The conceptual introduction of the “demyelinating Schwann cell” in peripheral demyelinating neuropathies
Abstract
Myelinating Schwann cells undergo irreversible demyelination in many demyelinating neuropathies that show complete demyelination of the internode. Dedifferentiation, reprogramming, and myelin clearance processes—which are specifically discussed in this article—appear to be shared by various demyelinating peripheral conditions, such as Wallerian degeneration, immune-mediated, and toxic demyelinating diseases. We propose to introduce the concept of the “demyelinating Schwann cell (DSC)” as a novel cell phenotype, which has specific properties required for myelin sheath clearance. We anticipate that the introduction of the DSC concept will provide a significant advance in understanding the pathophysiological mechanisms of demyelinating peripheral neuropathies.
1 INTRODUCTION
The myelin sheath of the peripheral nerve, which is produced by the Schwann cell (SC), is an essential structure for the saltatory conduction of axonal action potentials (Salzer, Brophy, & Peles, 2008). The myelin sheath covers a segment of axon, which is referred to as the internode, with each myelinated segment being flanked by the nodes of Ranvier. The glial regions that surround the node are named paranodal loops and cover axonal regions named paranodes (Salzer et al., 2008). Demyelination can be complete, meaning the internode is lost, such as in segmental demyelination, or partial, such as in paranodal demyelination when paranodal loops detach from the paranodes and retract from the axon. Both cases, however, result in slowing of nerve conduction velocity (NCV) and in conduction block that reduces peripheral nerve function causing devastating sensory and motor disabilities (Dalakas, 2011).
Demyelinating diseases have diverse causes including hereditary defects in myelin formation, autoantibodies to myelin and nodal or paranodal proteins, and other toxic attacks on the SC. As demyelination occurs as a result of distinct causes in various demyelinating neuropathies, demyelination mechanisms have been considered to be specific for each different demyelinating cause. For example, macrophage-mediated myelin stripping has been considered a prime mechanism of inflammatory demyelination; immune-independent processes such as endoplasmic reticulum stress and metabolic alterations are considered as the causes of toxic and hereditary demyelination (Calle, Berciano, Fernández, & Lafarga, 1999; Harry et al., 1989; Pennuto et al., 2008; Shin et al., 2010). However, several morphological changes associated with myelin destruction by the SC, such as paranodal retraction, collapse of Schmidt–Lanterman incisures (SLI), lysosomal induction, and even some molecular changes are commonly observed in the SC in diverse demyelinating conditions (Freidin, Asche-Godin, & Abrams, 2015; Jang et al., 2016; Jung, Cai, Jang, et al., 2011b; Klein, Groh, Wettmarshausen, & Martini, 2014; Lampert, 1969; Lampert & Garrett, 1971; Lee et al., 2014b). This suggests that there may be a general feature of demyelination reflecting the myelinolytic activity of SC regardless of the demyelination cause (Table 1). To clarify this question, here we review the common morphological and biochemical features of demyelination in three different paradigms of peripheral nerve demyelination.
| Common features of demyelination | |||||
|---|---|---|---|---|---|
| Changes in noncompact regions (PN and SLI) | Autophagy and lysosome | p75 or c-Juna | Hypertrophic or activated SC | Macrophage infiltration | |
| Nerve injury (Wallerian degeneration) | Webster (1965); Williams (1971); Ghabriel (1979); Jung (2011) | Holtzman, (1965); Gomez-Sanchez (2015); Jang (2016) | Jessen, (2008)b | Fisher (1963); Satinsky (1964); Stoll (1989); Jang (2016) | O'Daly (1967); Stoll (1989); Hirata (2002) |
| Inflammatory segmental demyelination | Ballin (1969b); Saida (1978) | Jang (2016, 2017) | Stoll (1993); Hutton (2011); Jang (2016) | Lampert (1969); Saida (1978); Jang (2016) | Lampert (1969); Saida (1978); Prineas (1981); Jang (2016) |
| Toxic demyelination (lysophosphatidylcholine, tellurium, diphtheritic, calcium ionophore) | Webster (1961); Allt (1969); Lampert (1971); Gregson (1973); Ghabriel (1981);b Smith (1988) | Weller (1966); Calle (1999) | Toews (1992); Stoll (1993) | Smith (1988); Griffin (1990); Calle (1999) | Webster (1961); Lampert (1971); Smith (1988); Calle (1999) |
- PN = paranodal region; SLI = Schmidt–Lanterman incisure.
