Apteranthes tuberculata's Antidiabetic Potential: Exploring Phytochemicals, Screening Antioxidant Activity, and Validating DPP-4 Inhibition Using In Vitro and In Silico Approaches
Funding: The authors received no specific funding for this work.
ABSTRACT
Diabetes is a chronic metabolic disorder that affects an increasing number of people worldwide, frequently managed with synthetic drugs that have side effects and can be costly. Apteranthes tuberculata (N.E.Br.) Meve & Liede, a plant with traditional medicinal use in Pakistan to treat diabetes, but its antidiabetic potential has not been scientifically validated. This research assessed the phytochemicals, antioxidant properties, and dipeptidyl peptidase-4 (DPP-4) inhibitory activity of A. tuberculata's methanolic extract. The extract was assessed through in vitro antioxidant assays, DPP-4 inhibition test, and metabolomic analysis via Fourier-transform infrared (FTIR) spectroscopy and liquid chromatography–mass spectrometry (LC–MS). The study used computational tools to visualize compound structures, protein-ligand interactions, and to measure pharmacokinetic parameters. Phytochemical analysis revealed significant levels of total phenols (71.991 ± 0.78 mg/g gallic acid equivalents) and flavonoids (66.216 ± 0.09 mg/g quercetin equivalents). Results showed a robust total antioxidant capacity (70.900 ± 2 mg/g ascorbic acid), total reducing power (72.000 ± 2.00 mg/g gallic acid equivalents), and DPPH IC50 value of 96.54 μg/mL. FTIR spectra showed the presence of carbohydrates and glycosides. The extract exhibited 70% DPP-4 inhibitory activity (IC50 value = 46.761 ± 0.043 μg/mL), comparable to Sitagliptin at 78% (IC50 value = 20.474 ± 0.407 μg/mL). LC–MS identified 24 bioactive compounds, including flavonoids and glycosides, with compounds like Kaempferol-3-O-rutinoside-7-O-glucoside and Kaempferol-7-O-rutinoside showing strong binding interactions with DPP-4. These results underscore the therapeutic potential of A. tuberculata as a natural source of DPP-4 inhibitors for managing diabetes.
1 Introduction
Apteranthes tuberculata, belonging to the Apocynaceae family, is a genus with up to 200 species (Al-Fatimi 2019). In Pakistan, two wild species, A. tuberculata and A. edulis, are highly drought-resistant, leafless, and succulent herbs, typically found growing under the canopy of Dodonaea viscosa on exposed bared rocks (Abdel-Sattar et al. 2007). The methanolic extract of A. tuberculata has been shown to possess antidiabetic, anti-inflammatory, phytotoxic, antioxidant, and cytotoxic potential, as well as a shielding effect against gastric mucosa injuries and hematoprotective properties (Poodineh and Nakhaee 2016; Saif et al. 2021). Extracts of A. tuberculata have also been demonstrated to have antidiabetic activity in alloxan-fed diabetic rabbits (Sultan et al. 2014), possibly through activation of insulin produced by pancreatic cells or extra-pancreatic activation of peripheral glucose usage, as well as lipid-lowering effects (Abdel-Sattar et al. 2011). A. tuberculata has been demonstrated to improve antioxidant enzyme activity, regulate oxidative markers, and inhibit oxidative stress in diabetic patients (Poodineh et al. 2015). This medicinal plant is composed of a variety of compounds, including flavone glycosides, pregnane glycosides, megastigmane glycosides, saponins, bitter principles, and triterpenes (Abdel-Sattar et al. 2013; Manzoor et al. 2022; Qasim et al. 2020). Gas chromatography (GC) analysis of A. tuberculata has further identified unstable compounds such as hydrocarbons, essential oils, and other fatty acids (Formisano et al. 2009; Zito et al. 2010). Metabolites, which are the end products of cell regulatory processes in response to environmental or genetic changes, are compiled in an organism's metabolome (Arshad and Mustafa 2023; Mustafa and Komatsu 2017, 2021). Metabolomics is a growing field that utilizes high throughput global investigation of targeted or untargeted metabolites to study their diverse functions in phytomedicines, toxicology, drug discovery, and development (Hasan et al. 2014; Zafar et al. 2024; Zaheer et al. 2023).
Plant metabolomes are thought to be more complex than those of animal cells, with an estimated number of metabolites exceeding 200,000. Each metabolite has a specific bioactivity corresponding to its structure (Sadaf et al. 2023; Shehzad et al. 2023). Recent interest in nutraceutical and pharmaceutical research has been sparked by the significant potential of plant secondary metabolites for drug discovery. While many such metabolites have been studied, very few novel drugs have been developed (Elshafie et al. 2023). Furthermore, plant metabolomics links phyto-compounds with their presumed biological activities, yielding novel and important insights into medicinal plants. The experimental results of A. tuberculata strongly suggest that the plant contains a significant amount of active metabolic compounds, mainly glycosides, which can be used in the formulation of anti-diabetic drugs (Hasan, Mehmood, et al. 2021; Ozturk et al. 2018). Diabetes mellitus (DM) is a complex metabolic disorder associated with various complications that reduce body activities due to serious damage to various body organs (Antar et al. 2023). Type 2 DM patients are often subject to serious complications such as cardiovascular disorders, renal disorders, blindness, amputation, myocardial infarction, and stroke (Lim and Park 2013). Various drugs are available for the treatment of DM, but no single drug can provide satisfactory results for all conditions of DM patients (Centers for Disease Control and Prevention 2014).
DPP-4 inhibitors are among the important therapeutic classes of DM drugs, and several such inhibitors, such as sitagliptin, saxagliptin, linagliptin, and alogliptin, have been approved by the FDA and are available on the market (Pawaskar et al. 2019; Wu et al. 2016). Yet presently available synthetic DPP-4 inhibitors are frequently linked with undesirable side effects, high costs, and limited long-term medication impact. These challenges have encouraged interest in the search for budget-friendly, safer, and more efficient plant-based alternatives.
