Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease
Funding information Cancer Research UK, Grant/Award Number: CRUK/A4308; Medical Research Council UK, Grant/Award Number: MC_U12266B
Abstract
How tissues are maintained over a lifetime and repaired following injury are fundamental questions in biology with a disruption to these processes underlying pathologies such as cancer and degenerative disorders. It is becoming increasingly clear that each tissue has a distinct mechanism to maintain homeostasis and respond to injury utilizing different types of stem/progenitor cell populations depending on the insult and/or with a contribution from more differentiated cells that are able to dedifferentiate to aid tissue regeneration. Peripheral nerves are highly quiescent yet show remarkable regenerative capabilities. Remarkably, there is no evidence for a classical stem cell population, rather all cell-types within the nerve are able to proliferate to produce new nerve tissue. Co-ordinating the regeneration of this tissue are Schwann cells (SCs), the main glial cells of the peripheral nervous system. SCs exist in architecturally stable structures that can persist for the lifetime of an animal, however, they are not postmitotic, in that following injury they are reprogrammed at high efficiency to a progenitor-like state, with these cells acting to orchestrate the nerve regeneration process. During nerve regeneration, SCs show little plasticity, maintaining their identity in the repaired tissue. However, once free of the nerve environment they appear to exhibit increased plasticity with reported roles in the repair of other tissues. In this review, we will discuss the mechanisms underlying the homeostasis and regeneration of peripheral nerves and how reprogrammed progenitor-like SCs have broader roles in the repair of other tissues with implications for pathologies such as cancer.
1 A BRIEF INTRODUCTION TO PERIPHERAL NERVE GLIAL CELLS
The peripheral nervous system (PNS) consists of a mixture of motor, sensory, and autonomic nerves that connect tissues and organs to the central nervous system (CNS; Zochodne, 2008). Similarly to the CNS, glial cells are found closely associated with axons and act to aid and support neuronal function, however in contrast to the CNS, all axons in the PNS are ensheathed in their entirety by a form of glia (Barres, 2008; Fields, Woo, & Basser, 2015; Zuchero & Barres, 2015).
Peripheral nerve glial cells are a heterogeneous population with distinct functions in different regions of the PNS (Figure 1). Peripheral nerve roots, trunks and terminals are associated with Schwann cells (SCs) that are by far the best characterised peripheral glial cell type and these can be further divided into three main classes, myelinating Schwann cells (mSCs), nonmyelinating Schwann cells (nmSCs), and terminal Schwann cells (tSCs; also called perisynaptic SCs or teloglia), although other less well-characterised sub-types are starting to be defined in tissues such as the skin (Gresset et al., 2015; Jessen & Mirsky, 2005; Li & Ginty, 2014).
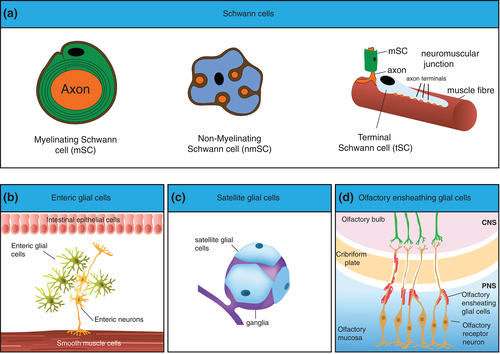
mSCs are the most abundant cells within the peripheral nerve trunk (Stierli et al., 2018). By forming a compact myelin sheath around large caliber axons (>1 μm), they provide electrical insulation which allows rapid, saltatory conduction of action potentials over the long distances covered by the PNS (Salzer, 2015). In contrast to oligodendrocytes (OLs), which are the myelinating cells of the CNS and are capable of myelinating multiple axons, mSCs ensheath single large caliber axons in a 1:1 ratio (Bercury & Macklin, 2015; Nave & Werner, 2014). The second most abundant cell population of the peripheral nerve trunk are nmSCs that individually ensheath several small caliber axons forming a structure known as a Remak bundle or ensheath single axons which have defasciculated toward their terminals. Compared to mSCs, nmSCs are less well-characterized due to the lack of suitable mouse models with specific lineage drivers (Harty & Monk, 2017). Nevertheless, nmSCs are reported to metabolically support smaller caliber axons to maintain axonal integrity (Viader et al., 2011).
Axon terminals, such as those at neuromuscular junctions (NMJs) or within the skin are enwrapped by highly specialized SCs called (tSCs). These cells have diverse morphologies depending on their site but all are thought to have active roles in the formation, maintenance, and repair of synaptic sites. For example, tSCs at NMJs––probably the best characterized of these cells––are required for the stable formation of the tripartite structure of the NMJ, exhibit plasticity to allow remodeling during adulthood and provide a substrate for regrowing axons to re-enter the former synaptic sites following nerve injury (Barik, Li, Sathyamurthy, Xiong, & Mei, 2016; Feng & Ko, 2008; Kang & Lichtman, 2013; Kang, Tian, Mikesh, Lichtman, & Thompson, 2014; Ko & Robitaille, 2015; Li & Ginty, 2014; Smith, Mikesh, Lee, & Thompson, 2013).