- a Dedifferentiation markers.
- b References there in.
2 EARLY DEMYELINATION PROCESSES IN WALLERIAN DEGENERATION
Physical nerve injury results in axonal degeneration and subsequent myelin destruction, a process known as Wallerian degeneration (WD). Although demyelination secondary to axonal degeneration appears to be distinct from primary demyelination in the presence of an intact axon, recent studies have suggested that the SC plays a pivotal role in myelin destruction in both conditions. Here, we first review how the SC contributes to remove the myelin sheath in WD. The early SC responses to nerve injury are represented by morphological changes of noncompact regions such as paranodal retraction and the dilatation of SLI, long before the appearance of obvious axonal degeneration (Causey & Palmer, 1953; Ghabriel & Allt, 1979; Webster, 1965; Williams & Hall, 1971). These phenomena are accompanied by the rapid expression of the lipolytic enzyme phospholipase A2 and activation of demyelinating mechanisms such as neuregulin-ErbB2 signaling in these regions, within hours following nerve injury; inhibition of these biochemical pathways suppresses demyelination in WD (De, Trigueros, Kalyvas, & David, 2003; Guertin, Zhang, Mak, Alberta, & Kim, 2005; Jung, Cai, Lee, et al., 2011b; Paul & Gregson, 1992). The SLI alterations are essential for myelin fragmentation as they result in the formation of the myelin compartments or chambers in which three-dimensional regional architecture of the myelin sheath is no longer maintained (Figure 1). It has been shown that the inhibition of actin polymerization in SLI prevents myelin fragmentation without affecting axonal degeneration (Jung, Cai, Lee, et al., 2011b), suggesting that actin polymerization by SC in SLI is also participating in myelin fragmentation during WD. These changes also involve the recycling of E-cadherin (Jung, Cai, Lee, et al., 2011b), an important junctional protein that organizes adherens junctions in SLI, paranodal loops, and at the outer cleft (Fannon et al., 1995; Tricaud, Perrin-Tricaud, Brusés, & Rutishauser, 2005).
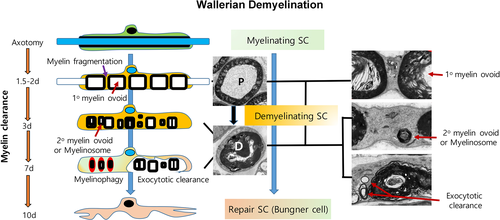
Once myelin compartmentation due to SLI changes is complete, the chamber is swollen by increased hydraulic pressure thereby displacing compact myelin outward and forming intracellular myelin primary ovoids (Johnson, McNabb, & Rossiter, 1949, 1950). At this point, the abaxonal cytoplasm and the Cajal bands are almost invisible due to the swollen myelin ovoids. We previously designated this type of degenerating nerve as the primary ovoid (P)-type fiber which is maintained for about 1 day (Jang et al., 2016; Figure 1). Shortly after, the compact myelin disintegrates forming out/in-foldings, and the abaxonal cytoplasm dilates until the apposition between the outer plasma membrane of the SC and the first layer of myelin is disrupted (Stoll, Griffin, Li, & Trapp, 1989). This inflated SC cytoplasm is filled with rough endoplasmic reticulum (RER) and various vesicular structures resulting from the alteration of membranous organelles with disintegrated compact myelin, and SC features have been described as “hypertrophic SC” in WD in previous reports (Fisher & Turano, 1963; Satinsky, Pepe, & Liu, 1964; Stoll et al., 1989; Takada, Yuasa, & Araki, 2011). The primary ovoids then shrink and the secondary ovoids or myelinosomes which were pinched off from the primary ovoid appear in the hypertrophic cytoplasm of the SC (Holtzman & Novikoff, 1965; Jang et al., 2016; Stoll et al., 1989; Figures 1 and 2). The heterogeneous morphologies of degenerating compact myelin membranes within SC cytoplasm are observed 3 days after injury and maintained for a week, and this was considered as morphological evidence for active myelin destruction by SCs during WD (Holtzman & Novikoff, 1965; Satinsky et al., 1964; Stoll et al., 1989; Williams & Hall, 1971; Figure 2). We previously designated this stage of myelin degeneration as demyelinating (D)-type (Jang et al., 2016), and a demyelinating SC (DSC) in Wallerian demyelination encompasses SC with both P- and D-type characteristics of degenerating nerve fibers (Figure 1).