Apteranthes tuberculata has been locally used in traditional medicine but remains mainly underexplored in scientific literature. Even though research has examined certain phytochemicals in this plant, no thorough investigation exists about its antidiabetic properties through DPP-4 inhibition. This research signifies the first thorough report from Pakistan assessing the in vitro DPP-4 inhibitory potential of A. tuberculata, supplemented by untargeted metabolomic profiling and computational validation, promoting its novelty and scientific contribution. Addressing a significant gap in current literature, as no prior studies have reported DPP-4 inhibition of this plant. Metabolomics, specifically untargeted using LC–MS and FTIR approaches, enables effective detection and identification of various bioactive metabolites found in medicinal plants. The methods reveal compounds that could affect DPP-4 inhibition as well as correlation with biological activity. This research integrates metabolomics with in vitro and in silico analysis to discover A. tuberculata compounds with lead potential for plant-based antidiabetic drug development. Taken together, the novelty of this study lies in being the first to report the DPP-4 inhibitory activity of A. tuberculata using omics and pharmacological approaches, filling a key gap in the literature about its therapeutic capability and supplementing its significance as a candidate for future drug discovery.
2 Materials and Methods
The present experiment was carried out in the Plant Proteomics laboratory at Quaid I Azam University Islamabad. The study was used to analyze the phytochemical properties, antioxidant potential, and metabolomic profiling of A. tuberculata. Fresh plant samples were collected from the Lower Dir district of Khyber Pakhtunkhwa (34°51′ N 71°51′ E). The plant specimen was taxonomically identified by Associate Prof. Dr. Muhammad Zafar. The botanical nomenclature was further verified using the WFO database (https://wfoplantlist.org/taxon/wfo-0000540983-2024-12?page=1).
2.1 Chemicals Used
Folin–Ciocalteu Reagent (Sigma-Aldrich, US), Na2CO3 (Sigma-Aldrich, US), Gallic Acid (Sigma-Aldrich, US), DPPH (Sigma-Aldrich, US), Ascorbic Acid (Sigma-Aldrich, US), Potassium Ferricyanide (Sigma-Aldrich, US), Gallic Acid (Sigma-Aldrich, US), Ammonium Molybdate (Sigma-Aldrich, US), Gly-Pro-Pnitroanilide (Sigma-Aldrich, US), DPP-4 (Sigma-Aldrich, US), Sitagliptin (Sigma-Aldrich, US), DMSO (Sigma-Aldrich, US), Methanol (Sigma-Aldrich, US), TCA (Sigma-Aldrich, US).
2.2 Extract Preparation
The Arial part of the plant was washed with tap water to remove soil particles and other impurities and shade dried. After the complete drying, the plant material was ground into fine powder in grinder (Silver Crest Powder Grinder Machine Model SC- 150G). The ground plant material of 15 g was dissolved in 150 mL methanol in a 250 mL Erlenmeyer flask. The flask was then kept in a shaker for 48 consecutive hours at room temperature. After that extract was filtered through Whatman No. 1 filter paper. The filtrate was evaporated using a rotary evaporator (Rotavapor R-300, BUCHI Labortechnik GmbH, Switzerland) and further dried in a vacuum oven (Shehzadi et al. 2022). The process was repeated twice. The resulting crude extract was stored at 4°C for further use. The extraction yield was calculated to be 26.67%.
2.3 Qualitative Phytochemical Analysis
Methanolic extract of A. tuberculata was tested for the presence or absence of bioactive secondary metabolites using a method previously reported by (Jabeen et al. 2023).
2.4 Quantitative Phytochemical Analysis
2.4.1 Total Phenolic Contents
The total phenolic contents TPC in plant extract were determined with a Folin–Ciocalteu assay. Aliquots of 20 μL of the extract were added to 90 μL of Folin–Ciocalteu reagent in a 96-well plate. Then, 20% of aqueous Na2CO3 solution (90 μL) was added and mixed well. Absorbance was recorded at 760 nm after 2 h. Using gallic acid as a standard, a calibration curve was prepared with various concentrations (15.625–500 μg/mL). The total phenolic contents were expressed as mg gallic acid equivalent (Farag et al. 2020; Hasan et al. 2020; Hasan, Gulzar, et al. 2021).
2.4.2 Total Flavonoid Contents
Total flavonoid contents in plant extract were estimated using (Farag et al. 2020) method. Aliquots of 20 μL of the extract were added to 10 μL of a 2% ethanolic solution of AlCl3 in a 96-well plate. Distilled water was added to make the mixture 200 μL. Absorbance was determined at 415 nm after incubation for 30 min at 37°C. Using quercetin as a standard collaboration curve was prepared with various concentrations (3.125–100 μg/mL). The amount of total flavonoid content was calculated from the quercetin calibration curve, and results were expressed as quercetin mg/g equivalent.
2.5 Radical Scavenging Assay
The antioxidant activity of A. tuberculata extract was estimated with the DPPH in vitro method with some modifications. An aliquot of 10 μL was mixed with a 0.004% methanolic solution of DPPH (190 μL). The mixture was left to stand for 30 min in the dark. Then, the absorbance was recorded at 517 nm. Ascorbic acid was used as a positive control. Serial dilutions of ascorbic acid were prepared at different concentrations (62.5–4000 μg/mL).
AS = sample absorbance at 517 nm (Farag et al. 2020).
2.6 Total Reducing Power
Reducing power of A. tuberculata methanolic extract was carried out according to the procedure (Farag et al. 2020) of with slight modifications. Various concentrations of methanolic extract (62.5–4000 μg) were dissolved in 1 mL of methanol with potassium ferricyanide (250 μL, 1%) and phosphate buffer (200 μL, 0.2 mol/L, and pH 6.6). Then 200 μL of 10% TCA (trichloroacetic acid) was added after incubation for 20 min at 50°C. The mixture was centrifuged at 3000 rpm for 10 min at room temperature. Then 50 μL of distilled water was added to the upper layer (150 μL) and ferric chloride (50 μL, 0.1%). Absorbance was recorded at 700 nm. Results were expressed as the equivalent of mg gallic acid (GAE/mg) equivalent.
