Open chromatin landscape of rat microglia upon proinvasive or inflammatory polarization
Funding information Narodowe Centrum Nauki, Grant/Award Number: 2013/09/B/NZ2/03170
Abstract
Microglia are brain-resident, myeloid cells that play important roles in health and brain pathologies. Herein, we report a comprehensive, replicated, false discovery rate-controlled dataset of DNase-hypersensitive (DHS) open chromatin regions for rat microglia. We compared the open chromatin landscapes in untreated primary microglial cultures and cultures stimulated for 6 hr with either glioma-conditioned medium (GCM) or lipopolysaccharide (LPS). Glioma-secreted factors induce proinvasive and immunosuppressive activation of microglia, and these cells then promote tumor growth. The open chromatin landscape of the rat microglia consisted of 126,640 reproducible DHS regions, among which 2,303 and 12,357 showed a significant change in openness following stimulation with GCM or LPS, respectively. Active genes exhibited constitutively open promoters, but there was no direct dependence between the aggregated openness of DHS regions near a gene and its expression. Individual regions mapped to the same gene often presented different patterns of openness changes. GCM-regulated DHS regions were more frequent in areas away from gene bodies, while LPS-regulated regions were more frequent in introns. GCM and LPS differentially affected the openness of regions mapped to immune checkpoint genes. The two treatments differentially affected the aggregated openness of regions mapped to genes in the Toll-like receptor signaling and axon guidance pathways, suggesting that the molecular machinery used by migrating microglia is similar to that of growing axons and that modulation of these pathways is instrumental in the induction of proinvasive polarization of microglia by glioma. Our dataset of open chromatin regions paves the way for studies of gene regulation in rat microglia.
1 INTRODUCTION
Rat is the primary model organism for toxicology and pharmacology research and the organism of choice for many behavioral, immunology, and tumor biology studies (Aitman et al., 2008; Shimoyama et al., 2016). Despite the popularity of mouse models, the use of rats as a model organism remains remarkably stable, comparable with that of mice and several-fold greater than for any other model organism (Dietrich, Ankeny, & Chen, 2014).
The choice of humans and mice as the two model organisms of the ENCODE consortium concentrated efforts on these two species (ENCODE Project Consortium, 2012; Yue et al., 2014). However, valuable transcriptomics data are also being generated in other model organisms, most notably rat. Realization of the full potential of such data for the elucidation of gene regulatory mechanisms requires matching of transcriptome data with experimentally identified cis-regulatory regions, such as open chromatin regions, which can be identified with a number of methods, including the identification of DNaseI-hypersensitive (DHS) regions with DNase-seq (John et al., 2011).
Microglia are brain-resident innate immune system cells that play beneficial roles in health and detrimental roles in brain pathologies. As their original description by Pío del Río-Hortega, recently republished in English (Sierra et al., 2016), these cells have been found to be instrumental in shaping brain plasticity, development, and pathological mechanisms, including functioning in synapse stripping and axonal guidance (reviewed by Kaminska, Mota, & Pizzi, 2016; Kettenmann, Hanisch, Noda, & Verkhratsky, 2011; Mosser, Baptista, Arnoux, & Audinat, 2017). The activity of microglia plays an important role in the early rewiring of the young brain (Pont-Lezica et al., 2014; Squarzoni et al., 2014). Microglia originating from the yolk sack have a different ontogeny than peripheral macrophages (Ginhoux, Schultze, Murray, Ochando, & Biswas, 2016; Prinz, Tay, Wolf, & Jung, 2014) and show a distinct transcriptomic signature from other macrophage populations (Butovsky et al., 2014; Sousa, Biber, & Michelucci, 2017). Experimental and clinical evidence has demonstrated the accumulation of microglia and peripheral macrophages in malignant gliomas, which are common deadly primary brain tumors. Glioma-secreted factors induce proinvasive and immunosuppressive activation of microglia in which these cells promote tumor growth, as reviewed by Hambardzumyan, Gutmann, and Kettenmann (2016).
Several studies have demonstrated molecular events underlying glioma-associated microglia polarization in vitro and in vivo (Markovic et al., 2009; Markovic, Glass, Synowitz, van Rooijen, & Kettenmann, 2005; Sliwa et al., 2007). More recently, the changes in the transcriptome induced in microglia by glioma have been characterized by our group and others (Ellert-Miklaszewska et al., 2013; Szulzewsky et al., 2015; Szulzewsky et al., 2016). However, the gene regulatory mechanism underlying the transcriptional changes induced in rat microglia by glioma are not known, at least partially due to a lack of experimentally identified cis-regulatory regions.
The landscape of active regulatory regions in peripheral macrophages and their relation to gene expression have been thoroughly studied in mice (Gosselin et al., 2014; Lavin et al., 2014) and humans (Saeed et al., 2014; Schmidt et al., 2016). In direct correspondence to the current work, open chromatin regions have been studied by ATAC-seq in tumor-associated macrophages and microglia in mice (Bowman et al., 2016). The rat presents distinct advantages over the mouse in glioma studies, permitting experimental procedures and interventions that are limited by the smaller size of the mouse brain as well as cross-species comparisons (Connolly et al., 2017, 2018).
Rat models of various brain pathologies show good clinical relevance to human pathologies. For example, transcriptomic profiling of rat C6 gliomas with Affymetrix microarrays indicated similarity of gene expression profiles to a mesenchymal subtype of glioblastoma, the most malignant human glioma (Gieryng et al., 2017). Additionally, the histopathological features of C6 gliomas, such as the presence of pseudopallisadic necrosis, neoangiogenesis, and a high content of cancer stem cells, are considerably similar to those of human gliomas. Furthermore, immune infiltrates in rat gliomas are more similar to human glioblastomas, with a prevalence of microglia and myeloid-derived suppressor cells (Gabrusiewicz et al., 2016), while peripheral macrophages predominate in murine gliomas. In the case of microglia under neuropathological conditions, researchers have noted that rat models of stroke more closely resemble human pathology than murine models. There are differences in immune infiltration after stroke in rats and mice, including more massive infiltration of bone marrow macrophages in mice (Lambertsen et al., 2011).
Here, we report the identification and analysis of DNaseI-hypersensitive open chromatin regions in primary rat microglia in vitro under control (untreated [UNTR]) conditions and 6 hr after treatment with glioma-conditioned medium (GCM) to induce proinvasive polarization, or lipopolysaccharide (LPS) to induce inflammatory polarization.