Other, less characterized glia have other specialized roles within the PNS. Satellite glial cells (SGCs) are glial cells that surround the cell bodies of peripheral ganglia creating a functional unit consisting of the cell body of the neuron and its associated SGC. While poorly understood, increasing evidence indicates SGCs modulate the neuronal environment and regulate neuronal activity within the ganglia (Hanani, 2010, 2012; Suadicani et al., 2010; Takeda, Takahashi, & Matsumoto, 2009). Enteric glial cells are a further subset of peripheral glia that reside in the intestinal wall, where they have multiple and diverse roles in maintaining the intestinal barrier, controlling intestinal motility and supporting enteric neuronal function and survival, all processes that are critical for the function of the gut. Morphologically distinct populations exist in different regions of the gut but the true diversity and functions of many of these cells remains unknown (Coelho-Aguiar Jde et al., 2015; Grubisic & Gulbransen, 2017; Rao & Gershon, 2018; Ruhl, 2005). A further class of peripheral nerve glial are olfactory ensheathing cells, which envelop olfactory axons in both the CNS and PNS (Barraud et al., 2010; Higginson & Barnett, 2011; Ramon-Cueto & Avila, 1998). These glial cells guide olfactory axons during development and are also required for the constant replenishment of the olfactory system that takes place in adult animals (Chehrehasa et al., 2012).
2 THE ORIGIN OF PERIPHERAL NERVE GLIAL CELLS
Peripheral glial cells originate from neural crest cells, an ectoderm-derived multipotent progenitor population that migrates away from the neural tube early in development, navigating along stereotypic pathways to distant sites in the embryo where they differentiate into multiple cell-types. These include all peripheral glia and other components of the PNS, such as autonomic and enteric neurons and endoneurial fibroblasts but also numerous other cell types such as melanocytes, cartilage, bone, and cardiovascular smooth muscle cells (Bronner & Simoes-Costa, 2016; Harris & Erickson, 2007; Le Douarin & Teillet, 1974; Zurkirchen & Sommer, 2017). Neural crest cells also give rise to boundary cap cells, which are a distinct transitional multipotent progenitor cell that produce many peripheral glia (Radomska & Topilko, 2017). Boundary cap cells are localized at embryonic cranial and spinal cord motor exit points and dorsal root entry zones and are a highly proliferative, migratory population that differentiate into both neuronal and glial cells. Moreover, they produce, in separate waves of genesis, almost all SCs within the dorsal and ventral nerve roots, SGCs in dorsal root ganglia and a substantial number of SCs within the skin. In contrast, peripheral nerve trunk SCs are predominantly derived directly from neural crest cells (Gresset et al., 2015; Maro et al., 2004; Radomska et al., 2018; Radomska & Topilko, 2017). Interestingly, boundary cap cells not only differentiate into neuronal and glial cells within the PNS, they also retain the capacity to differentiate into OLs, astrocytes and neurons when exposed to signals of the developing CNS, highlighting the plasticity of this progenitor population (Zujovic et al., 2011). Neither neural crest cells nor boundary cap cells appear to persist into adulthood and so cannot be the source of new cells in the adult (Kruger et al., 2002; Radomska & Topilko, 2017). This means that the homeostasis and regeneration of peripheral glia must involve distinct mechanisms to produce new cells.
The signals that direct the differentiation of neural crest cells into the different peripheral nerve cell types are poorly understood; however, some mechanisms have been identified. NRG1, a major regulator of SCs (Adlkofer & Lai, 2000; Esper, Pankonin, & Loeb, 2006; Garratt, Voiculescu, Topilko, Charnay, & Birchmeier, 2000; Meyer & Birchmeier, 1995; Riethmacher et al., 1997; Shah, Marchionni, Isaacs, Stroobant, & Anderson, 1994), notch signalling (Morrison et al., 2000) and transcription factors such as the SC identity factor Sox10 (Kuhlbrodt, Herbarth, Sock, Hermans-Borgmeyer, & Wegner, 1998) and Pax3 (Jacob et al., 2014) direct neural crest cells toward a SC precursor fate. Similarly to neural crest cells, SC precursors are migratory and proliferative but are distinguishable from neural crest cells by the expression of differentiation markers such as myelin protein zero (P0) and desert hedgehog (Dhh; Dong et al., 1999; Furlan & Adameyko, 2018; Jessen, Mirsky, & Lloyd, 2015; Meier, Parmantier, Brennan, Mirsky, & Jessen, 1999; Monk, Feltri, & Taveggia, 2015). However, despite their association with axons, SC precursors maintain a degree of plasticity, albeit more limited, in that they can also produce melanocytes (Adameyko et al., 2009; Nitzan, Pfaltzgraff, Labosky, & Kalcheim, 2013), endoneurial fibroblasts (Joseph et al., 2004), a proportion of enteric neurons (Uesaka, Nagashimada, & Enomoto, 2015) and parasympathetic neurons (Dyachuk et al., 2014; Espinosa-Medina et al., 2014). Within days however (E15-16 in the mouse), SC precursors convert to true SC progenitors that possess limited plasticity with their differentiation potential restricted to the SC lineage.