2.7 Total Antioxidant Capacity
To determine total antioxidant activity, the phospho-molybdenum method was followed. Plant extract 100 μL was added to 900 μL reaction solution (28 mM sodium phosphate, 0.6 M H2SO4, and ammonium molybdate 4 mM). Then the mixture was incubated for 90 min at 95°C. The mixture was then allowed to cool at room temperature and absorbance was determined at 695 nm. Results were expressed as ascorbic acid equivalent.
2.8 Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared spectroscopy is the most influential tool used to detect the functional groups or bonds present in plant compounds. The relevant form of the bond can be seen and interpreted from the spectrum when light is absorbed at a specific wavelength. The chemical bonds or functional groups present in a compound can be identified by deciphering the absorption spectrum (Nagarajan and Kumar 2017). The methanolic extract of plant 100 mg was loaded in an FTIR spectroscope (DW-FTIR-530A, China) for analysis. The absorbance of the sample was recorded on the wavelength range from 4000 to 400 cm−1.
2.9 Liquid Chromatography-Mass Spectrometry (LC–MS)
The fresh plant sample was taken, cut into pieces, and frozen in liquid nitrogen. 500 mg of frozen tissue was ground to a fine powder in a precooled pestle and mortar in liquid nitrogen. The powdered plant material was transferred to a 50 mL falcon tube (precooled). After that, 8 mL of chilled extraction solution was added. The mixture was vortexed for 10 s and sonicated for 15 min at room temperature. Then the centrifugation of the sample was done at 3000 rpm at room temperature. The supernatant was filtered through a 0.2-μm syringe filter in a 2 mL glass vial and was closed with a cap. Then the sample was analyzed using LC–MS (LCMS-9050 Shimadzu, Japan) (Iqbal et al. 2013, 2014; De Vos et al. 2007).
2.10 In Vitro DPP-4 Inhibition Assay
AS = sample absorbance.
2.11 Molecular Docking
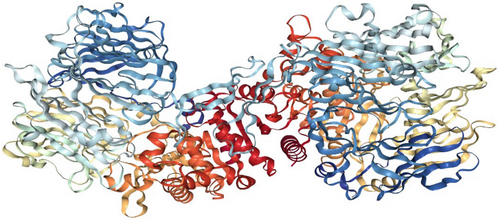
Experimentally identified 21 compounds were used as a dataset for molecular docking studies. Their two-dimensional structure was drawn by ChemDraw Ultra 12.0 (Table S3) (Cousins 2011). The three-dimensional structure of dipeptidyl peptidase-IV (DPP-IV) protein was retrieved from a protein data bank with accession ID 5I7U with a resolution 1.95 Å (Figure 5) (Wu et al. 2016). AutoDock Vina was used for the molecular docking of selected 21 compounds (Trott and Olson 2010). The initial step was followed by the preparation of the target protein and ligand structure for the calculation of physiochemical properties and converted from CDX (ChemDraw Exchange) files to mol format and then converted into PDB format by Open Bebel (O'Boyle et al. 2011). Ligand and protein structure files in PDB format were subjected to the AutoDock module for the generation of PDBQT files with the addition of charges and missing hydrogen atoms The grid set points were well-defined for MD, and after running the script, the top ten docked complex models were generated, with the lowest energy model selected based on the best ligand pose in the binding pocket.
2.12 Protein-Ligand Interaction Analysis
The selected best models with significant energy values were imported to Ligplot+ software (Laskowski and Swindells 2011) for the investigation of protein-ligand binding interactions. Their two-dimensional plots were generated, demonstrating the hydrogen and hydrophobic interactions within the distance range of 4 Å and the three-dimensional confirmations (Figures 6 and 7). The docked complex was visualized in chimera software showing the binding pocket of the target protein and the hydrophobic nature of the DDP-4 target protein (Pettersen et al. 2004).
2.13 Pharmacological Prediction
In computer-aided drug design protocols, in silico ADMET (absorption, distribution, metabolism, excretion, and toxicity) profile estimations are acceptable, so ADMET properties were calculated by SwissADME (Daina et al. 2017) and DataWarrior (Sander et al. 2015) tools are shown in Table 2.
2.14 Statistical Analysis
Statistical analysis was conducted using SPSS software (version 27; IBM Corp., Armonk, NY, USA). Data were expressed as mean ± standard deviation (SD) from triplicate assays (n = 3). One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was applied to determine statistically significant differences between group means at a significance level of p < 0.05.
3 Results and Discussion
3.1 Qualitative and Quantitative Analysis
Qualitative phytochemical analysis of A. tuberculata methanolic extract is given in (Table S1). The result indicates that flavonoids and phytosterols are present in higher amounts followed by saponins and tannins. Quantitative phytochemicals were analyzed such as phenolics and flavonoids. In our study, total phenolics and flavonoid content present in CTME were (71.991 ± 0.78 mg/g GAE) and (66.2162 ± 0.09) respectively (Table S2). (Maheshu et al. 2014) determined the total phenolic content of C. adscendens methanolic extract was (21.0 ± 0.59 mg/g GAE) and flavonoid content was (3.7 ± 1.2 mg/g QE). According to Chandran phenolic and flavonoid contents of C. diffusa were (6.26 g/100 g GAE) and (8.7 g/100 g RE) respectively (Chandran et al. 2014). When compared to the previous study the phenolic and flavonoid contents of A. tuberculata are expressively higher than other species of Caralluma. The flavonoid and phenolic contents observed in the current work might be helpful in free radical scavenging or antioxidant activity. The quantity of phenolics, specifically flavonoids content existing in the methanolic extract of the plant may evidence the antioxidant activity of the plant. Hence the occurrence of flavonoids and phenolics content supports that A. tuberculata can have antioxidant potential.