2 MATERIALS AND METHODS
2.1 Microglial cultures
Microglial cultures were prepared from 1-day-old Wistar rat pups as previously described (Ellert-Miklaszewska et al., 2013; Ellert-Miklaszewska et al., 2016). Cerebral cortices were dissociated mechanically and by trypsinization, and cells were plated at a density of 3 × 105 cells/cm2 on poly-l-lysine-coated flasks in Dulbecco's modified Eagle medium (DMEM) (with Glutamax, 4.5 g/L glucose, 10% heat-inactivated fetal bovine serum [FBS], 100 U/mL penicillin, and 0.1 mg/mL streptomycin). Cells were maintained at 37 C in a humidified atmosphere of 5% CO2/95% (Heraeus, Hanau, Germany). Culture medium was changed after 3 days and then twice a week. After 2 weeks, microglial cells were recovered from confluent cultures by mild shaking and centrifugation (300g for 5 min). Cell viability was determined by trypan blue exclusion. Cells were suspended in a culture medium and plated at a density of 2–3 × 105 cells/cm2 onto culture dishes for cells growing in suspension (Sarstaed, Nümbrecht, Germany) and incubated for 48 hr prior to experiments. Microglia were stimulated with 100 ng/mL LPS from Salmonella enteritidis (Sigma-Aldrich, Saint Louis, MO) or conditioned media from rat C6 glioma cultures (GCM). C6 glioma cells (ATTC) were grown in DMEM with 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA USA), and antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin). The medium was changed every 3–4 days. After seeding 1 × 106 C6 cells onto 100-mm dishes, standard culture media were exchanged for 8 mL of 10% FBS high-glucose DMEM with Glutamax and conditioned media were harvested after 24 hr from 85 to 90% confluent cultures and centrifuged at 300g for 10 min to remove cell debris.
2.2 DNase library construction
DNase library was constructed with a modified protocol of Song and Crawford (2010)). For full details, see Data S1. Briefly, 106 to 5 × 106 microglial cells (the same number of cells for each condition) were stimulated for 6 hr with 100 ng/mL LPS, GCM, or left UNTR. Then, cells were lysed, nuclei were collected and digested 10 min at room temperature with a series of DNaseI dilutions (0.2–3.2 U/reaction, Roche Diagnostics, Indianapolis, IN, USA). Digested DNA was enclosed in low melting point agarose blocks. DNAseI activity at different concentrations was determined using pulsed field gel electrophoresis and the CHEF-DRII apparatus (Biorad, Hercules, CA, USA), and DNAseI activity at three optimal concentrations was selected for further processing. DNA fragment ends were repaired and a “Linker 1” (High-performance liquid chromatography or HPLC-purified, annealed oligonucleotides: AGTTCCTTGGCACCCGAGAATTCCATCCGAC and GTCGGATGGAATTCTCGGGTGCCAAGG) using T4 DNA ligase (1 U/μL) and ligation buffer (10×) (Roche Diagnostics, Indianapolis, IN, USA). DNA with a ligated “Linker 1” was isolated from agarose blocks and digested with MmeI enzyme (2 U/μL; New England Biolabs, Ipswich, MA, USA). Fragments containing “linker1” were purified using streptavidin beads (Dynal M-280; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, a “Linker 2” (HPLC-purified, annealed oligonucleotides: GATCGTCGGACTGTAGAACTCTGAAC and GTTCAGAGTTCTACAGTCCGACGATCNN) was ligated to DNA on beads and the beads were treated with 0.15 M NaOH to denature DNA. The obtained DNA fragments attached to the streptavidin beads were used as a template for polymerase chain reaction (PCR) performed with primers containing the standard Ilumina library preparation sequences and indexes. PCR products were resolved in 3% agarose gels and purified from gel using diethylaminoethyl (DEAE)-cellulose chromatography papers, and sequenced on Illumina Hi-seq 1500 (San Diego, CA).
2.3 DNase library preparation and sequencing
Quality of DNaseI-seq processed DNA was assessed by gel electrophoresis and quantity was measured by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) and Quantus fluorometer and QuantiFluor double-stranded DNA System (Promega, Madison, WI, USA). In total, six DNaseI-seq processed DNA samples (two UNTRs, two LPS treated, two GCM treated) were sequenced. Mean size of DNA with adapters and index sequences was 144 bp. Libraries were run in the high output mode (v3 TruSeq) run single-end sequenced (26 base pair reads + 7 base pair index reads) on HiSeq 1,500 (Illumina). DNA cluster generation was done using cBot (Illumina). Sequencing was planned to obtain high coverage data, in total more than 400 M reads was obtained for each of the sample.
2.4 Preprocessing and mapping of sequencing reads
For each of six samples (two replicates for each of three conditions), raw sequence reads of 26 bp length were filtered by minimum threshold quality 30 in minimum 90% of the bases (−q 30 –p 90) with fastq_quality_filter from FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/). Adapter removal was done with cutadapt (Martin, 2011) (https://github.com/marcelm/cutadapt). The reads were then trimmed to length 20 by fastx_trimmer. After preprocessing, any reads of length less than 20 were discarded. The reads were mapped to the rat genome rn5 (University of California Santa Cruz/UCSC and Rat Genome Sequencing Consortium, obtained from: ftp://hgdownload.soe.ucsc.edu/goldenPath/rn5/) (Havlak et al., 2004) with bowtie (Langmead & Salzberg, 2012) with the following alignment and reporting options: “-v 2–m 1–all–best–strata–S.”
2.5 Finding DNase hypersensitive regions
We computed the uniquely mappable positions in the genome for tag length of 20 using scripts from the Hotspot tools package (John et al., 2011) (https://github.com/rthurman/hotspot). The same Hotspot algorithm was used for Hotspot calls, false discovery rate (FDR) thresholding (1%) and peak calling for all six samples. For each pair of samples, Pearson correlation between raw DNaseI cuts in defined regions was also computed within the SciPy framework (http://www.scipy.org/).