3 PERIPHERAL NERVE HOMEOSTASIS
Once the PNS is established and matured it forms a remarkably stable structure connecting tissues and organs to the interpreting machinery that constitutes the CNS. The trunks of major peripheral nerves consist of bundles of axons that are ensheathed by SCs (mSCs or nmSCs) embedded in the collagen-rich, vascularized extracellular matrix (ECM) of the endoneurium. The endoneurium is enclosed by highly-specialized fibroblast-like cells that polarise and tightly associate to form a protective cellular barrier known as the perineurial sheath and this unit is defined as a nerve fascicle. Larger peripheral nerves contain several fascicles that are surrounded by a common epineurium (Jessen et al., 2015; Zochodne, 2008). The majority of the cells in the peripheral nerve endoneurium are SCs with a 2:1 ratio of mSCs to nmSCs (Salonen, Aho, Roytta, & Peltonen, 1988; Stierli et al., 2018). In addition to SCs, the endoneurium also contains tissue-resident macrophages, endothelial cells and associated pericytes (Jessen et al., 2015). Furthermore, a new cell type was characterised recently that, while previously described as endoneurial fibroblasts (Joseph et al., 2004), is of unknown function in the homeostatic nerve, expresses markers of both pericytes and mesenchymal cells and makes contacts with different cell types within the endoneurium leading to them being re-named as tactocytes (Carr et al., 2019; Stierli et al., 2018).
Peripheral nerve is a highly quiescent tissue in the adult. Using cumulative long-term 5′ ethynyl-2′-deoxyurdine (EdU) labeling, together with immunostaining of endogenous cell-type specific markers, it was shown that that proliferation rates of all cell types within the mouse endoneurium is extremely low (Stierli et al., 2018). Most notably, the main cell type of the nerve, the mSC, appears not to divide at all, indicating that once this cell has matured it exists for the lifespan of the animal. nmSCs are also highly quiescent, although rare labeled cells showed these cells are occasionally replaced in the adult. However, even the most proliferative cell type, the resident macrophages, only turn over every 4 months (Figure 2).
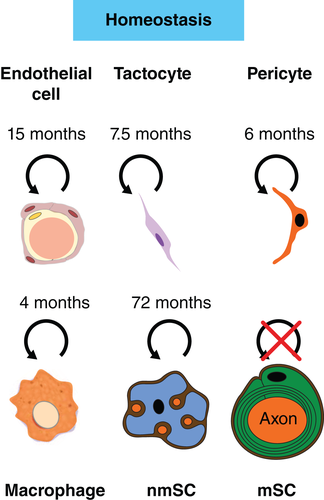
The quiescence of nerve tissue may reflect that peripheral nerves, once formed, tend to retain their structure and connections and also that peripheral nerves are relatively protected by the blood nerve barrier (BNB). Notably, however, peripheral nerves are more quiescent than the CNS, which is also protected by the similar blood brain barrier (Daneman & Prat, 2015). The CNS, however, likely requires greater adaptability than the PNS to ensure processes such as learning and memory continue in adulthood, which may require a higher level of cell turnover. An important aspect of this plasticity is thought to involve de-novo myelination of relevant axonal networks (Kaller, Lazari, Blanco-Duque, Sampaio-Baptista, & Johansen-Berg, 2017). In contrast to the PNS, in the CNS, an OL progenitor population (OPCs) is maintained throughout life that responds to a need for new myelination during adulthood (Birey, Kokkosis, & Aguirre, 2017). OPCs are a continually slowly-proliferating progenitor population dispersed throughout the CNS, that produce new OLs in response to a need for de-novo myelination that appears to be required for aspects of learning or as a result of demyelinating pathologies such as multiple sclerosis (Kaller et al., 2017; McKenzie et al., 2014; Saab & Nave, 2017; Xiao et al., 2016). However, there also appears to be a higher turnover of OLs in fully myelinated nerves, such as the optic nerve (Young et al., 2013). The reasons for this remain unclear, as the optic nerve is similarly protected by a blood barrier but may reflect increased damage to this region of the nervous system.
