Combination of four bacterial strains isolated from Yamahai-shubo in traditional Japanese sake brewing
Abstract
This study investigated the interactions of four bacteria strains isolated from Yamahai-shubo, the source of yeast used to produce a Japanese traditional rice wine, Yamahai-shikomi sake. The bacterial strains were nitrate-reducing Pseudomonas sp. 61-02, Leuconostoc mesenteroides LM-1, Lactiplantibacillus plantarum LP-2, and Latilactobacillus sakei LS-4. We examined fermentation factors for Yamahai-shubo and Yamahai-shikomi sake samples to compare the suitability of their bacterial combination (16 variations). As a result of principal component analysis, we found that two major groups were formed; one containing strain LP-2 and the other containing strain LS-4, and that strains LP-2 and LS-4 were important in the Yamahai-shikomi sake in the presence of strains 61-02 and LM-1. Then, we investigated the effects of strains LP-2 and LS-4 on the concentration of organic acids (pyruvic acid, citric acid, succinic acid, malic acid, and lactic acid) in Yamahai-shikomi sake. Only in lactic acid, a tendency to decrease with a smaller proportion of LS-4 strains in Yamahai-shubo was observed. Subsequently, their effect on the concentration of diacetyl, crucial for aroma, was investigated between the LP-2 and LS-4 strains. The sample prepared in the absence of strain LS-4 exhibited the lowest concentration of diacetyl. This result was supported by the statistical analysis for the sensory scores performed for aroma of each Yamahai-shikomi sake sample. In conclusion, strain LP-2 plays a more significant role in improving Yamahai-shikomi sake quality with strains LM-1 and 61-02 rather than strain LS-4 in Yamahai-shubo preparation and Yamahai-shikomi sake brewing.
1 INTRODUCTION
Japanese sake, a traditional rice wine, is produced by a multistep fermentation process mainly using well-studied strains of Saccharomyces cerevisiae with Aspergillus oryzae derived from koji (Japan Sake and Shochu Makers Association, 2011). The recent method for brewing sake employs a current starter, a so-called Sokujo-shubo that includes those S. cerevisiae strains, and is characterized by a shorter brewing period, although significant amounts of lactic acid are added to prevent contamination by other microorganisms. In contrast, the method of Yamahai-shikomi is one of the traditional methods of Japanese sake brewing (Tatsukami et al., 2018). It employs a traditional Yamahai-shubo that includes various and unidentified species of microorganisms (yeasts and bacteria) that are derived from each brewery. It is known to need more time than Sokujo-shubo because lactic acid is derived from lactobacilli included Yamahai-shubo, and it increases the risk of putrefaction of nonpreferable microorganisms, causing an off-flavor (Suzuki et al., 2008). On the other hand, it is most likely that the traditional method differentiates the quality of sake by the complex aromas and tastes derived from the microorganisms (Akaike et al., 2020); therefore, the produced sake often improves the original palatability due to the involvement of unidentified microbial habitat(s) in breweries. However, there are uncertain microbial diversities containing various species of bacteria and yeasts in Yamahai-shubo, and the diversities are dependent on the derived sources of breweries (Koyanagi et al., 2016).
The preparation of Yamahai-shubo is usually initiated in a lower temperature environment below 10°C (Obayashi & Kitahara, 1959); as a result, nitrate-reducing bacteria proceed to grow and convert trace, but significant amounts of nitrate derived from water (groundwater) into nitrite to prevent the growth of nitrite-sensitive microorganisms. In this early stage of the preparation of Yamahai-shubo, and the condition for bacterial cell growth is insufficient in the presence of nitrate-reducing bacteria. Thereafter, coccoid-form lactic acid bacteria (cocci-LAB), e.g., Leuconostoc mesenteroides, gradually and then preferentially grow, producing only a small amount of lactic acid. Subsequently, bacillus-form lactic acid bacteria (bacilli-LAB), e.g., Latilactobacillus sakei (Ashizawa & Saito, 1966a; Koyanagi et al., 2016), increasingly become predominant, producing a larger amount of lactic acid, which serves to decrease the pH of the medium. After the microbial transition, a well-studied strain of S. cerevisiae is added between 10 and 15 days for the preparation of Yamahai-shubo, while concomitant bacterial species become crucial for the characteristic brewing of Yamahai-shikomi sake. Therefore, although the brewing of Yamahai-shikomi sake with Yamahai-shubo is performed in the subsequent feeding processes (soe, naka, and tome) by adding koji and steamed rice with water, it becomes more significant to examine the diversity of bacteria as well as yeasts in the present brewing of Yamahai-shikomi sake with Yamahai-shubo to maintain the quality of the sake (Koyanagi et al., 2016). However, only a limited number of publications have reported on the identification of LAB from Yamahai-shubo samples (Ashizawa & Saito, 1966b; Bokulich et al., 2014; Momose & Kamao., 1993; Sotoike et al., 1964). This is mainly due to there being less information from research works on indigenous microbiota in Yamahai-shubo, and very little is known about the microbial transitions that occur during the preparation of Yamahai-shubo.
With this background, Pseudomonas sp. 61-02 was previously isolated from Yamahai-shubo as a nitrate-reducing bacterium and determined to be indispensable for the production of Yamahai-shikomi sake (Wakai et al., 1990). Thereafter, we isolated a cocci-LAB strain LM-1, a bacilli-LAB strain LP-2, and a bacilli-LAB strain LS-4 from Yamahai-shubo prepared at Kizakura Brewery, and from the 16 S rRNA sequence and several physiological properties, identified them as Le. mesenteroides, Lactiplantibacillus plantarum, and Ll. sakei, respectively (Fujiwara et al., 2013). Until now, there has been no publication showing the influence on aromas and tastes in sake produced with Yamahai-shubo using the nitrate-reducing Pseudomonas sp. 61-02 and three LAB strains. In the present study, we investigated the interactions of four bacterial strains (nitrate-reducing Pseudomonas sp. 61-02, Le. mesenteroides LM-1, Lp. plantarum LP-2, and Ll. sakei LS-4) in Yamahai-shubo preparation and showed the requirements of two strains, 61-02 and LM-1. In the evaluation process, we performed principal component analysis for samples of Yamahai-shubo and Yamahai-shikomi sake. Next, we examined how Lp. plantarum LP-2 and Ll. sakei LS-4 contributed to the quality of sake with the coexistence of Pseudomonas sp. strain 61-02 and Le. mesenteroides LM-1 in Yamahai-shikomi sake brewing. Finally, we showed the significance of Lp. plantarum LP-2 over Ll. sakei LS-4 and demonstrated that the quality of Yamahai-shikomi sake can be controlled by LAB through studying various combinations of those strains in Yamahai-shubo.