2.6 Deduplicating reads, preparation, and reversing of wigs, cut positions
As sequencing protocols use PCR amplification, next-generation sequencing (NGS) data are prone to unusually high peaks of duplicate reads. For this reason reads were deduplicated with Picard using default settings (Picard Tools version 1.952010 https://sourceforge.net/projects/picard/files/picard-tools/1.95/). As a result of the modified sequencing protocol, the DNaseI cut site was placed at the 3′ end of the read. As the most software works on 5′ end, the reads were reversed by swapping the 0 and 16 flags in “.sam” files. This action has changed the strandedness of the reads, but it's irrelevant as long as further analyses disregard the strandedness. The reversed reads were further used in generating “.wig” files (containing DNase cut counts for viewing in genomic browser) using dnase_wig_tracks from pyDNase toolbox (Piper et al., 2015). Two types of “.wigs” were created: summed cuts from “+” and “–” strand for individual samples, and summed cuts of both samples for the whole condition.
2.7 Reproducible regions of open chromatin
The common reference set of coordinates of open chromatin region was defined based on individual Hotspot regions obtained for each replicate using our software (with “makeRegions.py”) available at https://bitbucket.org/seventm/dnase. To build this reference set, first, separately for each condition we identified the condition reproducible regions as the intersection between both replicates. Second, we took the union of condition reproducible regions for every condition.
2.8 Differential analysis of open chromatin regions
To evaluate the statistical significance of differentially open chromatin regions common reference set of coordinates was used together with edgeR package (version 3.16.5) and our software available at https://bitbucket.org/seventm/dnase. First, we calculate the number of cuts (5′ ends of reads) in each region (using “countDNaseCuts.py”). Second, raw counts were converted into counts per million (CPM) and CPM per base (CPMB). Third, only regions with CPMB greater than 0.001 in at least two samples were used for further steps. Fourth, differential analysis with edgeR package was performed (Robinson, McCarthy, & Smyth, 2010). The methodology for differential analysis was based on DiffBind package (Ross-Innes et al., 2012). Normalization factors were calculated with calcNormFactors() function using trimmed mean method (TMM) and with doWeighting equal to FALSE. We estimated common dispersion with estimateCommonDisp(), GLM dispersion with estimateGLMCommonDisp(), and tagwise dispersion with estimateGLMTagwiseDisp(). To evaluate the model, we used glmFit() and glmLRT() functions for selected contrasts. Finally, differentially open chromatin regions were obtained with topTags() function with Benjamini-Hochberg (BH) as adjust.method (FDR). Logarithm of fold change (Log2FC) values were calculated with edgeR.
2.9 Hilbert plots, circos diagrams
We used R packages of HilbertVis and HilbertVisGUI (http://bioconductor.org/packages/HilbertVis/) for visualization of the genomic regions on two-dimensional Hilbert curve (Anders, 2009). For each chromosome, we overlaid data vectors of defined regions and expressed genes (transcription start site [TSS] ± 1 kb) in red and green channel, respectively. Each data vector was folded according to the Hilbert curve of eigth iteration, resulting in images of 28 × 28 = 256 × 256 pixels. Here, in chr1, 4,426 bps (chr1 length 290094216/65536 pixels) are represented by 1 pixel. We used circos (Krzywinski et al., 2009) for plotting Figures 2c and 3b.
2.10 Expression specificity of genes and DNaseI cut profiles
Gene-level expression (Relative log expression - transcript per kilobase million or RLE-tpm values) data for mouse (mm9) genes were obtained from publicly available FANTOM5 (FANTOM Consortium and the RIKEN PMI and CLST (DGT) et al., 2014) datasets (Phases 1 and 2 combined): http://fantom.gsc.riken.jp/5/datafiles/latest/extra/gene_level_expression/. There were 128 samples of primary cells. We dropped the genes that had zero as their minimum, maximum, and median expression values across all 128 samples. Expression specificity score of each gene was computed as log10 (median expression across all samples/maximum expression across all samples). Then we retrieved the rat orthologs of mm9 (Ensembl equivalent NCBIM37) genes from Ensembl BioMart (Ensembl 67) (Flicek et al., 2012). As Ensembl 67 contained rat genome version rn4 (or Baylor 3.4, Ensembl equivalent RGSC3.4), we also downloaded the unique rat rn5 (Ensembl equivalent Rnor_5.0) genes from Ensembl BioMart (Ensembl 79) (Cunningham et al., 2015). The corresponding orthologous rat gene ID for each mouse gene was then used to map the rat orthologs to corresponding genes in rn5. The DNaseI cut profiles in the gene_start_coordinate ±1 kb window of these orthologous genes were retrieved from merged bam files (both replicates) of each treatment condition separately, using a modified version of the pyDNase script dnase_wig_tracks.py (Piper et al., 2013). The genes were then classified by expression specificity scores and their mean DNaseI cut profile in the gene_start_coordinate ±1 kb window were plotted. Similarly, we plotted the distribution of expression values of the expressed genes (detected on microarray) classified by expression specificity scores. We obtained rn5 CpG island coordinates from UCSC table browser, and prepared the sets of CpG promoters and non-CpG promoters using bedtools intersect (intersecting CpG island coordinates and the above-mentioned gene_start_coordinate ±1 kb) with parameters “–wa–wb” and “–wa–wb–v,” respectively.
2.11 Annotating the regions, obtaining base pair overlap with features, mapping regions to nearest genes
We obtained the “.bed” coordinates of the rn5 ensembl genes in following categories from UCSC table browser (ensembl gene track): upstream by 10 kb, upstream by 2 kb, 5′ untranslated region (UTR) exons, coding exons, introns, 3′ UTR exons, downstream by 2 kb, and downstream by 10 kb. The complement set of distal regions, the rn5 genomic coordinates not covered by any of the features above, was also computed. The intersection and annotation of the open chromatin regions (rn5) with the above-mentioned genic features were performed using bedtools suite. The coordinates of all the Ensembl genes from rn5 were downloaded using Ensembl BioMart, and the closest gene in both directions of each region was determined using bedtools closest with parameter “–D b” (usage: bedtools closest – a < regions_coordinates> − b < genes_coordinates> − D b). Coordinates of ncRNA transcripts for rn6 were obtained from ftp://ftp.ensembl.org/pub/release-90/fasta/rattus_norvegicus/ncrna/. We moved the coordinates of open regions in rn5 to rn6 using the batch coordinate conversion tool (liftOver) from UCSC, and intersected them with ncRNA coordinates in rn6. The unique ID for each region (RID) kept track of the regions across different genome versions. Also, a comprehensive data table containing all regions, their fraction overlap with each of the features mentioned above, their CPMB values in each of the three treatment conditions, their FDR scores in each condition, their nearest genes, and corresponding expression values were created using custom python scripts.