While the SC population in peripheral nerve trunks is highly quiescent, it remains a possibility that SCs in other less characterized regions of the PNS behave differently. For example, SCs may be more exposed to damage in regions such as in the skin, where nerves can be defasciculated and presumably more vulnerable. Moreover, the tSCs that are associated with nerve terminals may have higher levels of turnover, as previous studies have reported structural remodeling is likely during processes such as exercise and ageing (Gonzalez-Freire, de Cabo, Studenski, & Ferrucci, 2014; Jang & Van Remmen, 2011; Li, Lee, & Thompson, 2011; Lloyd, 2013; Rudolf, Khan, Labeit, & Deschenes, 2014; Valdez et al., 2010).
4 REGENERATING A NERVE
In contrast to the CNS, peripheral nerves are able to efficiently regenerate following an injury (Mahar & Cavalli, 2018). Following an injury to a nerve, axons downstream of the injury site degenerate and thus the major goal of the regeneration process is for axons to regrow back to their targets. Following a transection injury, the directed regrowth of neurons back to their targets requires migration through two distinct environments; (a) the bridge region of new tissue that forms to repair the wound site and (b) the distal stump, which remains attached to the target tissues but requires remodeling to provide an environment conducive for axonal regrowth. Axonal migration through these two environments requires distinct, complex multicellular processes that act to guide and sustain the regeneration of axons back to their targets (Cattin & Lloyd, 2016; Zochodne, 2008).
In both environments, SCs play a critical role in orchestrating the regenerative process. This involves a remarkable reprogramming process in which quiescent and highly specialized adult SCs dedifferentiate en masse to proliferating, progenitor-like SCs, which orchestrate the regenerative response, with distinct roles in the different regions of the nerve (Cattin & Lloyd, 2016; Jessen & Arthur-Farraj, 2019). Lineage-analysis of adult mSCs following injury, demonstrated the efficiency of this process with all mSCs downstream of the injury re-entering the cell cycle within days of an injury (Stierli et al., 2018; Figure 3).
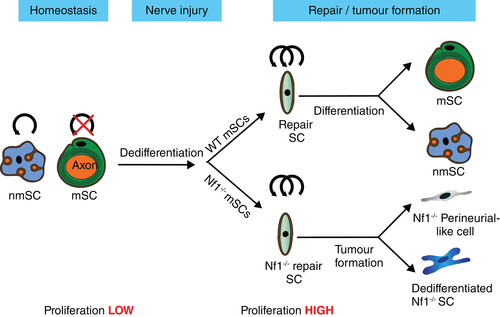
The ability of all mature SCs to proliferate following an injury should obviate the need for a further stem cell population to produce new SCs during the regeneration of peripheral nerves. Recent lineage analysis studies confirmed this by showing that the original mSC population appeared to be undiluted by an influx of stem cells from another source (Stierli et al., 2018). The lack of need for a further stem cell population to produce new SCs is further supported by studies that demonstrated that dedifferentiated SCs possess unlimited proliferative capacity (Mathon, Malcolm, Harrisingh, Cheng, & Lloyd, 2001). Moreover, a recent study reported that following depletion of ~70% of SCs, the remaining SCs were able to replenish the SC population, highlighting the apparent self-healing capacity of peripheral nerves (Gerber et al., 2019). These studies suggest that stimulating the intrinsic regenerative capacity of SCs is likely the best approach for improving nerve repair.
Less studied is how other regions of the PNS respond to injury, although this is also likely to involve SC plasticity. For example, during NMJ repair, tSCs undergo remodeling as they extend processes to previously unoccupied synaptic sites and guide axonal regrowth to these new sites (Kang et al., 2014). Moreover, tSCs are thought to modulate synaptic activity at the NMJ by responding to synaptic transmission, suggesting a further degree of plasticity. Whether this remodeling involves proliferation and/or the recruitment of SCs from other sources remains unclear (Auld & Robitaille, 2003; Todd, Darabid, & Robitaille, 2010).
Interestingly, Edu-labeling studies following nerve injury showed that all cell-types within the nerve endoneurium (resident macrophages, tactocytes, endothelial cells, and pericytes) were able to efficiently proliferate following injury to contribute to the regenerative response (Stierli et al., 2018). Thus, nerve tissue represents a further model by which a tissue can maintain itself during homeostasis and following injury, with the apparent lack of a separate stem cell/progenitor population to produce new cells in adulthood; rather all mature cell types within the nerve retain proliferative capacity and can replace themselves as required.