3 MYELIN PHAGOCYTOSIS, AUTOPHAGY, AND MYELINOPHAGY IN WALLERIAN DEMYELINATION
To properly describe the demyelination process driven by DSCs in WD, alternative terminologies for the demyelination process need to be considered. While “breakdown” and “disintegration” of the myelin sheath has been used to describe the initial destruction of the bulky structure of compact myelin (Blümcke & Niedorf, 1966; Johnson et al., 1950; O'Daly & Imaeda, 1967), myelin “digestion” has also been used to depict the biochemical degradation of myelin proteins and lipids to amino acids and fatty acids, respectively (Blümcke & Niedorf, 1966; Fernandez-Valle, Bunge, & Bunge, 1995; Gomez-Sanchez et al., 2015; Holtzman & Novikoff, 1965; Johnson et al., 1949). Although SCs appear to be able to digest their own myelin sheath with activated hydrolytic enzymes (De et al., 2003; Hallpike & Adams, 1969; Webster, 1973), a large proportion of the dismantled myelin sheath is actually expelled or rejected by the SC as a large deposit that also contains degenerated axoplasmic parts (Figures 1-3). These “chunks” of compact myelin are cleared by macrophages that begin to infiltrate the site from the blood, 3–4 days after injury (Beuche & Friede, 1984; Brosius Lutz et al., 2017; Crang & Blakemore, 1986; Hirata & Kawabuchi, 2002; O'Daly & Imaeda, 1967; Stoll et al., 1989). As such, myelin clearance is cooperatively performed by the SC and macrophages and reaches a peak around 7 days after injury. Most SCs no longer contain any more myelin debris within the cytoplasm 2 weeks after injury (Liu, Yang, & Yang, 1995), and Büngner bands can be visualized in cross-section at this time (Figure 1).
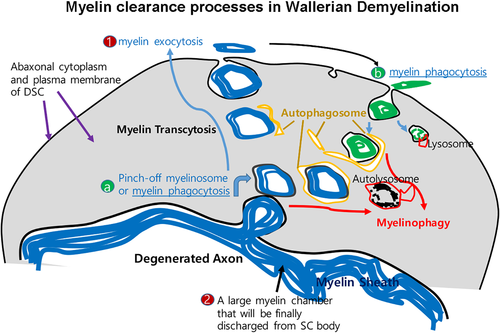
Myelin phagocytosis has long been considered as the main cellular mechanism by which DSCs digest their own myelin. Indeed the appearance of small intracellular myelin debris in vivo (Hirata, Mitoma, Ueno, He, & Kawabuchi, 1999; Liu et al., 1995) and the SCs' ability to uptake myelin in vitro (Bigbee, Yoshino, & DeVries, 1987; Salzer, Williams, Glaser, & Bunge, 1980) implicate phagocytosis by SC during WD. The upregulation of MAC2, a phagocyte marker, and the increased number of lysosomes in SCs following nerve injury also provide indirect evidence for SC phagocytosis in vivo (Holtzman & Novikoff, 1965; Jang, Cai, Jang, et al., 2011; Reichert, Saada, & Rotshenker, 1994). It has been recently reported that the receptor for phagocytosis, TAM receptor (Tyro3, Axl, and Mer), in the SC also plays a role in myelin phagocytosis after nerve injury (Brosius Lutz et al., 2017). However, how and when SC phagocytosis occurs during WD is still enigmatic. SC phagocytosis could occur during at least two different points during WD (Figure 2). First of all, the incorporation of small myelin clumps (called secondary ovoids or myelinosomes) into the cytoplasm of DSCs from the primary ovoid could be termed phagocytosis (Figure 2a). However, because phagocytosis is a process by which cells ingest extracellular materials, but myelin is initially an extension of the SC membrane, the terminology can be used to describe the generation of intracellular myelin clumps by a pinch-off process (Gomez-Sanchez et al., 2015; Goodrum & Bouldin, 1996; Stoll et al., 1989). Second, SCs can uptake myelin debris that has been discharged by the SC into extracellular space (but still within the endoneurial tube) in vivo during late stages of WD (Figure 2b). This seems to be in line with a recent finding showing that the ability of phagocytotic myelin digestion by SCs appears to peak around 1 week after injury (Brosius Lutz et al., 2017). Because this potential phagocytotic activity of DSCs temporally overlaps with phagocytotic myelin digestion by infiltrated macrophages (Brosius Lutz et al., 2017; Gomez-Sanchez et al., 2015; Hirata & Kawabuchi, 2002), it is still unclear whether the phagocytotic myelin digestion by DSCs during this period makes an important contribution to complete myelin digestion (Brosius Lutz et al., 2017; Goodrum & Bouldin, 1996; Holtzman & Novikoff, 1965). Indeed, the TAM receptor knockout mutant mice exhibited only a minimal and temporary delay in myelin digestion (Brosius Lutz et al., 2017), suggesting a nonessential role for this receptor in phagocytotic myelin digestion by SCs or a strong compensatory myelin digestion mediated by macrophages.