2.12 Functional analysis
Identification of overrepresented functional categories among the genes mapped to differentially open (FDR < 0.05) regions, separately for the GCM and the LPS treatments, was performed with gProfiler (Reimand et al., 2016) via its website interface (r1750_e91_eg38), using the default options with the following changes: hierarchical sorting was turned off, Benjamini–Hochberg FDR was used as the significance threshold, the gene list of all the genes that were mapped to any region was used as the statistical background. KeggAnim (Adler et al., 2008) was used to visualize the average Log2FC of the chromatin openness of the regions mapped to the genes in selected Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
2.13 Statistical tests
For the whole genome versus open chromatin regions, we defined “expected frequency” (vector) as number of base pairs covered by each of the nine genic feature annotations genome wide (Figure 3a). Then we defined “observed frequency” as the number of base pairs covered by the same nine genic feature annotations in all the open chromatin regions. A one-way chi-square test was then applied to test the null hypothesis that the categorical data have the given frequencies. We used the chi-square method from scipy.stats. We tested for “all open chromatin regions versus GCM-regulated regions,” “all open chromatin regions versus LPS-regulated regions,” and “LPS-regulated regions versus GCM-regulated regions” in the similar fashion as above, by defining expected frequency from the first category and observed frequency from the second category of regions in each case of comparison.
Mann–Whitney U test was performed to test the significance of difference in the openness between the set of detected genes and the remaining genes (Figure 5a). For microarray-detected genes and for each of the three treatment conditions (GCM, UNTR, and LPS) (Figure 6a), we computed Kolmogorov–Smirnov statistic for the DNase-I cuts profiles of high-specificity versus medium-specificity groups, high-specificity versus low-specificity groups, and medium-specificity versus low-specificity groups of genes. We used the ks_2samp method from scipy.stats. The null hypothesis that the samples are drawn from the same continuous distribution was rejected in every case. However, the null hypothesis could not be rejected with enough evidence for the same pairs of comparison (except high-specificity vs. low-specificity groups) in the set of nondetected genes. The expression distribution of high-specificity, medium-specificity, and low-specificity group of genes were also significantly different from each other in every treatment condition (Figure 6b). We computed Kolmogorov–Smirnov statistic on gene expression values of the pairs' high-specificity versus medium-specificity groups, high-specificity versus low-specificity groups, and medium-specificity versus low-specificity groups of genes. Also, Kruskal–Wallis H test (scipy.stats.kruskal) confirmed that the population medians of all three specificity groups were highly significantly different (p values 1.04e-79, 1.67e-82, and 1.72e-59 for UNTR, GCM, and LPS conditions, respectively).
2.14 Expression distribution of genes in mouse (FANTOM5 data)
We identified the undetected genes that are orthologs of mouse genes in rat. For each of those genes, mean expression value in three microglia replicates in FANTOM5 data (see Section 2.10) was computed. We also computed the mean expression values of detected genes in the same fashion. The differences in expression distribution between detected and undetected genes were then visualized through kernel density estimation plot. The same approach was used in order to differentiate between undetected-but-present-on-microarray and undetected-and-absent-on-microarray groups of genes.
2.15 Comparison of open chromatin with external mouse ATAC-seq datasets
We retrieved the bed files containing peaks from ex vivo and in vitro pools of ATAC-seq experiments from NCBI Gene Expression Omnibus (GEO) accession GSE89960. The file names were GSE89960_mouse_atac_exvivo_vsInput_no_style_factor_region_no_nfr_no_size_200_minDist_200_peaks.bed.gz and GSE89960_mouse_atac_invitro_vsInput_no_style_factor_region_no_nfr_no_size_200_minDist_200_peaks.bed.gz for ex vivo and in vitro, respectively.
We transformed the coordinates of our rat (rn5) open chromatin regions to the corresponding mouse (mm10) coordinates using the liftOver tool from UCSC. Out of 126,640 rn5 regions, 82,913 could be mapped to mm10. We performed a multi-intersection of the three sets of coordinates using bedtools, and performed the downstream analysis based on the output of the previous step.
3 RESULTS
3.1 Rat microglia harbor 127K reproducible open chromatin regions
We previously identified genes induced in rat microglia by either GCM or LPS along with the acquisition of a specific functional phenotype (Ellert-Miklaszewska et al., 2013). In the current study, using the same experimental setup, at the same time point (6 hr) following stimulation, we collected material to determine open chromatin patterns. Proinvasive and inflammatory polarizations were confirmed by the induction of the corresponding polarization markers (Figure S1a). We constructed two replicates of DNaseI-seq libraries for every condition: UNTR, GCM treated, and LPS treated; replicates were obtained from independent microglia cultures, and each culture originated from 10 to 15 rat pups. For each replicate, we independently identified open chromatin regions with the Hotspot program (John et al., 2011).
Figure 1 shows an example of our data for a 110-kb chromosome segment surrounding the Il1b-Il1a locus (Figure 1a,b) and the construction of our reference set of open chromatin regions (Figure 1c).
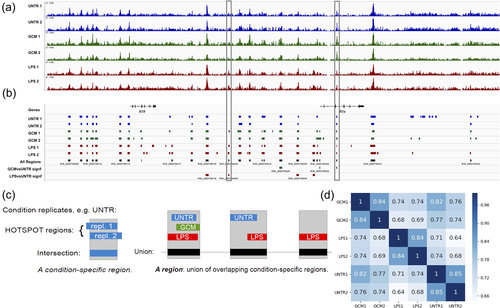
To quantitatively analyze the changes in openness induced by the treatments with statistical confidence, we needed a single common reference set of regions for subsequent application across all the replicates and conditions. To construct this set, we selected the union of all the segments that were reproducibly open under at least one condition. Technically, this set was constructed by first intersecting the Hotspot regions from both replicates for a given condition and then by selecting the union of the results from the first step across all the conditions (Figure 1c). This union yielded our reference set of 126,640 (127K) open chromatin regions.