2 MATERIALS AND METHODS
2.1 Microorganisms
Four bacterial strains [nitrate-reducing Pseudomonas sp. 61-02 and three LAB strains (LM-1, LP-2, and LS-4)] were used in this study. They were previously isolated from Yamahai-shubo described before (Fujiwara et al., 2013; Wakai et al., 1990). The yeast strain, KZ-06 U, used in this study was a strain of S. cerevisiae that was developed for industrial-scale sake brewing at Kizakura Co., Ltd.
2.2 Yamahai-shubo preparation
The initial mixture of Yamahai-shubo was prepared as follows. The mixture included 333 g of total rice [250 g of raw rice (102 g of koji and 231 g of steamed rice)] and 255 mL of water with the addition of KNO3 (60 mg/L). koji is a type of rice that is made from koji mold (A. oryzae FK-5, Konnomoyashi Co., Ltd., Hyogo, Japan) with polished rice made by the industrial koji manufacturing system (Churitsu Industry Co., Ltd., Tokyo, Japan). Fermentation with koji followed the same method described before (Aya, 1995). A polishing ratio of 70% was employed for the raw rice and applied to koji and steamed rice.
Four bacterial strains [nitrate-reducing Pseudomonas sp. 61-02 and three LAB strains (LM-1, LP-2, and LS-4)] were prepared as follows. Strain 61-02 was independently cultured in PN medium [1% (W/V) polypeptone, 1% (W/V) KNO3] for 3 days at 15°C (Wakai et al., 1990) and was adjusted to reach a cell concentration of 104 cells/mL. Three LAB strains were separately cultured in koji extract (sake meter −60) for 3 days at 20°C and were added to the initial mixture of Yamahai-shubo at a cell concentration of 103 cells/mL. Yamahai-shubo preparation was totally completed in 25 days with the initial mixture of Yamahai-shubo after adding any of the nitrate-reducing bacterial strain 61-02 and/or three LAB bacterial strains (a total of 16 variations depending on the bacterial strain(s) added; see Table 1).
| No. | Bacterial strain addeda | SM |
Alc % (v/v) |
TAb mL-0.1 N NaOH |
AAb mL-0.1 N NaOH |
Glcc % (g/V) |
EtAc |
iBuAc mg/L |
iBuOH mg/L |
iAAc mg/L |
iAOH mg/L |
EtCp mg/L |
iAAc/iAOH% (w/w) | Cell number of strain 61-02d (cells/mL) | Cell number of LABsd (cells/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 61-02 | LM-1 | LP-2 | LS-4 | mg/L | ||||||||||||||
| 1 | − | − | − | − | −75 | 12.2 | 12.6 | 5.4 | 9.7 | 612 | 0.16 | 97.1 | 0.87 | 126.2 | 0.06 | 0.69 | n. d. | 5.0 × 103 |
| 2 | − | + | − | − | −139 | 5.3 | 14.6 | 5.7 | 19.2 | 925 | 0.05 | 1.4 | 0.33 | 35.0 | 0.01 | 0.95 | n. d. | 2.6 × 106 |
| 3 | − | − | + | − | −62 | 12.0 | 14.8 | 4.4 | 7.4 | 206 | 0.12 | 118.6 | 0.72 | 134.6 | 0.09 | 0.54 | n. d. | n. d. |
| 4 | − | − | − | + | −75 | 12.2 | 11.6 | 5.6 | 9.5 | 695 | 0.21 | 106.6 | 1.18 | 129.3 | 0.08 | 0.92 | n. d. | n. d. |
| 5 | − | + | + | − | −60 | 14.0 | 13.4 | 4.5 | 7.1 | 248 | 0.14 | 125.2 | 0.74 | 142.6 | 0.12 | 0.52 | n. d. | 3.0 × 103 |
| 6 | − | + | − | + | −75 | 12.5 | 10.7 | 5.5 | 9.5 | 576 | 0.17 | 102.0 | 0.95 | 128.0 | 0.08 | 0.74 | n. d. | n. d. |
| 7 | − | − | + | + | −76 | 12.2 | 11.6 | 5.7 | 8.8 | 720 | 0.23 | 105.5 | 1.27 | 126.3 | 0.12 | 1.01 | n. d. | n. d. |
| 8 | − | + | + | + | −75 | 12.0 | 11.7 | 5.6 | 9.1 | 690 | 0.21 | 96.7 | 1.19 | 124.9 | 0.12 | 0.96 | n. d. | 6.0 × 103 |
| 9 | + | − | − | − | −71 | 12.9 | 9.3 | 6.4 | 8.8 | 495 | 0.22 | 105.4 | 1.04 | 117.9 | 0.14 | 0.88 | n. d. | n. d. |
| 10 | + | + | − | − | −148 | 4.1 | 9.5 | 7.5 | 19.1 | 1198 | 0.22 | 16.7 | 0.98 | 43.1 | 0.24 | 2.28 | n. d. | 4.4 × 107 |
| 11 | + | − | + | − | −54 | 14.3 | 13.4 | 4.3 | 6.3 | 109 | 0.12 | 116.5 | 0.77 | 136.8 | 0.23 | 0.56 | n. d. | 5.6 × 102 |
| 12 | + | − | − | + | −64 | 13.6 | 9.7 | 4.9 | 7.4 | 56 | 0.16 | 90.0 | 0.99 | 119.0 | 0.30 | 0.83 | n. d. | n. d. |
| 13 | + | + | + | − | −57 | 14.4 | 12.1 | 4.8 | 6.6 | 36 | 0.05 | 62.1 | 0.33 | 80.6 | 0.18 | 0.41 | n. d. | n. d. |
| 14 | + | + | − | + | −75 | 12.4 | 10.1 | 4.5 | 9.1 | 335 | 0.20 | 82.1 | 1.03 | 104.3 | 0.22 | 0.98 | n. d. | n. d. |
| 15 | + | − | + | + | −62 | 13.8 | 10.3 | 5.0 | 7.4 | 47 | 0.06 | 29.6 | 0.40 | 57.0 | 0.21 | 0.70 | n. d. | n. d. |
| 16 | + | + | + | + | −67 | 13.5 | 10.6 | 6.0 | 7.5 | 108 | 0.16 | 84.7 | 0.89 | 111.7 | 0.36 | 0.79 | n. d. | n. d. |
- Note: iAAc/iAOH. These fermentation factors were determined by the standard methods established by the National Tax Agency of Japan (National Research Institute of Brewing, 2017).