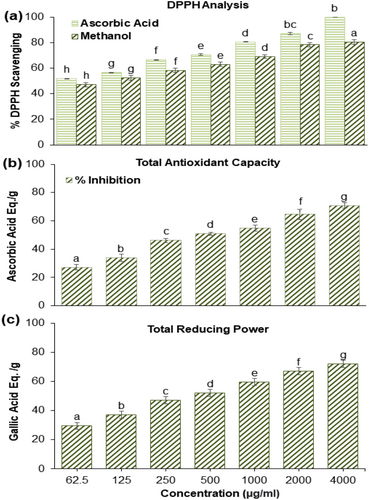
3.2 Antioxidant Activity
In the current study, the result shows that radical scavenging activity is dose dependent. The calculated IC50 value from the percentage inhibition of DPPH was 96.54 μg/mL for ATME. Figure 1a shows the percentage inhibition of ATME and ascorbic acid at different concentrations. Similarly, Figure 1b shows the reductive competence of ATME equated to gallic acid (GA). In the present study, the highest reducing power was observed at 4000 μg/mL, which was (72.000 ± 2.00 mg/g of gallic acid) and the lowest value was observed at a concentration 62.25 (29.625 ± 2.315 mg/g of GAE). The result shows that an increase in concentration increases the reducing capacity of the plant extract. Like total reducing capacity, total antioxidant capacity (TAC) also increased as the plant sample increased (Figure 1c). The present study reveals that maximum TAC was observed at a concentration of 4000 μg/mL, which was (70.900 ± 2 equivalent to ascorbic acid mg/g of extract) and lowest at 62.5 (27.233 ± 2.08 AAE mg/g of extract). The radical scavenging activity increased with an increase in concentration and the IC50 value recorded was 96.54 μg/mL for the methanolic extract of the plant sample. Our results are according to (Rauf et al. 2013), who also reported that the radical scavenging assay is dose dependent. According to (Al-Fatimi 2019), the IC50 value of C. adscendens methanolic extract was 66 μg/mL. Maximum antioxidant activity and total reducing power were observed at 4000 μg/mL, which is (70.900 ± 2 mg/g AA) and (72.000 ± 2.00 mg/g GAE) respectively, which is also reported by (Chandran et al. 2014) in C. diffusa.
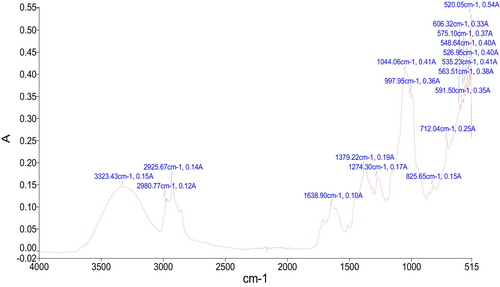
3.3 Fourier Transform Infrared Spectroscopy (FTIR)
In the current study, functional groups were examined using FTIR spectroscopy based on their absorbance. Figure 2 shows the characteristic FTIR spectra of A. tuberculata. The peaks obtained from the analysis were decoded, and the functional groups were determined. The functional groups present in the plant sample refer to a specific class of compounds (Table S4). The frequently occurring compounds were carbohydrates and glycogen, which refer to the presence of glycosides, a class of bioactive phytochemicals known for their antidiabetic potential. The valuable health-enhancing properties of A. tuberculata are due to the presence of bioactive compounds. The FTIR reveals that the major compounds in the plant sample are carbohydrates. Frequencies range from 3323 cm−1, 2980 cm−1, and 2925 cm−1 characterizing O-H stretching and the existence of carbohydrates and amino acids. Carbohydrates and glycosides are known to demonstrate glucose-lowering and antioxidant effects, aiding in the therapeutic properties of the plant (Khattak and Khan 2016). Frequency ranges from 1638 cm−1 characterize N-H bend and the existence of proteins, which may play a role in modifying enzymatic activities related to diabetes. Frequencies range from 1379 cm−1 exhibit C-H bend, indicating the presence of glycogen. Frequencies range from 1274 cm−1 and 1044 cm−1 represent C-N stretching and the presence of amino acids. Frequencies range from 997 cm−1 represent = C-H bend, indicating the presence of lipids, which are crucial for cell membrane integrity and may also induce insulin sensitivity (Rizevsky et al. 2022). Frequencies range from 825 cm−1 and 712 cm−1 represent C-H stretching and the presence of phenolics, extensively identified for their robust antioxidant potential (Johnson et al. 2020). The frequency ranges from 606 cm−1, 591 cm−1, 575 cm−1, and 563 cm−1 represent C-Cl stretching and the existence of chloro-compounds as previously reported and matched (Hasan et al. 2024; Mustafa et al. 2020, 2024; Shehzad et al. 2024). Frequencies range from 548 cm−1, 535 cm−1, 526 cm−1, and 520 cm−1 represent C-Br stretching and the existence of bromo-compounds. Similar results were also reported by (Nagarajan and Kumar 2017) from garlic by analysis through FTIR.
3.4 Liquid Chromatography-Mass Spectrometry (LC–MS)
In this study, we carried out an untargeted screening of bioactive secondary metabolites in A. tuberculata to evaluate its therapeutic potential. A total of 24 compounds were identified in Table 1 with retention time ranging from 1 to 20 min and mass-to-charge ratio (m/z) ranging from 152 to 1000 (Figure 3). Our result shows that acylated glycosides are the most abundant class of compounds, frequently present in all species of Caralluma. A. tuberculata is traditionally used for diabetes due to the presence of these acylated glycosides. In addition, we identified other important classes of secondary metabolites such as flavonoids, saponins, and sesquiterpene lactones. The LC–MS study of the methanolic extract of A. tuberculata displayed a diverse range of molecular ions spanning an m/z ratio of 152 to 1000 within the plant extract. Previous studies have explained structures of various acylated pregnane glycosides (7, 11, 13, 15, 19, 20, 21) using techniques such as FAB-MS, NMR, HMBC, and HMQC (Abdel-Sattar et al. 2008). The presence of acylated or nonacylated pregnane glycosides, such as russelioside C, raratuberside C, and russelioside B, in A. tuberculata indicates its antidiabetic activity. This is due to the blocking of G-6-Pase activity, stimulation of insulin release, improvement of glucose consumption, and inhibition of glucose uptake.