It is becoming increasingly clear that different tissues use distinct and varied mechanisms to maintain themselves in the adult and to repair following an injury with the classical model of slowly dividing stem cell populations generating the myriad of cell types to create new tissue being supplanted by more diverse models (Ge & Fuchs, 2018; Varga & Greten, 2017; Wells & Watt, 2018). These models include stem cells that divide frequently, precursor cell potential and reserve stem cell compartments that can be activated upon loss of the primary stem cell compartment (Tian et al., 2011) Furthermore, many cell-lineage committed cells possess the ability to dedifferentiate into more progenitor-like cells in tissues such as the skin (Donati et al., 2017; Stange et al., 2013), intestine (Beumer & Clevers, 2016) and stomach and trachea (Tata et al., 2013) Furthermore, postinjury, different stem or progenitor populations can be used depending on the insult (Ge & Fuchs, 2018; Ito et al., 2007; van Es et al., 2012; Varga & Greten, 2017; Wells & Watt, 2018). Peripheral nerve is most reminiscent of other highly regenerative tissues such as the liver or the vasculature, which are also normally relatively quiescent yet have remarkable regenerative capabilities that mainly involves the proliferation of mature cell types but while similar, unlike these tissues, peripheral nerve does not appear to have access to a further distinct stem cell population that can aid with the regeneration process following severe injury. In all tissues however, irrespective of the mechanism used to produce new cells, the processes needs to be tightly controlled in order to prevent pathologies such as cancer (Simon & Frisen, 2007).
It is particularly intriguing that the CNS and PNS use such distinct mechanisms to produce their respective glia. OLs in the CNS appear to be postmitotic and when new cells are required, they are produced from the slowly proliferating OPC population that is scattered throughout the CNS. The reasons for these differences can only be speculated upon but may reflect that a continually proliferating, progenitor population better serves the plasticity required by the CNS. A potential downside to this mechanism is that whilst providing a primed and ready source for rapid new myelination it also provides a pool more susceptible to tumor development. Consistent with this idea, malignant tumors are more frequent in the CNS than in the PNS, which perhaps reflects the presence of a more susceptible progenitor population.
5 THE MANY ROLES OF SCs DURING NERVE REGENERATION
The reprogramming of SCs to a pro-repair progenitor-like state is a poorly understood process. The initiating signal from the damaged axons remains unknown but leads to a strong sustained signal through the ERK-signaling pathway, lasting several days, which has been shown to be sufficient to drive the reprogramming process (Napoli et al., 2012). Whilst some positive and negative regulators of this process have been identified (reviewed in (Jessen & Arthur-Farraj, 2019)), the mechanistic pathways responsible for the reprogramming process remains poorly understood.
Once dedifferentiated, SCs have multiple roles in orchestrating nerve regeneration with different roles depending on their location (Cattin & Lloyd, 2016). The best-characterized are those within the newly-formed nerve bridge at the injury site and those that remain within the trunk of the distal stump. Consistent with these different roles, RNA-profiling can separate them into distinct populations showing that the microenvironment further changes the reprogramming of these cells (Clements et al., 2017).
The nerve bridge is new tissue that initially consists mostly of inflammatory cells (macrophages, neutrophils, and fibroblasts), that forms to re-join the two nerve stumps. This provides a large, apparently hostile, nondirectional environment through which re-growing axons need to find their way toward the distal stump. This involves a complex multicellular process, initiated by hypoxic macrophages within the bridge, which by the creation of a Vascular endothelial growth factor (VEGF) gradient leads to the formation of a newly-formed polarized vasculature across the bridge. SC cords then use this vascular track to carry re-growing axons across the wound site before entry into the distal stump (Cattin et al., 2015; Cattin & Lloyd, 2016; Parrinello et al., 2010).
Analysis of the bridge SC population showed that they differ from the distal stump population with an increased proliferation rate and enhanced mesenchymal characteristics (Clements et al., 2017). Consistent with a mesenchymal phenotype, SCs are migratory but to collectively migrate as cellular cords involves a further switch in their behavior, triggered by EphB2 signaling as a result of direct contact with Ephrin B-expressing fibroblasts upon entry into the wound. This results in the modification and stabilization of the stemness transcription factor Sox-2 leading to the relocalization of N-cadherin to cell–cell junctions, which mediates SC clustering into cords (Parrinello et al., 2010). Sox2 is induced as part of the SC reprogramming process, consistent with its well-characterized role as a “stemness” factor and Sox2 likely has multiple roles in the progenitor-like behavior of these cells (Feng & Wen, 2015). However, the requirement of Sox-2 to induce collective SC migration is a previously unappreciated role for a stemness factor to control other aspects of progenitor-cell behavior such as sustained migration. However, the expression of Sox-2 needs to be tightly regulated, as sustained expression of Sox-2 following nerve injury appears to be sufficient to maintain SCs in their dedifferentiated state (Roberts et al., 2017).
