify and target such factors, to promote the neurogenic potential of reactive astrocytes and their reconstruction of damaged neuronal circuits [for a complete review, see (Lei, Li, Ge, & Chen, 2019)].
Regarding the origin of reactive astrocytes, scar-forming reactive astrocytes were proposed to come from other proliferating cell types, like progenitors cells (Benner et al., 2013; Faiz et al., 2015) or ependymal cells (Sabelstrom et al., 2013). Fate mapping by Cre-reporters or viral targeting suggests that this phenomenon is marginal, with the majority of reactive astrocytes originating from previously labeled astrocytes (Buffo et al., 2008; Ren et al., 2017), but it may be insult-dependent and region-dependent (see Gotz et al., 2015, for a complete discussion).
These two questions are intimately linked to cell identity. At present, there is no single univocal marker for brain cell types [as discussed for reactive astrocytes in Section 6, see (Gotz et al., 2015), for a complete discussion]. For example, GFAP is also expressed by progenitor cells. A type of thalamic glial cell expresses both Connexin43 and Olig2, which are typical astrocyte and OPC markers, respectively (Griemsmann et al., 2015). Such blurred cell identities are even exacerbated in disease. Reactive astrocytes express nestin and other makers of neural stem cells (Gotz et al., 2015). Likewise, transcriptomic studies reveal that reactive astrocytes overexpress many genes characteristic of microglia (e.g., Cst7, Trem2, and Cts) (Ceyzériat et al., 2018; Orre, Kamphuis, Osborn, Jansen, et al., 2014). Indeed, cells co-expressing astrocyte and microglia markers are observed in the spinal cord of an ALS rat model (Trias et al., 2013), the deafferented mouse hippocampus or the brain of AD, Lewy body dementia, or stroke patients (Wilhelmsson et al., 2017). Moreover, reactive astrocytes may lose some of their defining functional features like input resistance, membrane currents, and gap-junction coupling, as described in human brains and in a mouse model of sclerotic mesial temporal lobe epilepsy (Bedner et al., 2015). They may also express lower levels of key astrocyte proteins, as discussed earlier (Sections 4 and 9), and as shown after mild repetitive TBI with loss of GLT1, GS, KIR4.1, and even GFAP (Shandra et al., 2019). This will complicate their identification as astrocytes.
When astrocytes become reactive, they undergo profound transformations, losing their original features and “disguising” as other cells. Refined fate mapping methods with specific drivers and tight time control will be valuable to trace the origin and future of single astrocytes in disease.
11 HOW DO REACTIVE ASTROCYTES INTERACT WITH OTHER INNATE IMMUNE CELLS?
The dialogue between reactive astrocytes and other cellular partners involved in inflammation and immunity (e.g., microglia, macrophages, and lymphocytes) is quite complex. It is generally admitted that microglial cells react first to many injury signals, in particular danger-associated and pathogen-associated molecular patterns, and then activate astrocytes through cytokine release (Sofroniew, 2014b). There are several reports of microglial cells driving astrocytes toward a specific reactive state. LPS-activated microglia produce IL1α, C1q, and TNF and induce A1 reactive astrocytes (Liddelow et al., 2017). But, microglia made reactive by methotrexate chemotherapy induce astrocytes to express A2 genes, not A1 (Gibson et al., 2019). Alternatively, when they release vascular endothelial growth factor B during EAE, microglial cells induce a possibly different deleterious type of reactive astrocyte, which needs to be fully characterized (Rothhammer et al., 2018). In TBI, microglia drives a beneficial form of astrocyte reaction through down-regulation of P2Y1 receptors (Shinozaki et al., 2017).
Overall, these studies suggest that microglia dictate the profile of reactive astrocytes, but it may go the other way around. Indeed, reactive astrocytes overexpress many cytokines, chemokines, and signaling molecules that could activate neighboring microglial cells [(Ceyzériat et al., 2018; Orre, Kamphuis, Osborn, Jansen, et al., 2014; Zamanian et al., 2012), see Figure 2l]. Astrocyte-specific NF-κB activation induces significant astrocyte reaction that secondarily causes microglia activation (Oeckl et al., 2012; Ouali Alami et al., 2018). Accordingly, astrocytes may become strongly reactive while microglia is barely affected after viral infection (Ortinski et al., 2010), or CNTF exposure (Lavisse et al., 2012), showing that astrocytes have the ability to become reactive on their own.