Using the reference set of coordinates of regions, we could quantitatively compare their openness across the replicates and conditions. The openness of a region, defined as the normalized number (CPM, see Section 2) of DNaseI cuts, was highly reproducible between the replicates for the same condition and markedly different between the conditions (Figure 1d), with LPS inducing greater changes than GCM when compared to the untreated condition. The raw sequence files and the processed data have been deposited in NCBI's Gene Expression Omnibus (Edgar, Domrachev, & Lash, 2002) and are accessible through GEO Series accession number GSE123328 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE123328). The processed data are also provided as tab-separated File S1 (GCM vs. UNTR) and File S2 (LPS vs. UNTR).
To identify the core profile of open chromatin regions, we performed multi-intersection analysis of the Hotspot regions from individual replicates. The result is provided as File S3. Using this result, it was easy to filter genomic sequence segments that were reproducibly open under just one condition, two conditions, or three conditions. The union of segments that were open under all three conditions (all six replicates) can be regarded as the core open chromatin profile of the rat microglia. This core has a cumulative size of 27.7 MB, corresponding to 45% of the total base pairs in our reference set of 127K open chromatin regions (62 MB).
To directly compare the rat open chromatin landscape to that of the mouse, we transformed our reference regions to the mouse with liftOver (Kuhn, Haussler, & Kent, 2013) and compared them to the ATAC-seq data from the published study (Gosselin et al., 2017). The results demonstrated that the rat open chromatin regions detected in our study show considerable similarity to the open chromatin landscape of mouse microglia. Notably, this rat-to-mouse similarity was comparable for mouse in vitro and ex vivo regions and was greater for rat core open chromatin regions (Figure S1b vs. c). However, when we compared the core and, in parallel, all the regions to the constrained elements from 39 eutherian mammals, the intersections had sizes of 2.97 and 5.8 MB, respectively, corresponding to 10.72% for the core and 9.34% for all the regions, indicating a similar level of deep conservation within and outside the core.
3.2 Stimulation with GCM or LPS induces distinct changes in chromatin openness
A total of 2,303 reference regions showed significantly (FDR < 0.05) changed openness after stimulation with GCM (Figure 2a) and 12,357 after stimulation with LPS (Figure 2b), with 802 regions being affected by both treatments. The regions with significantly changed openness at the Il1b-Il1a locus are indicated in the two bottom tracks (GCMvsUNTR signif, LPSvsUNTR signif) of Figure 1b. Following either treatment, approximately twice as many regions exhibited increased openness than decreased openness (Figure 2a,b). The changes were distributed evenly across all the chromosomes (Figure 2c).
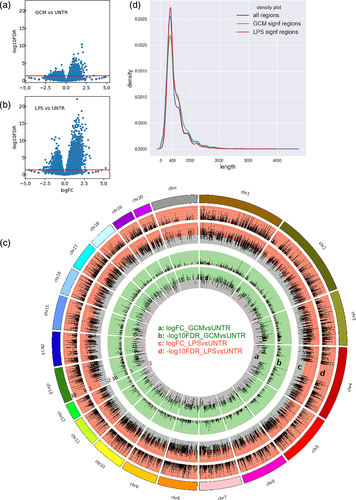
Similarly to the results for all the regions, the majority of the regions that were significantly regulated following either treatment were 200–400 bp long (Figure 2d). The regulated regions exhibited a lower openness density (CPMB) under the untreated conditions than all the regions (Figure S2), reflecting that the regions that were most open under the untreated conditions were not regulated (Figure S2 and data not shown).
We mapped each region to its nearest gene (gene body). Thus, 127K regions were mapped to 19,359 genes, among which the 12,357 regions that were significantly regulated by LPS were mapped to 6,755 genes, and the 2,303 regions that were significantly regulated by GCM were mapped to 1,682 genes. The 802 regions affected by both treatments were mapped to 689 genes. A comprehensive data table provided as File S4 contains all regions, their fractional overlap with each of the analyzed features, their openness values in each of the three treatment conditions, their FDR scores in each condition, their nearest genes, and the corresponding published microarray expression values (Ellert-Miklaszewska et al., 2013).
3.3 GCM-regulated regions are more frequent away from gene bodies, while LPS-regulated regions are more frequent in introns
We compared the distribution of base pairs in the whole genome and in open chromatin regions across gene-related genomic features (Figure 3a). The distribution of base pairs in open chromatin regions (blue bars) generally followed the distribution of all bases in the genome (gold outline), with the majority of open chromatin being located in introns (40%), distal regions (30%), regions 10 kb upstream (from the gene start site), and regions 10 kb downstream (from the gene end). The two distributions (blue vs. gold) were significantly different (chi-squared statistics 85,399,682, p value of 0), with the bases of open chromatin being overrepresented in all genic features, most strongly in promoters (regions 10 kb upstream and regions 2 kb upstream), followed by regions 10 kb downstream and then introns, and underrepresented in distal regions (not covered by any of the genic features). Among the DHS regions, 9.2% spanned exon–intron boundaries (data not shown).
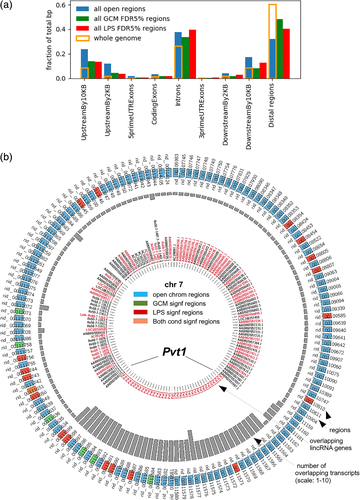
We then asked if the distribution of the open chromatin regions showing significantly changed openness following stimulation with GCM (Figure 3a, green) or LPS (Figure 3a, red) was identical to that of all the open chromatin regions. The distributions of GCM- and LPS-regulated regions were both different from the distribution for all open chromatin regions (chi-squared statistics 307,232, p value of 0; chi-squared statistics 909,298, p value of 0, respectively). Open chromatin regions regulated by either treatment were underrepresented in promoters (regions 10 kb upstream and regions 2 kb upstream) and in regions 10 kb downstream but overrepresented in distal regions. Finally, the regions regulated by the GCM and LPS treatments showed distinct localization preferences relative to the analyzed features (chi-squared statistics 63,566, p value of 0). The base pairs of GCM-regulated regions were more frequently located in distal regions, whereas the base pairs of LPS-regulated regions were more frequently localized in introns and regions 10 kb downstream.