Fibroblasts also secrete TGFβ at wound sites and this well-known initiator of epithelial mesenchymal transition (EMT; Xu, Lamouille, & Derynck, 2009), likely contributes to the more mesenchymal characteristics of the SCs found in the bridge region but is also appears to synergise with Ephrin signaling to promote SC collective migration by enhancing N-cadherin relocalization at SC cell: cell junctions (Clements et al., 2017). Consistent with this, loss of TGFβR2 in SCs decreased SC migration in the bridge leading to delayed and misdirected peripheral nerve regeneration, a phenotype reminiscent of loss of EphB2. Thus, while the reprogramming process of SCs is reminiscent of a classical EMT transition, the elevation of TGFβ signaling in the bridge further modulates the behavior of these cells (Arthur-Farraj et al., 2017; Clements et al., 2017). EMT is initiated in many epithelial tissues, such as skin, during wound repair and facilitates the phenotypic changes that induce the migration of cells into the damaged tissue (Savagner et al., 2005; Shaw & Martin, 2016). Thus, there appears to be clear parallels between these processes and the mechanisms by which SCs can become migratory following an injury.
Within the distal stump, dedifferentiated SCs have different roles in orchestrating the regeneration process and their distinct gene-expression profile reflects these different roles (Clements et al., 2017). SCs within the distal stump differ from both bridge SCs and immature SCs (which are the precursors to adult SCs during development). For example, dedifferentiated SCs express Olig1, Shh and GDNF at higher levels than immature SCs (Arthur-Farraj et al., 2012). Whether this means these different populations of SCs are intrinsically different or result from distinct environmental signals remains unclear but it seems particularly likely that the differences between bridge and distal SCs is the result of their local environment.
SCs within the distal stump have multiple cell autonomous and non-cell autonomous roles in orchestrating the efficient regrowth of axons back to their targets. Cell autonomous roles include the clearance of debris and dramatic changes in morphology, as SCs elongate along their basal lamina to provide a conducive substrate for axonal regrowth (Brosius Lutz et al., 2017; Gomez-Sanchez et al., 2015; Gomez-Sanchez et al., 2017). Non-cell autonomous effects result from the SCs secreting a number of factors, which control the behavior of other cell types important for the regeneration of a nerve (Napoli et al., 2012). Unknown signals downstream of the ERK-signaling pathway in SCs open the BNB and a variety of cytokines secreted in response to this signal, such as CCL2, are associated with a massive influx of inflammatory cells, which together with SCs, remodel the environment to ensure efficient axonal regrowth (Martini, Fischer, Lopez-Vales, & David, 2008; Napoli et al., 2012). SCs also secrete a number of neurotrophins such as artemin, GDNF and BDNF that appear to be essential for the survival and regrowth of axons. The secretion of these neurotrophins is under the control of the transcription factor c-jun that is upregulated in SCs following nerve injury, with loss of c-jun impairing neuronal survival leading to defects in axonal regeneration and reduced functional recovery (Arthur-Farraj et al., 2012; Fontana et al., 2012; Parkinson et al., 2008).
Once axons have regrown, in response to axonal signals, SCs exit the cell cycle and redifferentiate. Lineage-analysis studies showed that within a regenerated nerve, SCs maintain their identity but can switch between myelinating and nonmyelinating types (Gomez-Sanchez et al., 2017; Stierli et al., 2018). These findings are consistent with prior cross-anastomose experiments, in which myelinated nerves were joined to unmyelinated nerves, which suggested that SCs could switch from one form to the other (Aguayo, Epps, Charron, & Bray, 1976). Similarly to SCs, the other cell-types in the nerve exit the cell cycle and the inflammatory response resolves as the nerve returns to a homeostatic state. However, a regenerated nerve is clearly distinguishable from an uninjured nerve. There are more axons, reflecting increased sprouting during regeneration, moreover there is a three to fourfold increase in the density of all cell-types within the nerve (Fawcett & Keynes, 1990; Salonen et al., 1988; Stierli et al., 2018). Interestingly, the ratios of the cells remain constant suggesting that inter-cellular signals maintain their relative proportions (Stierli et al., 2018). A further difference is the increased levels of ECM that remain in a regenerated nerve, with high levels of fibronectin, laminin, and collagen suffusing the nerve (Stierli et al., 2018). ECM deposition is a ubiquitous aspect of a tissue injury (Eming, Wynn, & Martin, 2017) and it is likely that failure to clear injury-induced ECM contributes to imperfect recovery. Targeting the clearance of accumulated ECM could thus provide a strategy for improving tissue repair.