| S.No | tR/min | [M-H]− (m/z) | Formula | Identification |
|---|---|---|---|---|
| 1 | 1.4 | 152 | C5H5N5O+ | Deoxyguanosine |
| 2 | 4.8 | 316.0562 | C16H12O7 | Isorhamnetin |
| 3 | 5.2 | 325.0926 | C15H17O8 | Bilobalide |
| 4 | 7.4 | 423.1295 | C20H23O10 | Ginkgolide J |
| 5 | 7.9 | 447.0933 | C21H19O11 | Kaempferol-7-O-glucoside |
| 6 | 8.2 | 463.0877 | C21H19O12 | Quercetin-3-O-glucoside |
| 7 | 10.8 | 583.4977 | C51H76O17 | Caratuberside E |
| 8 | 11.21 | 593.1507 | C27H29O15 | Kaempferol-7-O-rutinoside |
| 9 | 11.63 | 609.1454 | C27H29O16 | Quercetin-3-O-rutinoside |
| 10 | 11.8 | 623.1612 | C28H31O16 | Isorhamnetin-3-O-rutinoside |
| 11 | 12.9 | 679.6 | C34H56O12 | Russelioside C |
| 12 | 14.6 | 755.2038 | C33H39O20 | Kaempferol-3-O-rutinoside-7-O-glucoside |
| 13 | 15.5 | 785.4129 | C43H62O14 | Russelioside G |
| 14 | 15.8 | 803.422 | C14H64O14 | Digoxin |
| 15 | 16.4 | 841.8 | C40H66O17 | Russelioside B |
| 16 | 17.3 | 879.257 | C40H47O22 | Ginkgolide C |
| 17 | 17.5 | 887.45 | C46H66O14 | Pregnane glycoside |
| 18 | 18.3 | 925 | C49H74O15 | Bouceroside—ADC |
| 19 | 18.4 | 929 | C50H47O17 | Russelioside F |
| 20 | 19 | 961 | C40H66H17 | Caratuberside E |
| 21 | 19.3 | 967.9 | C51H76O16 | Raratuberside C |
| 22 | 19.5 | 985.4896 | C54H74O15 | Isopropenyl Glycopyranosides |
| 23 | 19.6 | 989.521 | C51H76O16 | Tragopogonsaponin M |
| 24 | 19.9 | 999.5082 | C55H76O15 | 12,20-di-O-benzoyl boucerin |
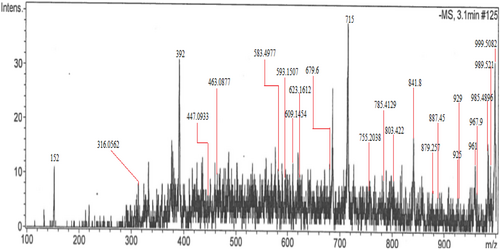
Furthermore, in this study the identified compounds such as isopropenyl glycopyranosides and 12,20-di-O-benzoyl boucerin, were previously reported by (Sultan et al. 2014) from chloroform extract of C. quadrangular. Using MS and NMR data, structures of compounds 17 and 22 were elucidated as 12-β-O-benzoyl-20-O-tigloyl boucerin-3-O-β-D-glucopyranosyl-(1 → 4)-β-D-cymaropyranoside and 12,20-di-O-benzoylboucerin 3-O-β-D-cymaropyranoside (Abdel-Sattar et al. 2008). Moreover, we identified flavonoid glycosides (compounds 5, 6, 8, 9, 10, and 12), including mono- and di-glycosides with basic structure. Although, these compounds have been previously reported by (Lim et al. 2023; Lim and Park 2013) (Zhou et al. 2014) from Ginkgo biloba but identified here for the first time in A. tuberculata. The structures of these compounds were elucidated through UHPLC-LTC-Orbitrap Elite (Ablajan and Tuoheti 2013). Compound 14 was identified as digoxin, a cardiac glycoside previously isolated from Digitalis lanata for the first time. Our results strongly indicate that A. tuberculata contains a significant amount of active metabolic compounds with medicinal properties, particularly for the treatment of diabetes and potentially other diseases.
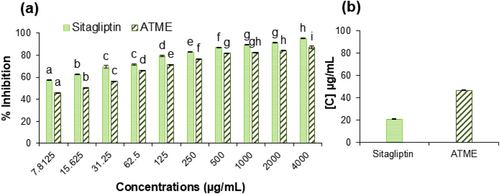
3.5 In Vitro DPP-4 Inhibition Assay
DPP-IV, or dipeptidyl peptidase-IV, is an enzyme that breaks down certain peptides (chains of amino acids) in the body, including the incretin hormones GLP-1 and GIP. These hormones are important for glucose metabolism and play a role in the body's response to insulin (Lok et al. 2022). Plant metabolites such as flavonoids, terpenoids, and alkaloids have been studied for their potential as DPP-4 inhibitors. For example, flavonoids such as quercetin, kaempferol, and luteolin have shown inhibitory effects on DPP-IV. Similarly, terpenoids such as glycyrrhizic acid and oleanolic acid are potent inhibitors of DPP-4 (He et al. 2019). Alkaloids such as berberine and piperine have also been studied for their potential as DPP-4 inhibitors. These plant metabolites play an important role in regulating glucose levels and thus possess therapeutic potential for the treatment of diabetes (Marahatha et al. 2021). The LC–MS result of A. tuberculata demonstrated high amounts of secondary metabolites such as flavonoids, terpenoids, and alkaloids. As a result, it showed a good DPP-4 inhibitory activity with an IC50 value of 46.761 ± 0.043 μg/mL (70% inhibition) (Figure 4a,b). This is the first report of DPP-4 inhibitory activity of A. tuberculata. To compare the effect, sitagliptin was used as a positive control and it showed an IC50 value of 20.474 ± 0.407 μg/mL (78% inhibition) (Figure 4a,b). The secondary metabolites present in the plant are known to have potent pharmacological properties, which could be responsible for its inhibitory activity. The IC50 value of the plant was slightly lower than the positive control suggesting that A. tuberculata could be a potential alternative to existing therapeutic agents. This inhibition of DPP-4 activity can lead to increased concentrations of GLP-1 and GIP in the body, which can improve glucose metabolism and help control blood sugar levels. In addition to the direct inhibition of DPP-IV, some plant metabolites also possess other actions relevant to diabetes, such as stimulation of glucose-stimulated insulin secretion, inhibition of alpha-amylase, activation of adenosine monophosphate-activated protein kinase (AMPK), and modulation of the expression of genes involved in glucose metabolism (Shehadeh et al. 2021). Furthermore, DPP-4 inhibitors have anti-inflammatory and antioxidant effects as well, which may be beneficial for patients with diabetes (Shastri and Gadhave 2022). In summary, plant metabolites have been shown to act as DPP-4 inhibitors and can help to improve glucose metabolism and reduce the risk of complications associated with diabetes which are further validated through molecular docking.