- Abbreviations: AA, amino acidity; Alc, ethanol; EtAc, ethyl acetate; EtCp, ethyl caproate; iAAc, isoamyl acetate; iAOH, isoamyl alcohol; iBuAc, isobutyl acetate; iBuOH, isobutyl alcohol; n.d., not detected; SM, sake meter; TA, total acidity.
- a The bacteria added for Yamahai-shubo preparation were indicated with + and those that were not added were indicated with −.
- b TA and AA are expressed as the amount of volume required to neutralize with 0.1 N NaOH solution in 10 mL of Yamahai-shubo samples.
- c Glucose (Glc) was measured by the method described previously (Fujiwara et al., 2013) using a specific instrument (Arkray ADAMS Glucose GA-1151, Kyoto, Japan).
- d The cell numbers of strain 61-02 and LABs (strains LM-1, LP-2, and LS-4) were determined by the method described previously (Fujiwara et al., 2013; Wakai et al., 1990).
The temperature during Yamahai-shubo preparation was kept at 8°C until day 4. After day 5, the preparation was warmed up by contact with hot water at 65°C to increase the temperature by one degree per day until it reached 21°C on day 16. After holding the temperature of the mixture at 21°C for another 5 days, the temperature was decreased to 15°C on day 21, to 13°C on day 22, to 12°C on day 23, and was then maintained at 12°C until day 25. The yeast strain KZ-06 U was separately cultured in koji extract (sake meter −60) for 2 days at 30°C and added between days 10 and 15 of the preparation to reach a cell concentration of 106 cells/mL when the nitrite concentration of the Yamahai-shubo preparation was less than 1.0 mg/L. The timing of yeast addition in the absence of strain 61-02 was the same as the timing of yeast addition in the presence of strain 61-02. The final preparation reached approximately 500 mL in volume and was called “Yamahai-shubo.” For use at the industrial scale, the process was considered complete at day 25, even if the disappearance of nitrite was delayed and the yeast growth period was shortened.
The cell numbers of strain 61-02 and three LAB strains (LM-1, LP-2, and LS-4) were determined by the method described previously (Fujiwara et al., 2013; Wakai et al., 1990).
2.3 Yamahai-shikomi sake brewing
The polishing ratio of raw rice was set at 70%, just as in the case for Yamahai-shubo preparation. Brewage ingredients (Yamahai-shubo, koji, steamed rice, and water) were mixed at three different times (soe, naka, and tome feeding procedures) to smoothly complete the fermentation (Anzawa et al., 2014; Furukawa et al., 2000). The soe procedure (23 mL of Yamahai-shubo, 9.6 g of koji, 26.6 g of steamed rice, and 33 mL of water were added) was carried out at 15°C from day 1 to day 2. Both the naka (day 3, 12.0 g of koji, 54.6 g of steamed rice, and 64 mL of water) and the tome (day 4, 14.4 g of koji, 99.4 g of steamed rice, and 112 mL of water) feeding procedures were carried out by adding the components at 10°C. After the tome procedure (170 g of raw rice, total volume 450 mL), sake brewing was monitored by measuring the weight reduction of the fermentation mixture, corresponding to CO2 evolution for 25 days. The temperature of the fermentation mixture was increased by about 0.5°C for another 6 days, kept at 13°C for an additional 7 days, and then promptly lowered to 10°C while the temperature was maintained for an additional 12 days. After sake brewing (25 days), the samples were separated by centrifugation (6800 × g, 20 min, 4°C), and the supernatants were called “Yamahai-shikomi” sake samples (a total of 16 types of Yamahai-shikomi sake samples depending on 16 variations of Yamahai-shubo; see Table 2). The volume of sake and the weight of sake lee were measured. Sake lees are the final sediments obtained in the sake brewing process and are alternatively called “sake-kasu.”
| No. | Bacterial strain added to “Yamahai-shubo”a |
Sake Vol. (mL) |
Sake Lee (g) |
Sake Lee/sake Vol. | SM |
Alc % (v/v) |
TAb mL-0.1 N NaOH |
AAb mL-0.1 N NaOH |
Glcc % (w/v) |
UV |
EtAc mg/L |
iBuAc mg/L |
iBuOH mg/L |
iAAc mg/L |
iAOH mg/L |
EtCp mg/L |
iAAc/iAOH% (w/w) | Sensory scored | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 61-02 | LM-1 | LP-2 | LS-4 | 260 Nm | 280 Nm | Aroma | Taste | ||||||||||||||||
| 1 | − | − | − | − | 272 | 106 | 0.39 | −11.9 | 17.9 | 3.1 | 2.3 | 1.4 | 11.4 | 10.0 | 45 | 0.07 | 41.3 | 3.0 | 142 | 0.34 | 2.09 | 2.7 | 2.5 |
| 2 | − | + | − | − | 256 | 125 | 0.49 | −17.3 | 16.6 | 5.4 | 2.8 | 1.7 | 11.1 | 10.5 | 41 | 0.01 | 15.6 | 1.0 | 113 | 1.64 | 0.91 | 3.3 | 3.5 |
| 3 | − | − | + | − | 266 | 118 | 0.45 | −12.9 | 17.3 | 3.3 | 2.1 | 1.4 | 10.5 | 9.7 | 58 | 0.10 | 47.6 | 3.9 | 158 | 0.53 | 2.49 | 2.3 | 2.3 |
| 4 | − | − | − | + | 273 | 113 | 0.41 | −9.9 | 18.3 | 3.0 | 2.1 | 1.3 | 10.8 | 9.8 | 78 | 0.15 | 56.8 | 5.2 | 173 | 0.61 | 3.00 | 2.8 | 2.8 |
| 5 | − | + | + | − | 274 | 110 | 0.40 | −11.0 | 18.0 | 3.2 | 2.1 | 1.4 | 10.9 | 9.9 | 47 | 0.07 | 32.5 | 3.1 | 125 | 0.43 | 2.51 | 2.3 | 2.7 |
| 6 | − | + | − | + | 286 | 99 | 0.35 | −8.7 | 18.2 | 3.1 | 2.3 | 1.3 | 11.7 | 10.2 | 53 | 0.09 | 54.5 | 3.7 | 168 | 0.50 | 2.18 | 3.0 | 2.7 |
| 7 | − | − | + | + | 279 | 106 | 0.38 | −10.6 | 18.0 | 3.0 | 2.2 | 1.3 | 10.9 | 9.8 | 75 | 0.14 | 56.0 | 5.0 | 172 | 0.61 | 2.94 | 3.0 | 2.8 |