Reactive astrocytes may also interact with peripheral immune cells. A recent study showed that regulatory T cells reduce astrocyte reaction (including A1 markers) after stroke, through amphiregulin, IL6, and STAT3 signaling (Ito et al., 2019). Alternatively, phospho-STAT3+ reactive astrocytes found around brain metastatic cells reduce CD8+ lymphocyte recruitment and increase the number of CD74+ macrophage/microglia, which are more permissive to metastatic proliferation [(Priego et al., 2018), Figure 2r]. Proliferating, juxta-vascular reactive astrocytes were also shown to inhibit monocyte infiltration following SWI (Frik et al., 2018). Using reporters to track expression of two genes typical of different phagocyte states, Locatelli et al. (2018) also showed that astrocytes release factors that convert polarized phagocytes expressing inducible nitric oxide synthase into arginase-expressing cells in vitro.
Overall, reactive astrocytes engage in complex bidirectional communications with other immune cells. It remains to be determined where and when these interactions take place, as peripheral immune cells entry into the brain is tightly regulated. Microglial cells and peripheral immune cells themselves show heterogeneous phenotypes, adding yet another layer of complexity to understand the role of reactive astrocytes in disease (Song & Colonna, 2018).
12 ARE AGING ASTROCYTES A TYPE OF REACTIVE ASTROCYTES?
The human aging brain displays higher GFAP immunoreactivity and mRNA levels [see references in (Munger et al., 2019)], and it was assumed that aging causes progressive astrocyte reaction. But, this idea is not consensual (see Table 1). It is possible, for example, that the analyzed subjects were exposed to some pathological stimuli (mild trauma, micro-infarcts, inflammation, or infection) that caused GFAP increase, not aging itself. Again, transcriptomics studies in mice provide more comprehensive insight into the changes occurring in astrocytes with aging. Results showed that aging astrocytes express higher levels of genes linked to immunity and inflammation (Boisvert et al., 2018; Orre et al., 2014). Another study showed that 2-year-old astrocytes express several pan and A1 genes at higher levels than young astrocytes (Clarke et al., 2018). Aging astrocytes also display reduced expression of some genes linked to cholesterol or ROS detoxification (Boisvert et al., 2018; Clarke et al., 2018). But overall, the molecular changes occurring in aging mouse astrocytes are rather limited. Interestingly, there are regional differences, with larger variations in hippocampal and striatal astrocytes than in their cortical counterparts, even if Gfap is induced in all brain regions (Boisvert et al., 2018; Clarke et al., 2018). A genome-wide study in Humans also evidenced significant, region-dependent changes in astrocyte-specific genes with aging (Soreq et al., 2017).
When analyzed in a healthy aging brain, astrocytes display mild reactive changes. However, aging astrocytes have a high probability to have encountered previous pathological conditions in their lifetime, and they may thus be in a primed state that will change their subsequent response (Cunningham et al., 2018). Astrocyte reaction and possible priming in the aging brain need to be thoroughly analyzed, as aging is a major risk factor for several brain diseases.
13 WHICH NEW APPROACHES AND TOOLS WILL MOVE THE FIELD FORWARD?
There are now many techniques and models to study astrocytes, some mentioned in the previous paragraphs [e.g., new transgenic mice, purification methods, and human induced pluripotent stem cells (iPSCs)-derived astrocytes (Almad & Maragakis, 2018; Guttenplan & Liddelow, 2019)]. In addition, refined cellular and molecular methods developed in other fields could prove very useful to study reactive astrocytes like spatial transcriptomics (Rodriques et al., 2019; Wang et al., 2018), scRNAseq (Svensson et al., 2018), Cas9 genome editing and screening (Shalem, Sanjana, & Zhang, 2015), and multiplexed immunostainings (Goltsev et al., 2018). Miniaturization (in terms of quantity of input sample), extension to large brain regions and multiplexing to hundreds or thousands of target genes or proteins will achieve unprecedented resolution to decipher how astrocytes react in a given disease context and define better molecular markers (see Section 6). But in the end, it is the function that matters. How will these molecularly defined reactive astrocytes perform their normal functions? This is easier tested in vitro where several functions can be quickly screened, [e.g., phagocytosis, glutamate uptake, catabolism of specific metabolites, production of cytokines/trophic factors/complement factors, toxicity towards other cell types, and effect on synapses, (Diaz-Amarilla et al., 2011; Escartin et al., 2007; Liddelow et al., 2017; G. E. Tyzack et al., 2014)]. It may be trickier to explore astrocyte functions in vivo or at least in ex vivo settings like acute brain slices or acutely dissociated astrocytes. It is important to combine functional assessment with some morphological or molecular markers to ascertain that probed astrocytes are indeed reactive and ideally provide some insight into their molecular features. Therefore, methods with cellular resolution like electrophysiology combined with fluorescent detection of markers, multidimensional cytometry, and two-photon imaging with functional sensors are methods of choice to establish how specific types of reactive astrocytes function. Several elegant studies recently succeeded in providing new insight into the complexity of astrocyte functions in the normal brain (Bindocci et al., 2017; Chung et al., 2013; Martin, Bajo-Graneras, Moratalla, Perea, & Araque, 2015; Nimmerjahn & Bergles, 2015) and in disease (Bardehle et al., 2013; Delekate et al., 2014; John Lin et al., 2017; Yu et al., 2018). Future studies will need to implement such refined methods in relevant disease models, to study reactive astrocyte responses at the single-cell level.