3.6 Molecular Docking and Protein-Ligand Interaction Analysis
Target protein and chemical compounds are the requirements to start the molecular docking procedure. Experimentally identified 24 compounds from A. tuberculata were used as a dataset for molecular docking studies. The two-dimensional structure of the selected 24 compounds was drawn very carefully by ChemDraw Ultra 12.0 software (Table S3) (Cousins 2011). The three-dimensional structure of the selected targeted protein, DPP-4 protein, was retrieved from the protein data bank (accession ID 5I7U) with resolution 1.95 Å (Figure 5) (Wu et al. 2016). These 24 extracted natural compounds from A. tuberculata were expected to have anti-DM potential so it was validated by the application of computer-aided drug design procedures, such as the calculation of protein-ligand binding interaction behavior with the DPP-4 protein target, which is one of the novel therapeutic agents to design potential drugs for DM. Several FDA-approved DPP-4 inhibitors are available in the market and used for the treatment of DM.
Selected dataset of 24 chemical compounds extracted from A. tuberculata used for molecular docking analysis has several biological activities, while in this study we focused on the activities of chemical compounds as DPP-4 inhibitors which could be significant anti-DM agents. Isorhamnetin could improve glycemic levels in DM patients and its related complications such as diabetic cataracts, hypertension, obesity, lipid peroxidation, and hyperglycemia (Li et al. 2016). Bilobalide is significant for the treatment of heart disease and DM; it is also a therapeutic agent for inflammation and insulin resistance to treat the complications of DM (Lim and Park 2013). While extensive research is required to confirm the Ginkgolide J activity as an anti-DM agent (Wang et al. 2006). Kaempferol-7-O-glucoside can improve insulin resistance and downregulation of glucose in blood and cholesterol profile in Type 2 DM (Nishina et al. 2019). Quercetin-3-O-glucoside can mediate the various metabolic activities, ameliorate the DM conditions, stimulate the insulin signaling pathway, and improve the lipid peroxidation in tissues (Panda and Kar 2007). The extract of A. tuberculata is used for DM treatment; thus, it is required to check the properties of Kaempferol-7-O-rutinoside chemical compound as an anti-DM drug (Abdel-Sattar et al. 2007). Quercetin-3-O-rutinoside is an important phenolic glycoside with various medicinal properties; it also includes anti-DM (Nazir et al. 2017).
Further research is required to confirm its mechanism of action for DM. Isorhamnetin-3-O-rutinoside is also a beneficial phenolic glycoside and useful dietary supplement and anti-DM agent (Jia et al. 2019). Extensive research is still required to understand its mechanism of action for DM. Russelioside C could significantly improve the fasting serum glucose and glycated hemoglobin and insulin sensitivity, and serum insulin and cholesterol profile in DM patients. Previous studies provide evidence of its use as an anti-diabetic and anti-hyperglycemic agent. Pregnane glycoside is useful to manage hyperglycemia in DM patients (Abdel-Sattar et al. 2016). Russelioside G is a pregnane glycoside, its diabetic properties are required to be investigated. Digoxin is usually prescribed to DM patients with heart-related complications but its mechanism of action concerning DM complications is necessary to be researched. Ginkgolide C is an active extract, which is used for various medicinal aspects, but its anti-DM activities are required to be investigated (Wang et al. 2006). Pregnane glycosides are useful for diabetic treatment. Bouceroside—ADC, Russelioside F, and Russelioside E are pregnane glycosides, its DM properties are required to be investigated.
DPP-4 protein shares several names such as adenosine deaminase complex protein 2 and CD26 protein also, encoded by DPP-4 gene, related to various biological activities such regulation of insulin signaling pathway, immune regulatory pathway, apoptosis, tumor suppression, and progression (Tanwar et al. 2014). In the case of diabetes mellitus, DPP-4 inhibition is beneficial to control hyperglycemia and improve insulin secretion and an additional benefit is there is no weight gain with control of lipid profile. Several approaches have been used to investigate the activities of the selected compounds previously, while we have developed the computer-aided drug design approach to analyze the binding behavior of DDP-IV protein with the selected compounds to validate its activity as anti-DM agents and also predicted their pharmacological behavior which could be helpful to understand the chemical nature of extracted 24 compounds when put forward to the drug development phase. A summary of the docking results of the selected 24 compounds is shown in Table S5, each compound is analyzed in detail concerning its binding energy, and its hydrophobic and hydrogen bonding interactions.
Protein-ligand binding interactions within the best pose with low energy models and more number of interactions were considered to be the best-docked complex visualized by Ligplot software (Wallace et al. 1995), hydrophobic and hydrogen bonding residues are shown in the two-dimensional plots, red spikes showing how the hydrophobic residues and green colored residues are making hydrogen bonds with the chemical compound within the vicinity of 4A in the active binding pocket of the target protein while the three-dimensional representation of ligand poses within the hydrophobic pocket is generated by Chimera software (Figures 6 and 7) (Pettersen et al. 2004). DPP-4 protein is hydrophobic, so most of the active pockets make hydrophobic interactions with the chemical compounds bonded to it.