The concept of SC myelin phagocytosis appears to be highly related to the ability of the SC to digest myelin (Bigbee et al., 1987), and it has been recently shown that autophagy-mediated myelin digestion termed “myelinophagy” plays a role in myelin destruction in WD (Gomez-Sanchez et al., 2015; Jang et al., 2016). Autophagy is a self-destructive mechanism in cells to remove damaged cell organelles or unused proteins (Menzies et al., 2017). The double membrane structure of the autophagosome encloses a target organelle and eventually fuses with a lysosome for digestion. An autophagosome-like structure has been previously identified in the hypertrophic D-type SC during WD, and high levels of lysosomal protein induction after nerve injury further supported the autolysosome-dependent myelin digestion by SC (Calle et al., 1999; Holtzman & Novikoff, 1965; Jang et al., 2016; Jung, Cai, Jang, et al., 2011a). The myelin targets to be destroyed by autophagy or myelinophagy could be derived from at least two sources. As the primary ovoid is too big to be encircled by an autophagosome, autophagosomes appear to surround the secondary ovoid or myelinosome which had been pinched off from the primary myelin ovoid (Figure 2a). Second, smaller myelin fragments, which have been phagocytosed from extracellular space during the late stages of WD (Figure 2b), could be digested by an autophagy-dependent mechanism as autophagy is known to play a role in phagosome digestion in other cells (Shui et al., 2008). In this sense, myelinophagy could be a final digestion mechanism of phagocytosed myelin debris, wherever the sources are, in the DSC. Conversely, myelinophagy and myelin phagocytotic digestion could be distinct from one another. For example, myelinophagy may be selective for the digestion of the myelin debris generated from the pinch-off process (Figure 2a). Thus, further cellular and molecular studies will be necessary to determine the mechanistic dichotomy of myelinophagy and myelin phagocytosis by DSCs during WD.
4 MYELIN TRANSCYTOSIS AND EXOCYTOTIC CLEARANCE IN WALLERIAN DEMYELINATION
Many descriptive works have long postulated the SCs' ability to expel myelin clumps at late stages of Wallerian demyelination (Beuche & Friede, 1984; Crang & Blakemore, 1986; O'Daly & Imaeda, 1967). Electron microscopic observation reveals that DSCs at the D-stage are filled with many intracellular membranous structures with inconsistent size and shape. Some of these show obvious myelin lamellae (Gomez-Sanchez et al., 2015; Holtzman & Novikoff, 1965; Williams & Hall, 1971), representing incorporated myelin debris after myelin fragmentation. Some of these myelinosomes contact abaxonal SC plasma membrane, to release myelin contents into the extracellular space as in exocytosis (Hirata & Kawabuchi, 2002). According to the epithelial-like nature of the myelinating SC structure (Ozçelik et al., 2010), the apical lumen of the SC is the axon and the abaxonal domain of the myelinating SC is the basolateral side. Therefore, the expulsion of myelins containing axonal debris to the abaxonal extracellular space can be considered as myelin debris transcytosis (Figure 2). In this sense, myelin transcytosis encompasses a form of myelin phagocytosis, which means the pinch-off process and the subsequent exocytosis (Figure 2). Even though the molecular mechanisms of this process are still largely unknown, various membranous structures observed in the cytoplasm of DSCs such as autophagosomes, endosomes, expanded endoplasmic reticulum, multivesicular bodies, and primary lysosomes could be implicated in the transcytotic myelin clearance (Brosius Lutz et al., 2017; Gomez-Sanchez et al., 2015; Holtzman & Novikoff, 1965).