6 SCs PLASTICITY IN TISSUE REPAIR
While SCs appear to retain their lineage during nerve regeneration, increasing evidence suggests they retain levels of multipotency that can contribute to the regeneration of other tissues. A number of studies have suggested that adult SCs retain the multipotency of SC precursors (Petersen & Adameyko, 2017; Real, Glavieux-Pardanaud, Vaigot, Le-Douarin, & Dupin, 2005; Widera et al., 2011). However, much of this work was performed in-vitro or alternatively in relatively nonphysiological conditions in-vivo, which suggests that the normal nerve microenvironment restricts the plasticity of adult SCs. Two separate studies are striking examples of how the environment can influence SC plasticity. In the first, an injured section of nerve was sutured into connective tissue adjacent to muscle. Away from the nerve environment, lineage-traced SCs lost their lineage restriction and were able to form melanocytes (Adameyko et al., 2009). In the second study, a tumorigenic environment influenced SC plasticity. In this model, neurofibroma tumors develop from Nf1−/− mSCs. Lineage-analysis showed that while these cells formed the majority SC-like component of the tumor, in addition, mSCs could be the cell-of-origin for many of the perineurial-like cells that are a known large component of these tumors (Ribeiro et al., 2013; Stierli et al., 2018). This increased plasticity was only seen within the tumour environment, as in other regions of the nerve, the Nf1−/− SCs retained their lineage. These findings implicate that both genetic changes and the microenvironment can synergise to increase the plasticity of these normally lineage-restricted cells.
Together, these findings emphasize the importance of the environment in restricting cell plasticity (Anderson, 2001) and demonstrate that modulating the physiological environment can release this restriction and may allow cells to produce other cell lineages. These findings are consistent with studies of other tissues, which have suggested that the apparent multipotency of progenitor cells when cultured in-vitro is not mimicked in-vivo (Anderson, 2001; Gabay, Lowell, Rubin, & Anderson, 2003; Guimaraes-Camboa et al., 2017). For example, the reported multipotency of pericytes in-vitro was contradicted by recent lineage analysis in the brain, which showed that pericytes maintain their cell lineage in their normal environment in-vivo (Guimaraes-Camboa et al., 2017).
Nevertheless, there are reports of SCs showing a degree of plasticity and contributing to the repair of other tissues in adulthood. There are for example, several reports that SCs contribute to CNS remyelination in both experimental models and in pathologies such as multiple sclerosis (Dusart, Marty, & Peschanski, 1992; Felts et al., 2005; Ghatak, Hirano, Doron, & Zimmerman, 1973; Guest, Hiester, & Bunge, 2005; Itoyama, Ohnishi, Tateishi, Kuroiwa, & Webster, 1985; Itoyama, Webster, Richardson Jr., & Trapp, 1983; Snyder, Valsamis, Stone, & Raine, 1975). However, recent fate mapping studies demonstrated that the majority of these cells are actually derived from OPCs or a poorly characterised Foxj1-expressing sub-set of peripheral SCs (Assinck et al., 2017; Ma et al., 2018; Zawadzka et al., 2010). The extent of PNS contribution to CNS remyelination seems to be dependent on the location of the injury with a higher contribution associated with locations close to the spinal roots. Notably this suggests that whereas OPCs can apparently switch lineage, SCs retain their characteristics once they enter into the environment of the CNS.
A more provocative report suggested that SCs contribute to the continuous growth of incisor teeth in the mouse (Kaukua et al., 2014). Lineage analysis indicated that SCs precursors during development and SCs in adulthood give rise to the mesenchymal stem cells that differentiate into the pulp cells and odontoblasts of the tooth. However, an alternative explanation could be that endoneurial fibroblasts/tactocytes are the responsible cell, as a recent study showed these cells can act as mesenchymal precursor cells in the skin (Carr et al., 2019). Further work is required to ascertain the true multipotent potential of adult SCs in all regions of the PNS.
7 SCs AS PROMOTERS OF TISSUE REGENERATION
While it is well documented that SCs have remarkable regenerative capabilities and orchestrate the regeneration of nerves, a more unappreciated role is the requirement of innervation and specifically SCs for the regeneration of other tissues and their contribution to the microenvironment of stem cell niches (Carr & Johnston, 2017). This apparent requirement for innervation to create new tissue in the adult may have important implications for the development and spread of many types of cancers (Figure 4).

Newts have remarkable regenerative properties in that they can regenerate an entire limb. This involves the formation of a blastema at the cut site, which consists of the stem cells that will recreate the limb (Tanaka, 2016). It was known for decades that innervation was required for the regeneration process but more recent work showed that it was actually dedifferentiated SCs that were required and they acted by secreting a factor (newt anterior gradient), that was required to sustain the proliferation of the blastema (Kumar & Brockes, 2012; Kumar, Godwin, Gates, Garza-Garcia, & Brockes, 2007). Remarkably, a similar SC requirement is seen for the regeneration of the digit tip in mammals. The distal digit tip is the only multi-tissue part of the body capable of such a regenerative process (Borgens, 1982; Han, Yang, Lee, Allan, & Muneoka, 2008; Neufeld & Zhao, 1995). This also involves the formation of a heterogeneous blastema containing the precursors of several cell lineages (Lehoczky, Robert, & Tabin, 2011; Rinkevich, Lindau, Ueno, Longaker, & Weissman, 2011). Digit tip regeneration also depends on nerve innervation, with dedifferentiated SCs playing an important role (Johnston et al., 2016). They also act by secreting growth factors that promote the self-renewal of precursor cells in the blastema but in this case, the relevant factors are OSM and PDGF-AA (Johnston et al., 2016).