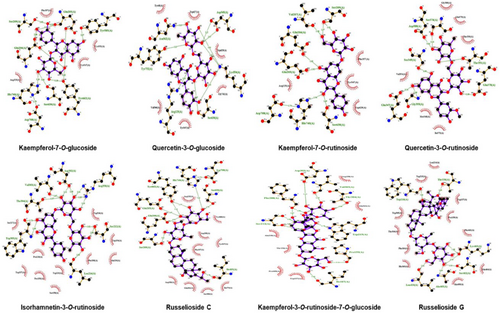

After the application of molecular docking, it clearly understood the DPP-4 inhibitory nature of each selected compound, and nine compounds demonstrated very significant modes of binding interactions within the binding pocket of the target protein. Kaempferol-7-O-rutinoside and Kaempferol-3-O-rutinoside-7-O-glucoside show the best binding mode of interaction with DPP4 target protein presenting 12 hydrogen bonds, 38 hydrophobic bonds with −9.4 binding energy score while Quercetin-3-O-glucoside also presented significant docking results with 11 hydrogen bonds, 27 hydrophobic bonds with −7.8 binding energy score, Quercetin-3-O-rutinoside presented 9 hydrogen bonds and 37 hydrophobic bonds with −8.8 binding energy score, Russelioside C 8 hydrogen bonds and 41 hydrophobic bonds with −8.9 binding energy score, Russelioside G 7 hydrogen bonds and 36 hydrophobic bonds with −10.3 binding energy score, Bouceroside–ADC 7 hydrogen bonds and 37 hydrophobic bonds with −9.8 binding energy score, Isorhamnetin-3-O-rutinoside 7 hydrogen bonds and 53 hydrophobic bonds with −8.9 binding energy score, and Kaempferol-7-O-glucoside 7 hydrogen bonds and 27 hydrophobic bonds with −8.2 binding energy score, two dimensional and three dimensional.
3.7 Pharmacological Prediction
Pharmacological estimations account for the ADMET properties of drugs in the initial phase of development (Ntie-Kang et al. 2013), but in the case of natural products mostly some properties are not fulfilled, as for the significant information helpful for the future synthesis and drug development. It is preferred to calculate the pharmacological properties in this study. Table 2 presents the pharmacological predictions in terms of drug-likeness, and toxicity estimations concerning mutagenic, tumorigenic, reproductive, and irritant effects, pharmacokinetics, water solubility, and indications beneficial for medicinal chemistry synthesis of chemical compounds. As from the selected 24 compounds extracted from A. tuberculata, usually, everyone does not fulfill the drug-like properties, such as Lipinski rule (Lipinski et al. 2012) and Veber rule (Veber et al. 2002) because natural compounds consist of different heavy atoms and functional groups, so its weight is generally more than 500 Da, but natural compounds are highly active and previously proved their medicinal status in terms of important biological activities (Newman and Cragg 2020). Bilobalide, Ginkgolide J, Quercetin-3-O-glucoside, Caratuberside E, Quercetin-3-O-rutinoside, Isorhamnetin-3-O-rutinoside, Digoxin, Ginkgolide C, Caratuberside E, Isopropenyl Glycopyranosides, Tragopogonsaponin M has presented no toxic effects in terms of mutagenicity, tumorigenicity, reproductive and irritant effects. Pharmacokinetics profile of each compound is evaluated to confirm the bioavailability of chemical compounds such as GI absorption, BBB permeability, P-gp substrate, CYP1A2 inhibitor, CYP2C19 inhibitor, CYP2C9 inhibitor, CYP2D6 inhibitor, CYP3A4 inhibitor, Log Kp value for skin permeability, and water solubility estimation.
| Chemical compounds | Drug-likeness | Toxicity estimation | Pharmacokinetics | Water solubility | Medicinal chemistry |
|---|---|---|---|---|---|
| Deoxyguanosine |
Lipinski = No violation Veber = No violation Bioavailability =0.55 |
Mutagenic effects = high Tumorigenic effects = high Reproductive effects = high Irritant effects = high |
GI absorption = high BBB permeant = No P-gp substrate = No CYP1A2inhibitor = No CYP2C19 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −7.16 cm/s |
Highly soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 2.08 |
| Isorhamnetin |
Lipinski = No violation Veber = No violation Bioavailability =0.55 |
Mutagenic effects = high Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = high BBB permeant = No P-gp substrate = No CYP1A2inhibitor = Yes CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = Yes CYP3A4 inhibitor = Yes Log Kp (skin permeation) = −6.90 cm/s |
Soluble |
PAINS = No alert Break = No alert Synthetic accessibility = 3.26 |
| Bilobalide |
Lipinski = No violation Veber = No violation Bioavailability =0.55 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = high BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.48 cm/s |
Highly soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 5.41 |
| Ginkgolide J |
Lipinski = No violation Veber = One violation Bioavailability =0.55 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.16 cm/s |
Soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 6.39 |
| Kaempferol-7-O-glucoside |
Lipinski = Two violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = high Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = No CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.52 cm/s |
Soluble |
PAINS = No alert Break = No alert Synthetic accessibility = 5.24 |
| Quercetin-3-O-glucoside |
Lipinski = Two violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = No CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.88 cm/s |
Soluble |
PAINS = One alert Break = One alert Synthetic accessibility = 5.32 |
| Caratuberside E |
Lipinski = Two violations Veber = Two violations Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.18 cm/s |
Moderately soluble |
PAINS = No alert Break = Two alerts Synthetic accessibility = 9.46 |
| Kaempferol-7-O-rutinoside |
Lipinski = Three violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = high Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.91 cm/s |
Soluble |
PAINS = No alert Break = No alert Synthetic accessibility = 6.43 |
| Quercetin-3-O-rutinoside |
Lipinski = Three violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −10.26 cm/s |
Soluble |
PAINS = One alert Break = One alert Synthetic accessibility = 6.52 |
| Isorhamnetin-3-O-rutinoside |
Lipinski = Three violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −10.12 cm/s |
Soluble |
PAINS = No alert Break = No alert Synthetic accessibility = 6.64 |
| Russelioside C |
Lipinski = Three violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.75 cm/s |
Soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 8.21 |
| Kaempferol-3-O-rutinoside-7-O-glucoside |
Lipinski = Three violations Veber = four violation Bioavailability =0.17 |
Mutagenic effects = high Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −12.18 cm/s |
Soluble |
PAINS = No alert Break = No alert Synthetic accessibility = 7.65 |
| Russelioside G |