Importantly, it is now clear that human astrocytes are more complex than their rodent counterparts and express specific genes (Oberheim et al., 2009; Y. Zhang et al., 2016). In vitro, human and mouse astrocytes are reactive to different stimuli and overexpress different genes [(Tarassishin et al., 2014), Table 3]. It is thus important to eventually go back to human samples to confirm findings obtained in animal models (Arranz & De Strooper, 2019). Patient brains are a valuable resource but with associated bias due to post mortem delay, comorbidities, and other confounding factors. Derivation of human astrocytes from iPSCs taken from patients is opening new research opportunities to probe disease-specific changes (Tyzack, Lakatos, & Patani, 2016). However, the study of reactive astrocytes may be particularly difficult, as iPSC-derived astrocytes (and cultured astrocytes in general) tend to be already reactive, especially when exposed to serum. It is thus required to optimize differentiation and culture protocols to obtain a mature phenotype, reactive to subsequent stimulation (Perriot et al., 2018). It will also be important to define which subtype of reactive astrocytes is modeled and to confirm that this subtype truly exists in vivo.
Research on astrocytes is at an exciting stage, with potent approaches, new models, and refined concepts to study their role in the normal and diseased brain.
14 CONCLUDING REMARKS
It is now becoming obvious that broad and quite old expressions like Astrocyte reactivity or Astrogliosis hide in fact a striking complexity with subtle regulations. Our survey highlights that several aspects of astrocyte reaction are still unclear or even controversial, starting with their very name. But, there is a strong interest in better understanding this specific transformation of astrocytes in response to a pathological stimulus. Reactive astrocytes may have significant impact on disease progression, diagnosis, or even treatment. Therefore, we should now aim to disentangle this widespread and complex brain response to disease and define which aspects are amenable to therapeutic improvement.
ACKNOWLEDGMENTS
We are extremely grateful to all researchers who positively replied to our request and provided their own definition of reactive astrocytes. We thank them for their valuable input and support. By alphabetical order: Nicola Allen, Alfonso Araque, Luis Barbeito, Dwight Bergles, Giorgio Carmignoto, Colm Cunningham, Marc Freeman, Elena Galea, Vittorio Gallo, Magdalena Götz, Philip Haydon, Helmut Kettenmann, Schuichi Koizumi, Andras Lakatos, C. Justin Lee, Shane Liddelow, Albee Messing, Keith Murai, Christopher Norris, James O'Callaghan, Seiji Okada, Stéphane H. R. Oliet, Aude Panatier, Luc Pellerin, Gertrudis Perea, Gabor Petzold, Frank Pfrieger, Stefanie Robel, David Rowitch, Alberto Serrano-Pozo, Swetlana Sirko, Michael Sofroniew, Harald Sontheimer, Christian Steinhauser, Raymond Swanson, Alexei Verkhratsky, and Andrea Volterra. Their full affiliation and reply are provided as Supplemental. We apologize for not being able to cite all original studies relevant to this review, due to space limitations. We thank Prof. C. Barcia, Dr. E. Hernández-Garzón, Dr. S. Liddelow, Prof. M. Sofroniew, Dr. M. Valiente, and Prof. A. Verkhratsky for providing original images for Figure 2. We thank Dr. Lucile Ben Haim for her critical review of the manuscript. Our team is supported by CEA, CNRS, and grants from the French National Research Agency (Grants # 2010-JCJC-1402-1, 2011-BSV4-021-03, and ANR-16-TERC-0016-01), from Fondation Vaincre Alzheimer (Grant # FR-15015), Fédération pour la Recherche sur le Cerveau and Association Huntington France. O.G. is a recipient of a doctoral fellowship from the CEA.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
C.E. wrote the review, performed the survey, and generated Table 1, Table 2, and Figure 3. O.G. generated Table 3. M.A.C.S. produced Figures 1 and 2. All authors edited and finalized the review.