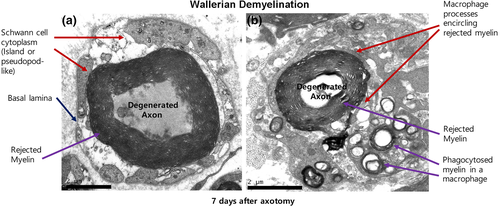
Large clumps of myelin that contain the degenerated axon in their centre are also often observed in the extracellular space in the late stage of WD (Beuche & Friede, 1984; Goodrum & Bouldin, 1996; Jang et al., 2017; Ohara, Takahashi, & Ikuta, 1986; Stoll et al., 1989; Figures 2 and 3), and they can be clearly visualized following abrogation of macrophage infiltration as the rejected myelin would be rapidly removed by macrophages (Beuche & Friede, 1984; Goodrum & Bouldin, 1996; Jang et al., 2017; Figure 3). The terminology “discharge or rejection” has been used to describe this big chunk of myelin; however, the process could also be an exocytotic clearance in the respect of extracellular shedding of the intracellular large myelin clump from the SC body. This exocytotic shedding of large myelin clumps would inevitably require the fission/fusion of plasma membranes. This suggests that SCs could activate the machinery for membrane remodeling during WD. Lysosomes, which play an essential role in plasma membrane remodeling and repair (Corrotte, Castro-Gomes, Koushik, & Andrews, 2015; Reddy, Caler, & Andrews, 2001), may also be involved in this process. Indeed, lysosomal exocytosis, a mechanism of plasma membrane repair, was reported to participate in peripheral nerve repair (Chen et al., 2012). Moreover it has recently been shown that SC autophagy is involved not only in myelinophagy but also in the myelin rejection process during WD (Jang et al., 2017). Thus, besides the role of the canonical autolysosome in myelin digestion, the autophagosome and lysosomes could also contribute to membrane remodelling for exocytotic myelin clearance.
Therefore, myelin clearance by the DSC following myelin fragmentation is likely to rely on two representative mechanisms, myelinophagy and myelin exocytotic clearance, to effectively and completely remove myelin debris from SCs. Thus, the SC demyelination program could be considered as “irreversible” in terms of complete myelin clearance from the SC once the program had been activated.
5 DEMYELINATING SCHWANN CELLS IN INFLAMMATORY SEGMENTAL DEMYELINATION
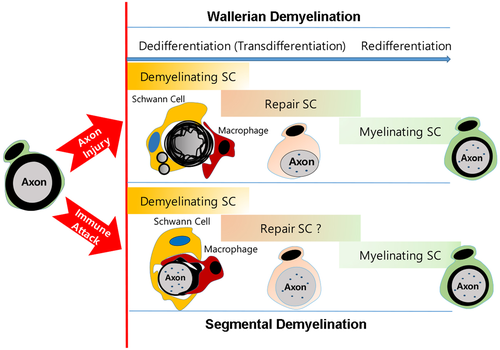
In contrast to WD, in inflammatory, hereditary, and toxic demyelinating neuropathies, demyelination can occur sparsely, uniformly, partially, or segmentally. However, in such neuropathies, a completely demyelinated SC appears like a Büngner cell in WD (Figures 4 and 6). This suggests demyelination can be accomplished through an irreversible demyelination process similar to those observed in WD. The term “irreversible” here means that the demyelination process driven by the DSC progresses unidirectionally toward the complete removal of intracellular myelin sheath debris. It does not indicate that remyelination is not possible.