| 8 | − | + | + | + | 270 | 115 | 0.43 | −12.9 | 17.3 | 3.0 | 2.0 | 1.3 | 10.4 | 9.7 | 53 | 0.09 | 43.3 | 3.5 | 152 | 0.53 | 2.31 | 2.8 | 2.5 |
| 9 | + | − | − | − | 261 | 120 | 0.46 | −14.1 | 17.2 | 3.0 | 1.9 | 1.4 | 10.5 | 9.8 | 55 | 0.12 | 46.0 | 4.1 | 153 | 0.63 | 2.69 | 3.0 | 2.7 |
| 10 | + | + | − | − | 266 | 115 | 0.43 | −16.5 | 16.7 | 3.9 | 2.4 | 1.5 | 10.7 | 9.9 | 53 | 0.05 | 37.2 | 2.1 | 137 | 0.42 | 1.51 | 2.7 | 2.8 |
| 11 | + | − | + | − | 274 | 112 | 0.41 | −11.7 | 17.7 | 3.1 | 2.0 | 1.4 | 10.6 | 9.7 | 68 | 0.13 | 47.8 | 4.7 | 160 | 0.70 | 2.92 | 2.3 | 2.3 |
| 12 | + | − | − | + | 285 | 99 | 0.35 | −9.9 | 18.2 | 3.0 | 2.0 | 1.4 | 11.9 | 10.3 | 80 | 0.18 | 58.1 | 5.9 | 174 | 0.71 | 3.36 | 2.5 | 2.3 |
| 13 | + | + | + | − | 279 | 105 | 0.37 | −11.6 | 17.8 | 3.2 | 2.0 | 1.4 | 11.4 | 10.3 | 65 | 0.13 | 50.1 | 4.4 | 162 | 0.66 | 2.69 | 2.3 | 2.5 |
| 14 | + | + | − | + | 268 | 116 | 0.43 | −12.9 | 17.8 | 3.0 | 1.9 | 1.4 | 10.8 | 9.8 | 80 | 0.17 | 52.0 | 5.4 | 167 | 0.74 | 3.23 | 2.5 | 2.7 |
| 15 | + | − | + | + | 267 | 116 | 0.43 | −13.4 | 17.5 | 3.1 | 1.9 | 1.3 | 10.4 | 9.8 | 54 | 0.12 | 45.3 | 4.1 | 158 | 0.71 | 2.58 | 2.5 | 2.7 |
| 16 | + | + | + | + | 280 | 103 | 0.37 | −10.8 | 18.1 | 3.1 | 2.0 | 1.4 | 10.9 | 9.8 | 86 | 0.18 | 49.4 | 5.9 | 168 | 0.84 | 3.49 | 2.2 | 2.5 |
- Note: iAAc/iAOH. These fermentation factors were determined by the standard methods established by the National Tax Agency of Japan (National Research Institute of Brewing, 2017).
- Abbreviations: AA, amino acidity; Alc, ethanol; EtAc, ethyl acetate; EtCp, ethyl caproate; iAOH, isoamyl alcohol; iBuAc, isobutyl acetate; iBuOH, isobutyl alcohol; SM, sake meter; TA, total acidity; UV, Ultraviolet absorption.
- a The bacteria added for Yamahai-shubo preparation were indicated with + and those that were not added were indicated with -. The patterns of strains added to Yamahai-shubo are the same as those in Table 1.
- b TA and AA are expressed as the amount of volume required to neutralize with 0.1 N NaOH solution in 10 mL of Yamahai-shikomi sake samples.
- c Glucose (Glc) was measured by the method described previously (Fujiwara et al., 2013) using a specific instrument (Arkray ADAMS Glucose GA-1151, Kyoto, Japan).
- d The sensory scores of sake samples held at 20°C consisted of qualitative evaluations of aromas and tastes according to five different grades (1, very good; 2, good; 3, normal; 4, bad; 5, very bad). Sensory evaluations were performed by six well-trained panelists.
2.4 Analysis of fermentation factors in Yamahai-shubo preparation and sake samples
Basic fermentation factors for sake meter (SM), ethanol (Alc), total acidity (TA), amino acidity (AA), and Ultraviolet absorption (UV) were determined by the standard method established by National Research Institute of Brewing (National Research Institute of Brewing, 2017), while glucose (Glc) was measured by the method described previously (Fujiwara et al., 2013) using a specific instrument (Arkray ADAMS Glucose GA-1151, Kyoto, Japan). Fermentation factors for volatile organic compounds [ethyl acetate (EtAc), isobutyl acetate (iBuAc), isobutyl alcohol (iBuOH), isoamyl acetate (iAAc), isoamyl alcohol (iAOH), ethyl caproate (EtCp), and isoamyl acetate/isoamyl alcohol (iAAc/iAOH)] were determined by following the same standard methods (National Research Institute of Brewing, 2017). Other fermentation factors, including concentrations of other organic acids (pyruvic acid, citric acid, succinic acid, malic acid, and lactic acid), were determined by the method described previously (Kohsaka et al., 2016): 25 μL of sample was taken in a 10 mL screw-top test tube, and 1 mL of 0.3 M HCl/1-propanol solution was added. It was heated at 100°C for 1 h. After cooling, 1 mL of methyl tert-butyl ether containing 4 μg of methyl laurate as an internal standard and 1 mL of distilled water were added and mixed. The ether layer was analyzed by a gas chromatography GC-2014 (Shimazu Corporation, Kyoto, Japan). Diacetyl was derivatized by Shinwa DS-DA (Shinwa Chemical Industries, Ltd., Kyoto, Japan, https://shinwa-cpc.co.jp/products/ds/) and analyzed using the same gas chromatography system. All data are the averages of two or three independent experiments.
The qualities of Yamahai-shikomi sake samples were graded by sensory scores evaluated by four or six well-trained panelists. The sensory scores of sake samples held at 20°C consisted of qualitative evaluations of aromas and tastes according to five different grades (1, very good; 2, good; 3, normal; 4, bad; 5, very bad).
2.5 Principal component analysis (PCA)
Principal component analysis (PCA) was performed with Excel Statistical analysis 2008 software for Windows (Social Survey Research Information Co., Ltd., https://www.bellcurve.jp/), employing fermentation factors (SM, Alc, TA, AA, Glc, UV, EtAc, iBuAc, iBuOH, iAAc, iAOH, EtCp, and iAAc/iAOH) and sensory scores at the final point (25 days) of Yamahai-shubo (Table 1) and Yamahai-shikomi sake samples (Table 2). In addition, the data for sake volume and sake lee, sake lee/sake volume, were used for the analysis.