Lipinski = Two violations Veber = two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.78 cm/s |
Soluble |
PAINS = No alert Break = Two alert Synthetic accessibility = 8.44 |
| Digoxin |
Lipinski = Three violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −10.78 cm/s |
Moderately soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 8.81 |
| Russelioside B |
Lipinski = Three violations Veber = Four violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −11.87 cm/s |
Soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 9.27 |
| Ginkgolide C |
Lipinski = One violations Veber = One violation Bioavailability =0.55 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.95 cm/s |
Soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 6.48 |
| Pregnane glycoside |
Lipinski = two violations Veber = Three violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = high |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.18 cm/s |
Soluble |
PAINS = No alert Break = Three alert Synthetic accessibility = 8.80 |
| Bouceroside – ADC |
Lipinski = Two violations Veber = Two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = No CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.77 cm/s |
Moderately soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 9.41 |
| Russelioside F |
Lipinski = Two violations Veber = Two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.37 cm/s |
Soluble |
PAINS = No alert Break = Two alert Synthetic accessibility = 9.55 |
| Caratuberside E |
Lipinski = Two violations Veber = Two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.35 cm/s |
Moderately soluble |
PAINS = No alert Break = Two alert Synthetic accessibility = 9.63 |
| Raratuberside C |
Lipinski = Two violations Veber = Two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = low Irritant effects = high |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −9.53 cm/s |
Moderately soluble |
PAINS = No alert Break = Two alert Synthetic accessibility = 9.79 |
| Isopropenyl Glycopyranosides |
Lipinski = Two violations Veber = Two violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = Yes Log Kp (skin permeation) = −5.61 cm/s |
Insoluble |
PAINS = No alert Break = One alert Synthetic accessibility = 8.89 |
| Tragopogonsaponin M |
Lipinski = Three violations Veber = Three violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = none Reproductive effects = none Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = Yes CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8,07 cm/s |
Poorly soluble |
PAINS = No alert Break = two alert Synthetic accessibility = 9.33 |
| 12,20-di-O-benzoyl boucerin |
Lipinski = Two violations Veber = One violation Bioavailability =0.17 |
Mutagenic effects = none Tumorigenic effects = low Reproductive effects = low Irritant effects = none |
GI absorption = Low BBB permeant = No P-gp substrate = No CYP1A2inhibitor = No CYP2C9 inhibitor = No CYP2C9 inhibitor = No CYP2D6 inhibitor = No CYP3A4 inhibitor = No Log Kp (skin permeation) = −8.36 cm/s |
Poorly soluble |
PAINS = No alert Break = One alert Synthetic accessibility = 9.61 |
Log Kp values are acceptable for the selected 24 compounds; most of them are soluble in nature, and medicinal chemistry properties are calculated to highlight problematic fragments of chemical compounds, such as PAINS and break alerts, which indicate the highly reactive functional groups that could be prerequisites for the synthesis of these chemical compounds in the laboratory so each compound could be optimized to manage these alerts and make the drug orally bioavailable without any harmful effects, and the synthetic accessibility score depends on each structural fragment of the chemical compound and is helpful for the easy synthesis of chemicals in the laboratory. Out of 24 selected chemical compounds, Isorhamnetin, Kaempferol-7-O-glucoside, Kaempferol-7-O-rutinoside, Isorhamnetin-3-O-rutinoside, and Kaempferol-3-O-rutinoside-7-O-glucoside have demonstrated no PAINS and break alerts, while other chemical compounds have some indications for the medicinal chemist to research and optimize these chemical compounds in the laboratory to deliver a safe drug to market.
4 Conclusion
In conclusion, this study has demonstrated the potential antidiabetic activity of A. tuberculata and identified new phytochemicals with a high antidiabetic potential. The phytochemical analysis revealed the presence of various classes of glycosides, flavonoid glycosides, flavonols, cardiac glycosides, and triterpenoids which are responsible for the antioxidant and antidiabetic activity of the plant. The results of in vitro DPP-4 assay, molecular docking, and pharmacological prediction studies agreed with the findings that kaempferol-7-O-rutinoside and kaempferol-3-O-rutinoside-7-O-glucoside have the potential to be used as antidiabetic agents. Out of 24 selected chemical compounds, Isorhamnetin, Kaempferol-7-O-glucoside, Kaempferol-7-O-rutinoside, Isorhamnetin-3-O-rutinoside, Kaempferol-3-O-rutinoside-7-O-glucoside, has demonstrated no PAINS, and Break alerts, indicating the safe use of these compounds for medical purpose. The untargeted metabolomics analysis revealed that A. tuberculata has a wide range of bioactive secondary metabolites, which can be further studied to develop new antidiabetic drugs. Additionally, the antioxidant activity of A. tuberculata could be beneficial in the prevention of obesity and diabetes-related complications. Further, in vivo studies are needed to establish the safety, efficacy, and pharmacokinetic properties of the compounds for potential therapeutic use.
Author Contributions
Ilham Khan: conceptualization (equal), methodology (equal), writing – original draft (equal). Shabana Bibi: data curation (equal), formal analysis (equal), investigation (equal), writing – original draft (equal). Junaid Shehzad: investigation (equal), writing – original draft (equal). Zarqa Riaz: software (equal), validation (equal), writing – original draft (equal). Muhammad Saad Khan: formal analysis (equal), software (equal), writing – original draft (equal). Mansour Ghorbanpour: writing – original draft (equal). Murtaza Hasan: resources (equal). Ghazala Mustafa: conceptualization (equal), resources (equal), supervision (equal).
Ethics Statement
The authors have nothing to report.
Consent
The authors agreed to publish this work. All the authors agreed to participate in this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data and materials will be available on request.