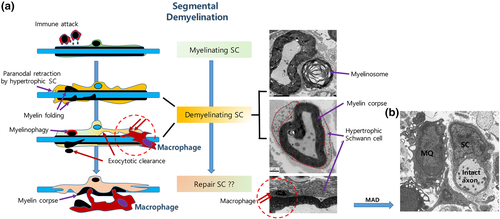
Segmental demyelination is a prototype of complete demyelination found in inflammatory demyelination that occurs in Guillain–Barré Syndrome or chronic inflammatory demyelinating polyneuropathy (CIDP). In these immune diseases, pathological data suggest macrophage-associated demyelination (MAD) because macrophages predominantly invade the endoneurial tube and significantly participate in the clearance of myelin debris (Midroni & Bilbao, 1995; Prineas, 1972, 1981; T. Saida et al., 1982). In addition to MAD, previous studies also described a reactive change in the SC (termed “activated SC”) in response to the immunological attack (Carpenter, 1972; Lampert, 1969). The reactive SC is a hypertrophic SC with high amounts of RER and diverse structures of degenerating myelin, very similar to DSCs in WD. Furthermore, morphological evidence of myelin destruction in SCs was observed, even in the absence of invading macrophages, in animal models of inflammatory demyelination (Ballin & Thomas, 1969b; Lampert, 1969; Saida, Saida, Brown, Silberberg, & Asbury, 1978a), suggesting SC demyelination could be possible without a direct contact of macrophage processes with the myelin sheath.
Recent studies have confirmed the active involvement of SCs in myelin clearance during inflammatory segmental demyelination. First, SC dedifferentiation, which is intimately associated with DSCs in WD, also occurs in human CIDP nerves and animal models of inflammatory demyelination (Hutton et al., 2011; Jang et al., 2016). In both WD and inflammatory demyelination, the dedifferentiated SC shows downregulation of myelin gene expression and upregulation of immature SC gene expression patterns (Jang et al., 2017). In addition, the hypertrophic SCs in inflammatory demyelination also show other typical features of DSCs in WD, such as paranodal retraction, E-cadherin down regulation in SLI, F-actin changes, and autophagolysosome activation (Ballin & Thomas, 1969b; Jang et al., 2016, 2017; Saida, Saida, Brown, et al., 1978a). This reaction of SCs to inflammatory demyelinating insults might lead to the generation of myelinosomes that are to be expelled, or digested by autophagy.
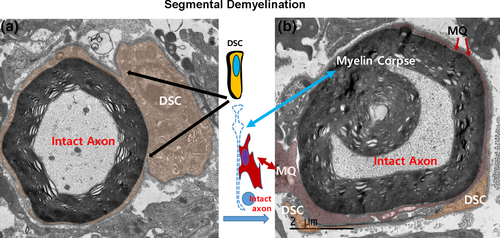
The mechanism used by macrophages to remove the myelin sheath from SCs in inflammatory demyelination was thought to be stripping of myelin lamellae by macrophage processes (Lampert, 1969; Midroni & Bilbao, 1995; Prineas, 1972). Macrophages can also be found in the newly generated space between hypertrophic SC cytoplasm and the outermost myelin lamella (Jang et al., 2017; Lampert, 1969; Figure 4). In addition, macrophage processes that completely encircle the intact axon–myelin sheath structure has been frequently found (Figure 5; Saida, Saida, Silberberg, & Brown, 1978b), suggesting that this myelin sheath can no longer be considered as a part of the SC (Jang et al., 2017; Prineas, 1981). The myelin debris object, which is no longer regarded as part of the SC proper, is termed the “myelin corpse” (Jang et al., 2017). Macrophages could invade the cleft left between the myelin corpse and the SC abaxonal cytoplasm and then phagocytose the severed myelin corpse without attacking the axon (Jang et al., 2017; Figures 4-6). It has been suggested that membrane and cytoplasm remodeling during generation of the myelin corpse is mediated by proteases and lipases released from macrophages (Trotter & Smith, 1986). However the phenotypic transition of SCs may also induce membrane remodeling that helps SCs sever the myelin sheath, thereby ensuring macrophage insinuation into the cleft between SC body and outmost myelin lamellae (Jang et al., 2017; Figures 4-6). Lysosome inhibition, which delays demyelination in WD (Gomez-Sanchez et al., 2015; Jung, Cai, Jang, et al., 2011a), also inhibited myelin clearance in DSCs in an animal model of inflammatory segmental demyelination (Jang et al., 2017). Thus, the core process of inflammatory demyelination involves myelin clearance through cooperative processes by DSCs and macrophages.