3 RESULTS
3.1 Effect of four bacterial strains (nitrate-reducing pseudomonas sp. 61-02, Le. Mesenteroides LM-1, Lp. Plantarum LP-2, and Ll. Sakei LS-4) on Yamahai-shubo preparation samples
The four bacterial strains previously found in Yamahai-shubo were examined to evaluate their effects along the time course of Yamahai-shubo preparation (a total of 25 days). Changes in three fermentation factors are depicted in Figure 1, and others are shown in Table 1. In Figure 1, three factors—nitrite concentration (a-1 and a-2), acidity (b-1 and b-2), and amino acid concentration (c-1 and c-2)—were described in the absence (a-1, b-1, and c-1) and presence (a-2, b-2, and c-2) of nitrate-reducing Pseudomonas sp. 61-02, following the method as described in Materials and Methods. Nitrite is known to be essential in Yamahai-shubo preparation process; it is converted from nitrate, which is derived from PN medium for the preparation of strain 61-02 (see Materials and Methods) and inhibits the growth of wild yeasts that are concomitant in the initial mixture for Yamahai-shubo. As shown in Figure 1a-1,a-2, strain 61-02 was responsible for nitrite production; its concentration increased in the first half of Yamahai-shubo preparation and drastically decreased before the second half. However, the coculture of strain LM-1 only with strain 61-02 was found to retard the decrease in the concentration of nitrite (Figure 1a-2). It is noted that the delay is a characteristic of strain LM-1 when it is cocultured with strain 61-02. The acidity increased along the time course of Yamahai-shubo preparation in the absence and presence of strain 61-02, which is necessary for the growth of yeast in sake brewing of strain KZ-06 U; on the other hand, there was a slower rise in acidity for the coculture of strain LM-1 only with strain 61-02 and the simple culture of strain 61-02 without any LAB strains (Figure 1b-1,b-2). Furthermore, there seemed to be no remarkable difference in amino acid concentration in the presence and absence of strain 61-02 (Figure 1c-1,c-2). In addition, with the exceptions of Yamahai-shubo preparations contained strains 61-02 and LM-1 (No. 10 in Table 1) and only strain LM-1 (No. 2 in Table 1), the viable cell numbers of four bacterial strains (61-02, LM-1, LP-2, and LS-4) in Yamahai-shubo became extremely decreased after the preparation for 25 days (Figure 1d-1,d-2 and Table 1). In the first half of the preparation of Yamahai-shubo, there seemed to be no significant difference in cell number of LABs (strains LM-1, LP-2, and LS-4) in the presence and absence of strain 61-02 (Figure 1d-1,d-2). However, Yamahai-shubo preparations contained only strain 61-02 (No. 9 in Table 1) and no addition of any strains (No. 1 in Table 1) had no LABs on day 1, but they increased LABs on day 5. Although the growth of LABs is confirmed in the industrial scale Yamahai-shubo preparation without the addition of microorganisms (Ashizawa & Saito, 1966a; Koyanagi et al., 2016), it is known that the process is not stable at the industrial scale. On the other hand, we have previously confirmed that Yamahai-shubo can be stably incubated at an industrial scale by adding cocci LAB and bacilli LAB (Fujiwara et al., 2013) without being affected by environmental microorganisms, and therefore, as a negative control, we used Yamahai-shubo preparations contained only strain 61-02 and no addition of any strains. After yeast strain KZ-06 U was added in Yamahai-shubo preparation between day 11 and day 19, alcohol concentration changes were almost the same at the 16 patterns (data not shown).
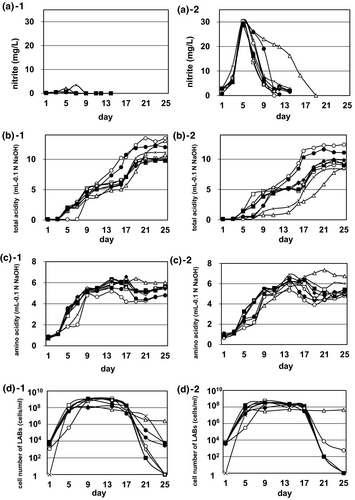
Comparing the components at the final point (25 days) of Yamahai-shubo preparation in Table 1, the addition of strain LP-2 (Nos. 5 and 11) contributed to the highest level of the alcohol concentration (Alc) and total acidity (TA), simultaneously resulting in essential requirements of strains 61-02 and/or LM-1 for Yamahai-shubo utilizing the advantages of strain LP-2 without strain LS-4.
We further investigated fermentation factors for Yamahai-shikomi sake similarly as Yamahai-shubo (Table 2) and applied them to principal component analysis (PCA).
3.2 Principal component analysis (PCA) for Yamahai-shubo and Yamahai-shikomi sake
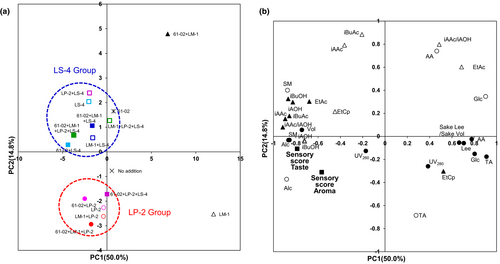
To investigate the suitability of the bacterial combination of the nitrate-reducing Pseudomonas sp. 61-02 and three LAB strains (LM-1, LP-2, and LS-4), we performed principal component analysis (PCA) by using fermentation factors for 16 samples of Yamahai-shubo and Yamahai-shikomi sake, as shown in Tables 1 and 2. As a result of PCA score plotting of fermentation factors, the contribution ratios of principal component 1 (PC1) and principal component 2 (PC2) were 50.0% and 14.8%, respectively (Figure 2). In addition, the eigen values of principal component 1 (PC1) and principal component 2 (PC2) were 15.5 and 4.6, respectively.