3.4 GCM and LPS treatments affect chromatin openness in lincRNA gene bodies
A total of 1,823 DHS regions overlapped with known transcripts of 899 lincRNA genes, which constituted the largest fraction of nonprotein-coding RNAs intersecting microglial open chromatin regions (Figure S3a). Forty-three lincRNA genes hosted at least one region that changed significantly upon GCM treatment, and 149 lincRNA genes hosted regions significantly changed by LPS. The Myc-regulating Pvt1 lincRNA-encoding gene contained the highest number of open chromatin regions, at 37, out of which 3 showed significantly changed openness after GCM treatment and 6 after LPS treatment (Figures 3b and S3b,c).
3.5 GCM and LPS differently affect openness of genes in functional pathways related to developmental and immune roles of microglia
We performed overrepresentation analysis of the functional annotations of the genes that mapped to regions that were significantly (FDR < 0.05) regulated by GCM or, separately, by LPS compared to the total set of all the genes that were mapped to any region using gProfiler (Reimand et al., 2016). The results are provided as tab-separated File S5 (GCM vs. UNTR) and File S6 (LPS vs. UNTR).
This analysis revealed that both sets of genes were significantly (FDR < 0.05) enriched in genes annotated to a large number of gene ontology (GO) terms, a majority of which (we focused on the Biological Process ontology) can be classified into two broad categories: (a) terms related to nervous system development and function (including nervous system development, neuron differentiation, synapse) and (b) terms related to cell motility/adhesion/migration/response to wounding (including cell motility, cell adhesion, ameboidal-type cell migration, chemotaxis, and response to wounding), while terms related to immune/defense responses (including immune response and defense response) were only significantly enriched among the genes that mapped to the regions significantly regulated by LPS.
The same analysis conducted for KEGG (Kanehisa, Sato, Kawashima, Furumichi, & Tanabe, 2016) pathways identified a smaller number of pathways, whose members were significantly (FDR < 0.05) enriched among the genes regulated by either GCM (including: ECM–receptor interaction) or LPS (including Toll-like receptor (TLR) signaling pathway and axon guidance) or among both the GCM- and LPS-regulated genes (including pathways in cancer and glutamatergic synapse).
The changes of chromatin openness were aggregated by gene, as the average Log2FC values for either treatment over all regions mapped to a particular gene. When these values were visualized in diagrams of the overrepresented KEGG pathways, it was apparent that (a) both treatments affected the openness of a large fraction of genes in these pathways and (b) in general (data not shown), the patterns of up-downregulation were similar for the two treatments with some notable exceptions (Figures 4 and S4). These exceptions were more frequent in two pathways related to neuronal differentiation and function, the axon guidance (Figure 4a,b) and glutamatergic synapse (Figure S4a,b) pathways, than in the remaining inspected pathways, such as pathways in cancer (Figure S4c,d). Specifically, in the axon guidance pathway, seven genes, Ntn1 and Dcc (netrin and its receptor), Ptpn11, Robo1, Robo2, Rac1, and Bmpr1b, showed changes in their aggregated openness in the opposite directions following the two treatments (Figure 4a,b). In the glutamatergic synapse pathway, three genes, Kcnj3, Grm1, and Grm3, showed changes in their aggregated openness in the opposite directions following GCM and LPS treatments. The majority of genes in this pathway had their openness downregulated by both treatments (Figure S4a,b).

Another significantly overrepresented pathway with a noticeably greater frequency of up/down differences in the direction of regulation between the GCM and LPS treatments was the TLR signaling pathway (Figure 4c,d). In this pathway, stimulation with GCM and LPS differentially affected the openness of regions mapped to genes encoding receptors such as Tlr3 and Tlr7; downstream pathway components such as Irak4, Chuk (alias IKK-alpha), Map2k1, and Map2k4; and downstream effectors such as cytokines or cell surface ligands, including Ccl5, Tnf, and Cd40.
3.6 Expression requires open promoters, but there is no direct dependence between openness and expression
We previously published microarray gene expression data obtained under the same experimental settings (Ellert-Miklaszewska et al., 2013). Utilizing Affymetrix microarray technology, we reliably measured the absolute expression of 8,667 genes (R oligo MAS5 call: present), among which 7,632 were mapped to open chromatin regions. Therefore, among the 19,359 genes that were mapped to the open chromatin regions in the current work, 19,359 – 7,632 = 11,727 genes were not detected in our previous microarray experiment, among which 2,989 genes were represented on the microarray (Affymetrix rat 230_2) but were not expressed/expressed at low levels that were not reliably detectable with microarray technology (MAS5 call: Absent or Marginal), while the remaining 8,738 genes were not represented on the microarray. Comparison to the FANTOM5 expression data confirmed the lower expression (of the mouse orthologs) of the nondetected genes compared to the detected genes (Figure S5a).
We sought to study whether there was a difference between the detected and the nondetected genes in their chromatin openness or its variation. We aggregated the openness per gene (by sum) and its log2 change (by average) for all the regions mapped to a particular gene. We found a clear difference in openness between the set of detected genes and the remaining genes (Mann–Whitney test p < 10−268). The aggregated openness was higher for the detected genes than for the nondetected genes (Figure 5a). However, within the detected gene set, we found little or no dependence between the aggregated openness of all the regions mapped to a gene and its absolute expression rank, as indicated by a flat moving median plot (orange) for the detected genes (ranks 11728–19359) ranked regarding their absolute expression (Figure 5b). Conversely, we looked for dependence between the aggregated openness ranks of genes and their log2 expression for several different aggregating functions (sum, average, and maximum) (Figure S5b–d). As demonstrated by moving median plots, flat-fitted linear response lines, and very small R2 values, openness had only a very small effect on expression. This effect was most noticeable for genes with the lowest openness ranks. Similarly, we found no dependence between the ranks of the genes on the average fold change of the openness of regions that mapped to them and their Log2FC in expression (Figure S5e,f). We further checked whether there was such dependence for the genes in the KEGG axon guidance or TLR signaling pathways. For the axon guidance genes, represented by green points, there was clearly no dependence following either treatment, whereas for the TLR pathway genes, represented by red points, there was a tendency for the upregulated genes (including Il6, Ccl5, Cxcl11, Cxcl10, and Cxcl9) to exhibit increased average openness, but this tendency was not statistically significant (p = 0.26, chi-square test).