6 INFLAMMATORY PARANODAL DEMYELINATION AND DEMYELINATING SCHWANN CELLS
Demyelination, functionally and clinically, results in the slowdown of NCV and/or conduction block. The same electrophysiological features can also be seen in paranodal demyelination, which is a retraction of the myelin sheath from the node and paranodes (Dalakas, 2011). The destruction of axo-glial interactions around nodal and paranodal regions alters the normal distribution of voltage-gated sodium and potassium channels, which are essential for the generation of action potentials in the nodes of Ranvier, leading to abnormal conduction of the electrical impulse (Arroyo, Sirkowski, Chitale, & Scherer, 2004; Doppler et al., 2015; Hafer-Macko et al., 1996; Pollard & Armati, 2011; Salzer et al., 2008). One of the main mechanisms that leads to paranodal demyelination is mediated by autoantibodies (Dalakas, 2011; Devaux, 2012; Querol, Devaux, Rojas-Garcia, & Illa, 2017; Uncini & Vallat, 2017). Indeed several recent studies have revealed the presence of autoantibodies against paranodal proteins such as neurofascin-155 and contactin-1 in the serum of CIDP patients (Lonigro & Devaux, 2009; Manso, Querol, Mekaouche, Illa, & Devaux, 2016; Querol et al., 2013). However this antibody-mediated mechanism of demyelination may not be the only mechanism involved in paranodal demyelination (Feldman, Hughes, & Willison, 2015; Mathey et al., 2015). In some cases, the occurrence of structural disturbances in paranodal regions after administration of pathologic antibodies requires preceding abnormalities in paranodes such as pre-existing paranodal retraction or concomitant mild inflammation (Lonigro & Devaux, 2009; Manso et al., 2016). These pre-existing or concomitant alterations in SC phenotype may favor the accessibility of paranodal regions to pathologic antibodies, therefore participating in paranodal demyelination.
While a difference may exist between paranodal retraction and paranodal demyelination, the resulting widening of the node is not specific to inflammatory demyelination. Indeed such reactive changes have been found in a wide variety of demyelinating conditions, including demyelination by toxic agents (Griffin et al., 1987; Jones & Cavanagh, 1983; Webster, Spiro, Waksman, & Adams, 1961), and agents inducing axonal injury (Ballin & Thomas, 1969a; Holtzman & Novikoff, 1965). Moreover, the plastic changes that occur in paranodes appear to be reversible in some conditions, and in this case, paranodal demyelination does not lead to complete demyelination. It was recently shown that CIDP patients with autoantibodies showed a selective paranodal demyelination without segmental demyelination, whereas CIDP without autoantibodies did not show paranodal demyelination, but exhibited classical segmental demyelination with MAD (Koike et al., 2017), illustrating that the pathomechanisms of paranodal demyelination and complete demyelination are likely different.
7 MYELINOLYTIC FUNCTION OF DEMYELINATING SCHWANN CELLS IN OTHER TYPES OF DEMYELINATING NEUROPATHIES
SC demyelination can be induced by diverse toxic insults inflicted by compounds such as tellurium and heavy metals. This specific demyelination is characterized by a complete and homogeneous demyelination, exhibiting paranodal retraction, SLI abnormality, and a dedifferentiation profile in the absence of axonal degeneration (Ghabriel & Allt, 1980; Griffin et al., 1987; Lampert & Garrett, 1971; Toews et al., 1992). In addition, DSCs in toxic neuropathies also display autophagosomes and recruit macrophages to clear myelin debris (Calle et al., 1999; Goodrum & Bouldin, 1996; Lampert & Garrett, 1971). In diphtheritic neuropathies, paranodal retraction and lysosomal activation have been observed even though the origin of the demyelinating insult is not an immune attack (Allt & Cavanagh, 1969; Webster et al., 1961; Weller & Mellick, 1966). The forced rise of free calcium level within SCs via a calcium ionophore also induces segmental demyelination and macrophage-mediated myelin clearance (Smith & Hall, 1988). The injection of lysophosphatidyl choline, a product of phospholipase activity, into peripheral nerves has been used as an inducible model of demyelination, as it leads to complete demyelination without affecting the survival of SCs and axonal integrity (Gregson & Hall, 1973; Griffin, Stocks, Fahnestock, Van Praagh, & Trapp, 1990; Stoll, Li, Trapp, & Griffin, 1993). While interpreting all these models, Gregson and Hall introduced the concept of “threshold” in the demyelination process: When part of an SC is damaged and the damage reaches a threshold, demyelination of the complete internodal segment is engaged by a “self-perpetuating,” irreversible, myelinolytic mechanism (Gregson & Hall, 1973). This concept of a demyelination threshold is consistent with the cell reprogramming mechanism that is engaged during the demyelination process (Jessen, Mirsky, & Arthur-Farraj, 2015).