 ), addition of the strain LP-2 (No. 3); LS-4 (
), addition of the strain LP-2 (No. 3); LS-4 ( ), addition of the strain LS-4 (No. 4); LM-1 + LP-2 (
), addition of the strain LS-4 (No. 4); LM-1 + LP-2 ( ), addition of the strains LM-1 and LP-2 (No. 5); LM-1 + LS-4 (
), addition of the strains LM-1 and LP-2 (No. 5); LM-1 + LS-4 ( ), addition of the strains LM-1 and LS-4 (No. 6); LP-2 + LS-4 (
), addition of the strains LM-1 and LS-4 (No. 6); LP-2 + LS-4 ( ), addition of the strains LP-2 and LS-4 (No. 7); LM-1 + LP-2 + LS-4 (
), addition of the strains LP-2 and LS-4 (No. 7); LM-1 + LP-2 + LS-4 ( ), addition of the strains LM-1, LP-2, and LS-4 (No. 8); 61-02 (*), addition of 61-02 (No. 9); 61-02 + LM-1 (▲), addition of strain No.61-02 and LM-1 (No. 10); 61-02 + LP-2 (
), addition of the strains LM-1, LP-2, and LS-4 (No. 8); 61-02 (*), addition of 61-02 (No. 9); 61-02 + LM-1 (▲), addition of strain No.61-02 and LM-1 (No. 10); 61-02 + LP-2 ( ), addition of the strains 61-02 and LP-2 (No. 11); 61-02 + LS-4 (
), addition of the strains 61-02 and LP-2 (No. 11); 61-02 + LS-4 ( ), addition of the strains 61-02 and LS-4 (No. 12); 61-02 + LM-1 + LP-2 (
), addition of the strains 61-02 and LS-4 (No. 12); 61-02 + LM-1 + LP-2 ( ), addition of the strains 61-02, LM-1, and LP-2 (No. 13); 61-02 + LM-1 + LS-4 (
), addition of the strains 61-02, LM-1, and LP-2 (No. 13); 61-02 + LM-1 + LS-4 ( ), addition of the strains 61-02, LM-1, and LS-4 (No. 14); 61-02 + LP-2 + LS-4 (
), addition of the strains 61-02, LM-1, and LS-4 (No. 14); 61-02 + LP-2 + LS-4 ( ), addition of the strains 61-02, LP-2, and LS-4 (No. 15); 61-02 + LM-1 + LP-2 + LS-4 (
), addition of the strains 61-02, LP-2, and LS-4 (No. 15); 61-02 + LM-1 + LP-2 + LS-4 ( ), addition of the strains 61-02, LM-1, LP-2, and LS-4 (No. 16). (b) Loading plot of the samples of Yamahai-shubo preparation and Yamahai-shikomi sake are shown in Tables 1 and 2. Thirty-one fermentation factors were obtained as shown in Materials and Methods. Open symbols: those data were from Yamahai-shubo samples. Closed symbols: those data were from Yamahai-shikomi sake samples. Circle; basic fermentation factors (SM, sake meter; Alc, ethylalcohol; TA, total acidity; AA, amino acidity; Glc, glucose concentration; UV260, Ultraviolet absorption at 260 nm; UV280, Ultraviolet absorption at 280 nm; Vol, sake volume; Lees; sake lees; Lee/Vol, sake lees/sake volume). Triangle; fermentation factors in aroma (EtAc, ethyl acetate; iBuAc, isobutyl acetate; iBuOH, isobutyl alcohol; iAAc, isoamyl acetate; iAOH, isoamyl alcohol; EtCp, ethyl caproate; iAAc/iAOH). Square; sensory scores in aroma and taste for Yamahai-shikomi sake.
), addition of the strains 61-02, LM-1, LP-2, and LS-4 (No. 16). (b) Loading plot of the samples of Yamahai-shubo preparation and Yamahai-shikomi sake are shown in Tables 1 and 2. Thirty-one fermentation factors were obtained as shown in Materials and Methods. Open symbols: those data were from Yamahai-shubo samples. Closed symbols: those data were from Yamahai-shikomi sake samples. Circle; basic fermentation factors (SM, sake meter; Alc, ethylalcohol; TA, total acidity; AA, amino acidity; Glc, glucose concentration; UV260, Ultraviolet absorption at 260 nm; UV280, Ultraviolet absorption at 280 nm; Vol, sake volume; Lees; sake lees; Lee/Vol, sake lees/sake volume). Triangle; fermentation factors in aroma (EtAc, ethyl acetate; iBuAc, isobutyl acetate; iBuOH, isobutyl alcohol; iAAc, isoamyl acetate; iAOH, isoamyl alcohol; EtCp, ethyl caproate; iAAc/iAOH). Square; sensory scores in aroma and taste for Yamahai-shikomi sake.In the score plot (Figure 2a), when Yamahai-shubo preparations and Yamahai-shikomi sake samples contained strains 61-02 and LM-1 (No. 10 in Tables 1 and 2) or only strain LM-1 (No. 2), their plots were located in the first and fourth quadrants, respectively, apart from other combinations of strains that occurred in the second and third quadrants. In the second and third quadrants, two major groups were formed. Plots for the group containing strain LP-2 (LP-2 group; Nos. 3, 5, 11, 13, and 15) occurred in the third quadrants, forming one cluster. In contrast, plots for the group including strain LS-4 (LS-4 group; Nos. 4, 6, 7, 12, and 14) occurred in the second quadrant. The sample only contained strain 61-02 (No. 9) was located in LS-4 group. Interestingly, combinations of three LAB strains (LM-1, LP-2, and LS-4) with (No. 16) and without strain 61-02 (No. 8) were included in this LS-4 group. These results showed that the characteristics of strain LS-4 in the LS-4 group were predominant in the second quadrant when strain LP-2 was present with strain LM-1.
In order to evaluate the fermentation factors that contribute to the principal component scores, we confirmed the factor loadings by their plots (Figure 2b). The factor loadings are the correlation coefficients between the principal components and the variable fermentation factors. The closer the values of factor loading are to 1 or −1, the more the factors contribute to the principal components. On the PC1 axis, the factor loading of TA for Yamahai-shikomi sake was 0.91. On the other hand, the factor loading of the sensory score for taste showed a negative value of −0.79. On the PC2 axis, the factor loadings of iBuAc for Yamahai-shubo and of EtAc for Yamahai-shikomi sake were 0.88 and 0.30, respectively. Since EtAc and iBuAc generally provide aromas undesirable for sake products (Yoshizawa, 1999), their positive values are disadvantageous in the quality of sake. In contrast, the factor loading of the sensory score for aroma of Yamahai-shikomi sake showed a negative value of −0.31 (Figure 2b). The factor loading plots showed that negative values of sensory evaluation contributed to the principal components of the PC1 and PC2 axes (Figure 2b). In the score plot, since the LP-2 group showed negative values on the PC1 and PC2 axes (third quadrant, Figure 2a), strain LP-2 was confirmed to be responsible for a group with a higher sensory score. On the other hand, the sample containing strain 61-02 and LM-1 (No. 10) showed positive values on the PC1 and PC2 axes (first quadrant, Figure 2a). EtAc and iAAC/iAOH for Yamahai-shubo were both located in the first quadrant (Figure 2b). Although iAAC/iAOH is the indicator of the good quality of sake, EtAc for Yamahai-shubo may decrease sake quality while iAAC/iAOH for Yamahai-shubo may be less effective in improving sake quality.