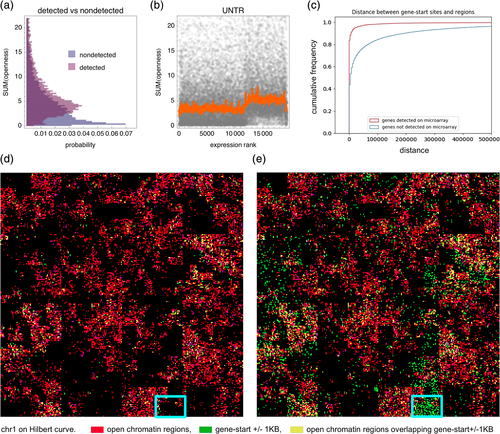
The cumulative distribution of the distance from the TSS of a gene to the nearest open chromatin region was very different for the 8,667 detected genes and the remaining rn5 genes (Figure 5c). For 80% of the detected genes, this distance was less than 5 kb, indicating an open chromatin region overlapping with or located near a gene start site (i.e., an open promoter), whereas the same was true for only 40% of the nondetected genes.
We used the Hilbert plot (Anders, 2009; Gu, Eils, & Schlesner, 2016) to visualize the genomic localization of the set of 127K open chromatin regions and either the 8,667 genes detected as transcriptionally active in microglia (Figure 5d) or all rat (rn5) genes (Figure 5e). Most of the detected genes were located within chromosomal segments containing open chromatin regions, whereas many nondetected genes were located within chromosomal segments devoid of open chromatin regions (highlighted in Figure 5d,e).
3.7 Genes classified by expression specificity display distinct DNaseI cut profiles around TSSs
Previous studies in humans and mice have demonstrated a relationship between the shape of the open chromatin peak at a promoter and the expression specificity of a gene (FANTOM Consortium and the RIKEN PMI and CLST (DGT) et al., 2014). We stratified the genes according to expression specificity (of their mouse orthologues) across a panel of primary cell types into three expression specificity bins: high, medium, and low. Note that this high specificity can be for any cell type(s), not necessarily microglia. After stratification, performed separately for the detected and nondetected genes, we compared the shapes of the average DNaseI-cut profile around the gene start site. We observed differences in the shape of the average DNaseI-cut profile around the gene start site, with high-specificity genes exhibiting the tallest peaks and low-specificity genes presenting the lowest and broader peaks (Figure 6a, top row). These differences were robust, as they were significant (p value < .001, Kolmogorov–Smirnov test) for every culture condition and pair of specificity bins (high vs. low, high vs. medium, medium vs. low). For the nondetected genes, the differences between the specificity bins were smaller/not significant (Figure 6a, bottom row). The differences observed for the detected genes were similar for CpG and non-CpG promoters (Figure S6).
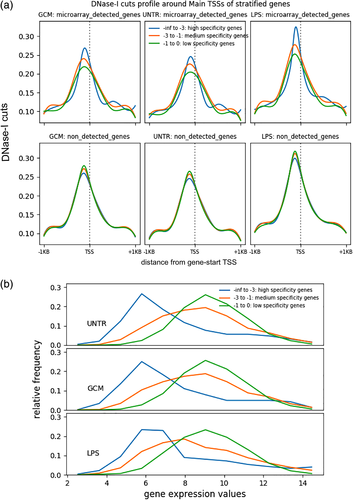
Under every culture condition, the genes in the low-specificity group exhibited the highest average expression in the rat microglia, while the genes in the high-specificity group exhibited the lowest expression (Figure 6b). However, some of the high-specificity genes were highly expressed in microglia (thick right tail of the distribution), with their fraction in the highest expression bin increasing after LPS treatment. Functionally, the low-specificity group was overrepresented in housekeeping genes (data not shown).
3.8 GCM and LPS induce different patterns of openness changes in individual regions mapped to immune checkpoint genes
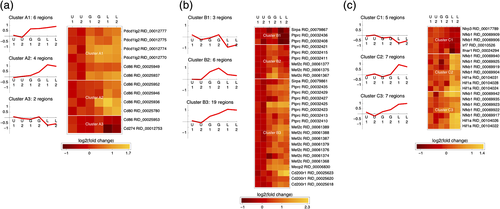
Finally, to identify potential major differences between the LPS-stimulated (inflammatory) and GCM-stimulated (proinvasive and anti-inflammatory) microglia, we decided to examine the changes in the openness of individual regions mapped to immune checkpoint genes. Figure 7 shows the patterns of openness changes for open chromatin regions with significantly altered openness following either treatment (FDR < 0.05) that mapped to immune checkpoint genes, clustered to reveal similarities. We used three (disjoint) lists of immune checkpoint proteins/genes from two recent reviews on this topic describing proteins/genes that are involved in antigen presentation to T cells (Dyck & Mills, 2017) (Figure 7a) or participate in immune checkpoint mechanisms specific to microglia (Deczkowska et al., 2018) under either physiological (Figure 7b) or pathological conditions (Figure 7c). Several useful observations can be made from these data: (a) openness patterns are shared across regions mapped to different genes; (b) individual regions mapped to the same gene show different patterns of openness changes (e.g., Figure 7a; Cd86); (c) GCM treatment generally increases the openness of (regions mapped to) several genes involved in antigen presentation (Figure 7a; Pdcd1lg2 and Cd86) and immune checkpoint genes operating in microglia under physiological conditions (Figure 7b; Ptprc, Mef2c, Sirpa, Mecp2, and Cd200r1), with the openness of several regions mapped to Ptprc and Mef2c being specifically increased after GCM treatment; (d) only LPS and not GCM induces changes in the openness of (regions mapped to) immune checkpoint genes operating in microglia under pathological conditions (Nlrp3, Nfkb1, Irf67, and Hif1a).
4 DISCUSSION
Rat microglia harbor 127 thousand open chromatin regions, among which 2% and 10% show a change openness after treatment with GCM or LPS, respectively (Figures 1 and 2). Similar to the findings of Saeed et al. (2014)), who studied macrophage activation by LPS, we observed that the openness of promoters remained largely unchanged after this treatment and after stimulation by GCM (Figure 3a).