Dedifferentiated SCs also appear to have an important role in promoting wound healing in the skin (Johnston et al., 2013; Parfejevs et al., 2018). Following a punch-injury to the skin, the repair process also requires a multicellular response to repair the wound. Dedifferentiated SCs can be observed migrating into the wound bed of the regenerating dermis, often but not always accompanied by axons. Moreover, depletion of these cells inhibits wound closure by impairing both epithelial proliferation and the myofibroblast contraction necessary for wound closure. Again, SCs appear to act by modulating the behavior of other cell-types involved in the repair process in this case by the secretion of factors that enhance TGFβ signaling to increase the myofibroblast phenotype within the repairing wound (Parfejevs et al., 2018).
SCs also appear to control the behavior of stem cells in the hematopoietic niche but in this situation reportedly act by maintaining the dormancy of the stem cells (Yamazaki et al., 2011; Yamazaki & Nakauchi, 2014). Hematopoietic stem cells (HSCs) were recently observed to be in close contact with SCs within the bone marrow niche and denervation of the niche resulted in the loss of HSCs from the bone marrow (Yamazaki et al., 2011). Subsequently, it was shown that SCs contribute to HSCs dormancy by activating latent TGFβ that is sequestered in the ECM of the bone marrow niche and which maintains HSC dormancy.
Together these results show an important role for nerves, with many of the effects mediated by SCs, in modifying the behavior of stem cells and are often a required microenvironmental signal for the formation of new tissue in the adult. Unsurprisingly therefore, innervation is emerging as an important component of the tumor microenvironment. Moreover, tumors once innervated are presented with an alternative route to spread and perineural invasion is increasingly appreciated as route of cancer dissemination (Bapat, Hostetter, Von Hoff, & Han, 2011; Boilly, Faulkner, Jobling, & Hondermarck, 2017; Liebig, Ayala, Wilks, Berger, & Albo, 2009; Saloman, Albers, Rhim, & Davis, 2016).
Cancers are often referred to as unrepaired wounds and like the repair of a tissue, cancer development requires the co-operation (or co-option) of other cell-types to develop a conducive environment to support the expansion of the tumour cells. The so-called tumor microenvironment is now well established as a critical component of a developing tumor and has emerged as an attractive therapeutic target because of the greater genetic stability of the nontumor cells (Arwert, Hoste, & Watt, 2012; Chen et al., 2015; Dvorak, 1986; Hanahan & Weinberg, 2011). Whilst vascularization and inflammatory cells are accepted components of the tumor microenvironment, the role of innervation has largely been ignored, probably as it was a little counter-intuitive that a tumor would require innervation. However, the regenerative field has clearly demonstrated that innervation is an important component of the microenvironment required to make new tissue in the adult and thus would seem likely to contribute to tumor development.
In a seminal study, autonomic nerves were shown to have a critical role in the development and spread of prostate cancer and since this study, innervation has been shown to be an important component of the development of gastric, pancreatic and other cancers (Faulkner, Jobling, March, Jiang, & Hondermarck, 2019; Magnon et al., 2013). How tumors become innervated is unknown with most research focusing on the attraction of neurons and their potential regulation of the tumor microenvironment (Faulkner et al., 2019). However, it seems likely that tumour innervation is likely to recapitulate normal nerve regeneration and/or the innervation processes that take place during tissue repair. Thus, SCs are likely to play an important role in both initiating tumor innervation and then contributing to the microenvironment that can promote and maintain tumor development. While this field is in its infancy, targeting tumor innervation and its resulting contribution to the tumor microenvironment is an attractive new target for therapeutics to inhibit the development and spread of cancer.
8 CONCLUSIONS
Peripheral nerves show remarkable regenerative capabilities that allow both the repair of damaged nerves and also contribute to the repair of other tissues. Underlying these regenerative capacities is the remarkable plasticity of adult SCs that can be reprogrammed during injury to either orchestrate peripheral nerve repair or migrate to injured tissue to aid in the repair process. Understanding the mechanisms underlying these complex autonomous and non-cell autonomous behaviors has important therapeutic implications for improving nerve and tissue regeneration but also for the future treatment of pathologies such as cancer.
ACKNOWLEDGMENT
This work was supported by a program grant from Cancer Research UK (C378/A4308).
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.