Judging from these results, it was found that strains LP-2 and LS-4 were important in the Yamahai-shikomi sake in the presence of strains 61-02 and LM-1.
3.3 Effect of Lp. Plantarum LP-2 and Ll. Sakei LS-4 in Yamahai-shubo preparation on the concentration of organic acids in Yamahai-shikomi sake
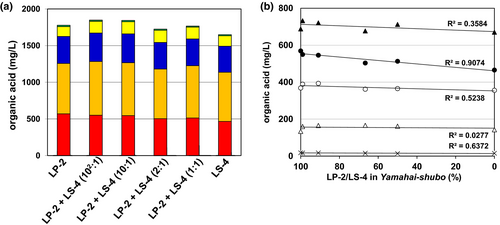
The sample coexisting strains LP-2 and LS-4 was located in the LS-4 group (Figure 2a). So, to evaluate the effect of coexisting bacilli-LABs on sake quality, Yamahai-shubo samples were prepared by adding different proportions of strains LP-2 and LS-4 to its initial mixture in the presence of conventional amounts of strains 61-02 and LM-1. After sake brewing with those Yamahai-shubo samples for 25 days, the Yamahai-shikomi sake samples were withdrawn and prepared by following the method as described in the Materials and Methods section, and then concentrations of organic acids (pyruvic acid, citric acid, succinic acid, malic acid, and lactic acid) in Yamahai-shikomi sake and Yamahai-shubo samples were determined for each sample (Figure 3). Although the sake samples were produced in the same way as those Yamahai-shubo samples including bacterial strains with different proportions of strains LP-2 and LS-4, there seemed to be no significant difference in the concentration of five organic acids (Figure 3a). However, taking into account the change in each acid relative to the proportion of strains LP-2 to LS-4 strains in Yamahai-shubo preparation, only lactic acid was found to decrease with an adequate correlation to the decrease in the ratio of LS-4 strain (R2 = 0.9074; Figure 3b). Although the proportion of strains LP-2 and LS-4 was unequal, there seems to be a tendency for lactic acid concentration to increase as the mixing ratio of strain LS-4 decreases.
 ) for pyruvic acid, (
) for pyruvic acid, ( ) for citric acid, (
) for citric acid, ( ) for succinic acid, (
) for succinic acid, ( ) for malic acid, and (
) for malic acid, and ( ) for lactic acid. Yamahai-shikomi sake was obtained by the method as described in Materials and Methods with different Yamahai-shubo samples prepared by adding various proportions of strains LP-2 and LS-4 to its initial mixture of Yamahai-shubo with the strains 61-02 and LM-1. (b) The change of each acid [(×) pyruvic acid, (Δ) citric acid, (▲) succinic acid, (○) malic acid, and (●) lactic acid] relative to the proportion of LP-2 to LS-4 strains occurring in Yamahai-shubo. The dots on the line for each organic acid correspond to LP-2, LP-2 + LS-4 (102:1), LP-2 + LS-4 (10:1), LP-2 + LS-4 (2:1), LP-2 + LS-4 (1:1), and LS-4 in Figure 2 a from left to right. The values of R2 represent the correlation coefficients.
) for lactic acid. Yamahai-shikomi sake was obtained by the method as described in Materials and Methods with different Yamahai-shubo samples prepared by adding various proportions of strains LP-2 and LS-4 to its initial mixture of Yamahai-shubo with the strains 61-02 and LM-1. (b) The change of each acid [(×) pyruvic acid, (Δ) citric acid, (▲) succinic acid, (○) malic acid, and (●) lactic acid] relative to the proportion of LP-2 to LS-4 strains occurring in Yamahai-shubo. The dots on the line for each organic acid correspond to LP-2, LP-2 + LS-4 (102:1), LP-2 + LS-4 (10:1), LP-2 + LS-4 (2:1), LP-2 + LS-4 (1:1), and LS-4 in Figure 2 a from left to right. The values of R2 represent the correlation coefficients.3.4 Effect of Lp. Plantarum LP-2 and Ll. Sakei LS-4 in Yamahai-shubo preparation on the concentration of diacetyl in Yamahai-shikomi sake
We investigated their effect on the concentration of diacetyl. The sake brewing was carried out in the same way as shown in Figure 3, and the concentrations of diacetyl in Yamahai-shikomi sake samples were determined. As shown in Figure 4, the sample prepared in the absence of strain LS-4 significantly exhibited the lowest concentration of diacetyl. Lower concentrations of diacetyl are so favorable for improving sake quality that the use of strain LS-4 in Yamahai-shubo preparation had an undesirable effect on the final sake samples. Although diacetyl concentration of LP2 + LS4 (102:1) was higher than that of LP2 + LS4 (2:1), there was no significant difference between them (Figure 4). This result was supported by the statistical analysis for the sensory scores for aroma of each Yamahai-shikomi sake sample (Figure 5). The sensory scores for aroma showed that Yamahai-shikomi sake prepared with strain LP-2 had significantly lower values than those with strain LS-4, with the exception of the case with a proportion of LP-2:LS-4 = 102:1.

 ), LP-2:LS-4 = 102: 1 (
), LP-2:LS-4 = 102: 1 ( ), LP-2:LS-4 = 10: 1 (
), LP-2:LS-4 = 10: 1 ( ), LP-2:LS-4 = 2: 1 (
), LP-2:LS-4 = 2: 1 ( ), LP-2:LS-4 = 1: 1 (
), LP-2:LS-4 = 1: 1 ( ), LS-4 only (
), LS-4 only ( ). After the sake brewing with seven types of Yamahai-shubo, Yamahai-shikomi sake samples were withdrawn and prepared by following the method as described in Materials and Methods and then concentrations of diacetyl were determined by the method described in Materials and Methods. The data were represented with error bars from the average of the three data. The Student's t-test was used for statistical analysis. **p < .01; *p < .05.
). After the sake brewing with seven types of Yamahai-shubo, Yamahai-shikomi sake samples were withdrawn and prepared by following the method as described in Materials and Methods and then concentrations of diacetyl were determined by the method described in Materials and Methods. The data were represented with error bars from the average of the three data. The Student's t-test was used for statistical analysis. **p < .01; *p < .05.