8 THE INTRODUCTION OF THE CONCEPT OF THE “DEMYELINATING SCHWANN CELL”
All the above morphological and biochemical data suggest a common SC mechanism of myelin clearance, in a wide variety of complete demyelination conditions, even though demyelinating triggers are heterogeneous. Accumulating evidence indicates that SCs exhibit a similar dedifferentiated or transdifferentiated status when peripheral nerves undergo demyelination irrespective of the demyelinating cause. The prototype of SC dedifferentiation is found in Wallerian demyelination, which occurs during axonal degeneration. As recently reviewed (Tricaud & Park, 2017), it is characterized by the upregulation of several SC dedifferentiation markers and the shutdown of myelin associated gene expression, such as myelin proteins and lipid biosynthetic enzymes. SC dedifferentiation indicates that the demyelinating SC changes phenotype and does not express mature myelinating behavior or markers anymore (Jessen & Mirsky, 2008). However the end phenotype is not of an immature SC, but a distinct repair cell phenotype (Jessen & Mirsky, 2016). SCs undergoing WD express several neurotrophic factors, that are not expressed in immature SCs, thereby playing an important role in sensory neuronal survival and axonal regeneration after injury (Fontana et al., 2012; Jessen & Mirsky, 2016). To distinguish between the acquisition of a novel phenotype by demyelinating cells, Kristjan Jessen and Rhona Mirsky introduced the term “transdifferentiation into the repair cell”, underscoring the implication of these neurotrophic SCs in nerve regeneration (Jessen & Mirsky, 2016; Figure 6). Therefore, the DSC is not an SC phenotype that has lost myelin but appears to be a novel cell phenotype with specific properties for active myelin clearance. In line with this conceptual advance, it would then be necessary to designate the dedifferentiating SCs' ability to activate several myelin destructing processes such as intrinsic and extrinsic phagocytosis, autophagy, and myelin exocytosis as the characteristics of a novel cell phenotype. Thus, by reviewing common myelin clearance processes performed by DSCs in diverse demyelinating conditions (Table 1), we would like to introduce the concept of the “demyelinating Schwann cell”. This novel SC phenotype, arising from reprogramming of SCs, holds specific properties required to clear the myelin sheath during WD. It therefore plays a pivotal role in myelin clearance in many demyelinating neuropathies displaying complete demyelination either with or without intact axons. Even though both the repair SC and the DSC could be the outcomes of transdifferentiation of SC, it is unclear at the moment how much the repair SC and the DSC are identical. Indeed they may partly or completely share their characteristics in WD but they may also be different cell types in particular in other demyelinating neuropathies (Figure 6). Thus, future studies are required to distinguish the two cell phenotypes in different demyelinating conditions.
The molecular mechanisms of SC plasticity including dedifferentiation during WD have been discussed in several recent reviews (Boerboom, Dion, Chariot, & Franzen, 2017; Jessen & Mirsky, 2016; Tricaud & Park, 2017). Even though there is no compelling evidence for a causal relationship between SC dedifferentiation and demyelination, the systematic coincidence of the two processes indicates that the molecular mechanism of phenotypic transition of SC into DSC is tightly related to that of SC dedifferentiation. By further investigating how mechanisms of SC dedifferentiation—such as MAP kinases and c-Jun (Hu et al., 2018; Lee, Shin, & Park, 2014a; Napoli et al., 2012)—drive DSC phenotype acquisition, we might be able to better understand the molecular processes of demyelination in peripheral neuropathies.
ACKNOWLEDGMENTS
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (2016R1A5A2007009) to HTP. NT is grateful for the support of European Research Council (FP7-IDEAS-ERC 311610) and ATIP-Avenir Program. The authors declare that they have no conflict of interest. The authors thank Prof. Hugh Willison (Glasgow University) for valuable comments on the article.