Interestingly, LPS induced changes in openness with a preference for regions that were intronic or located within 10 kb immediately downstream of the transcription end site (Figure 3a). These findings are in agreement with the results of a recent chromatin proteomics study that identified a subpopulation of intronic enhancers involved in inflammatory gene expression (Soldi et al., 2017). In contrast, the GCM treatment preferentially induced openness changes in open chromatin regions distant from the genes' bodies.
We observed clear dependence between the presence of a nearby open chromatin region and the fact that a gene is expressed (Figure 5a,c), as previously described (Frank, Manandhar, Gordân, & Crawford, 2016). While we documented extensive and complex changes in chromatin openness, there was no direct dependence between the aggregated openness of open chromatin regions near a gene and its expression (Figures 5b and S5), with exception of genes with lowest openness ranks, consistently with the need for an open promoter. Our findings are therefore in agreement with those of Schmidt et al. (2016), who demonstrated that the macrophage transcriptional response to activation was regulated in the context of broadly accessible chromatin, but they contrast with results for some other systems involving nuclear receptors (He et al., 2012). Simple dependence between changes in chromatin openness and gene expression may not occur. Instead, chromatin openness reflects the changing state of individual open chromatin regions (cis-regulatory modules), which affects gene expression in a manner that is not simply proportional to a region's openness.
We identified a large number of lincRNA genes containing open chromatin regions, including regions that were regulated following the treatments, exemplified by the Myc-Pvt1 locus (Figure S3). In our previous transcriptomics study (Ellert-Miklaszewska et al., 2013), we demonstrated that LPS treatment, but not GCM treatment, significantly increased the expression of genes assigned to the GO terms immune response and defense response. These findings are corroborated by our current results for open chromatin regions, in that the same two GO categories were overrepresented among the genes mapped to the regions showing significant changes (in either direction) in openness in response to LPS, but not the genes that showed a change in openness in response to GCM.
To further characterize the differences between LPS (proinflammatory) and GCM (anti-inflammatory) microglia, we examined the patterns of chromatin openness changes in the genes involved in immune checkpoint mechanisms in detail. We report that GCM increased the openness of (regions mapped to) immune checkpoint genes that are known to operate in microglia under physiological conditions or to be involved in antigen presentation, while only LPS induced changes in the openness of immune checkpoint genes known to operate in microglia under pathological conditions.
The two treatments differentially affected the openness of regions near genes within functional KEGG pathways related to the interactions between microglia and neurons (glutamatergic synapse and axon guidance) and to the immune functions of microglia (TLR signaling) (Figures 4 and S4). Microglia contact synapses and harbor receptors for neurotransmitters, including glutamate (Kettenmann, Kirchhoff, & Verkhratsky, 2013; Madry & Attwell, 2015). Our data show that in response to stimulation with either GCM or LPS, microglia regulate two genes at the chromatin openness level that encode components of the glutamatergic synapse machinery, namely Grm1, encoding the metabotropic glutamate receptor, and Grm3, encoding an 2-Amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) glutamate receptor, both of which known to be expressed on microglia (Christensen, Ha, Sun, Bresnahan, & Beattie, 2006; Hagino et al., 2004).
The greatest number of differences in the patterns of openness changes between the GCM and LPS groups was observed for the functional pathways of TLR signaling and axon guidance, suggesting that these two pathways in microglia are “hijacked” by glioma (i.e., they are particularly affected during the proinvasive polarization of microglia). The importance of the TLR pathway in microglia–glioma interactions is well established (Dzaye et al., 2016; Hu et al., 2014; Hu et al., 2015; Vinnakota et al., 2013). Among the differentially regulated genes in this pathway, Ccl5 is known to promote the migration of microglia (Pham, Luo, Liu, & Harrison, 2012). Chuk (alias IKK-alpha) encodes a component of the IKK complex that negatively regulates NFKB in microglia (Nguyen & Benveniste, 2002).
While it is known that microglia can affect axonal guidance, it has been assumed that they do so by expressing guidance cues and acting on the extracellular matrix (Mosser et al., 2017). We now demonstrate that in response to stimulation, microglia regulate many genes at the chromatin openness level that encode proteins involved in axonal guidance. In particular, our data show that cultured microglia not only regulate the chromatin openness of genes encoding axonal guidance cues, such as Netrin 1, but also regulate that of their previously described receptors on the axonal side, including Dcc, Robo, and Bmpr1b, and their downstream effectors, including Ptpn11 and Rac1, among which Bmpr1b was previously shown to be expressed on microglia (Miyagi et al., 2012). Thus, our dynamic open chromatin data indicate that microglia regulate genes encoding components of the guidance machinery used by the growing axons. We speculate that this machinery is expressed by microglia and used to navigate their dynamic protrusions and microglia motility.
In vivo, microglia form mobile protrusions that continuously scan the neuropil, growing, branching, and retracting (Davalos et al., 2005). Moreover, the process of protrusion formation is closely linked to the motility of microglia when they invade the cortex during development (Swinnen et al., 2013), by what is known as a saltatory migration pattern, in which microglia repeatedly “send out one or multiple processes, and displace their soma in the direction of one of the protrusions while retracting the others” (Smolders et al., 2017). Stimulation of microglia in vivo by glioma-secreted factors induces profound morphological changes, specifies an ameboid shape and increases mobility (Gabrusiewicz et al., 2011).
The two treatments differentially affected genes encoding molecular links between the axon guidance pathway, TLR pathway, and microglial mobility. Tlr7 regulates microglial chemotaxis, with Rac1 as its effector (Ifuku, Buonfiglioli, Jordan, Lehnardt, & Kettenmann, 2016). Rac1 has also been characterized as a Tlr4 target in the context of M1/M2 microglia polarization (Yao et al., 2017). Our DNaseI-seq data therefore reflect the multifaceted function of microglia in the normal brain and suggest how glioma repurposes these programs to cause microglia to support invasive glioma growth.
ACKNOWLEDGMENTS
The authors would like to thank Jakub Mieczkowski for discussion and Bartek Wilczyński for critical reading of the manuscript. This work was supported by grant 2013/09/B/NZ2/03170 (to M.D.) from the National Science Center (Poland).