Through this investigation, in order to reduce the diacetyl concentration in Yamahai-shikomi sake, it was found that the addition of strain LS-4 should be avoided in Yamahai-shubo preparation, and it is likely that strain LP-2 plays a more significant role in reducing the concentration of diacetyl with strains LM-1 and 61-02 in Yamahai-shubo preparation and Yamahai-shikomi sake brewing.
4 DISCUSSION
The steady supply of Yamahai-shikomi sake employing Yamahai-shubo has been requested by many sake producers and consumers, while it requires various developments in technological and scientific fields. Therefore, the use of microorganisms (yeasts and bacteria) isolated from Yamahai-shubo samples is very important for producing Yamahai-shikomi sake. In particular, the isolation of different bacteria from Yamahai-shubo samples and their practical use in sake brewing make it possible to produce Yamahai-shikomi sake under artificial control in the recent process.
Yamahai-shubo samples contain various kinds of identified and unidentified bacteria, and their bacterial diversities vary depending on the sources from which they are derived. It is well known that the quality of Yamahai-shikomi sake depends on the diversities of those bacteria. Therefore, it is necessary to grow advantageous bacteria to prepare appropriate Yamahai-shubo samples and to achieve the desired sake quality.
We previously isolated four bacterial strains (nitrate-reducing Pseudomonas sp. 61-02, cocci-LAB Le. mesenteroides LM-1, bacilli-LAB Lp. plantarum LP-2, and bacilli-LAB Ll. sakei LS-4) from the Yamahai-shubo sample (Fujiwara et al., 2013; Wakai et al., 1990). In our previous report, we confirmed that strain LP-2 is industrially available, but it remained unconfirmed how the Yamahai-shubo preparation and the Yamahai-shikomi sake brewing change under the coexistence of strains LP-2 and LS-4 (Fujiwara et al., 2013). In the present study, we confirmed that the nitrate-reducing strain 61-02 was necessary for sufficient nitrite production, whereas the decrease in nitrite was delayed where only the cocci-LAB strain LM-1 was present with the nitrate-reducing strain 61-02 (Figure 1a-2). Sato et al. cultivated lactic acid bacteria by adding nitrite to two different types of culture media and confirmed that the reduction in nitrite was closely linked to the decrease in the pH of the culture (Sato et al., 2016). They attributed the decrease in the pH of the media to lactic acid produced by LAB, resulting in a fall in nitrite due to a nonenzymatical diazotization reaction with various amino groups occurring in the environment (Yoshizawa, 1999). They also reported that the reduction in nitrite was more retarded by the presence of Le. mesenteroides than that of Ll. sakei, since the production of lactic acid was much slower in the presence of Le. mesenteroides than of Ll. sakei. It indicates that the reduction in nitrite is delayed only in a cocci-LAB strain (Le. mesenteroides) that produces lactic acid more slowly. We clarified the turnover of those bacteria, along with the amount of nitrite, through this study.
In Yamahai-shubo samples after 25 days (Table 1), the addition of strain LP-2 provided higher acidity than the addition of LS-4 in the presence of strains 61-02 and LM-1 (see the results of Nos. 13 and 14). It has already been confirmed that the acid production of strain LP-2 in Yamahai-shubo is higher than that of strain LS-4 (Fujiwara et al., 2013). At the same reason, the addition of strain LP-2 provided higher acidity than the addition of LS-4 (see the results of Nos. 11 and 12) in the presence of strain 61-02 and the absence of stain LM-1. After sake brewing with the Yamahai-shubo samples, the acidity of Yamahai-shikomi sake samples when strain LP-2 was added to Yamahai-shubo was higher than that when strain LS-4 was added (see TA of Nos. 13 and 14 in Table 2). Since the volume of Yamahai-shubo accounted for only 5% of the total volume of Yamahai-shikomi sake after the tome procedure (23 mL-Yamahai-shubo/450 mL-total volume; see Materials and Methods), Yamahai-shubo was diluted nearly 20 times in Yamahai-shikomi sake. Thus, the higher acidity of Yamahai-shubo was supposed to be unable to directly keep the acidity of Yamahai-shikomi sake. On the other hand, the majority of the acids in Yamahai-shikomi sake were most likely produced by yeasts coexisting during Yamahai-shikomi sake brewing under higher acidic environment, resulting in increased acidity. This was consistent with the fact that the viable cell numbers of most bacteria in Yamahai-shubo became extremely decreased after 25 days (Table 1); therefore, acids produced by the bacteria de novo during the brewing of Yamahai-shikomi sake was negligible.
In Figure 2, the LP-2 group showed preferable sensory score values for aroma and taste. Diacetyl (2,3-butanedione) is a butter-flavored diketone produced as a by-product of yeast valine metabolism during beverage fermentation and affects evaluations (Krogerus & Gibson, 2013; Sato et al., 1981). Since the cognitive threshold of the diacetyl concentration in sake was reported to be 0.2 mg/L (Utsunomiya et al., 2004), its concentrations in the sake samples in Figure 4 indicate that sake prepared with only strain LP-2 had a favorable value below the limit in the sensory score; on the other hand, those with strain LS-4 exceeded the limit and were found to be unsuitable for Yamahai-shikomi sake preparation. Diacetyl also has a typical unfavorable flavor in sake, beverages, and foods, while thought to be produced by LAB (Clark & Winter, 2015; Inoue, 2004). The general pathway for diacetyl synthesis is recognized to be a result of valine anabolism in bacteria and yeasts, since they arise from the spontaneous nonenzymatic oxidative decarboxylation of α-acetolactic acid that is an intermediate in the valine biosynthesis pathway (Chuang & Collins, 1968; Horie et al., 2010; Suomalainen & Ronkainen, 1968). However, in the present study for Yamahai-shikomi sake brewing, it was presumably produced by sake yeasts S. cerevisiae because most LAB had died off after completing the preparation of Yamahai-shubo (Table 1). Although the detailed mechanism remains unknown, we assume that the coexistence of sake yeast with lactic acid bacteria may have changed its properties.
The significance of LAB as well as yeasts has been recognized in winemaking. In particular, the contribution of Ll. plantarum in malolactic fermentation is highly evaluated (Hernández et al., 2007; Mónica et al., 1999; Olsen et al., 1991). Recently, several reports have been published demonstrating that LAB in Kimoto (the same brewing method as Yamahai-shubo) and Sokujo-shubo affect sake qualities; e.g., yeasts grown in Yamahai-shubo showed more enhanced tolerance to ethanol than those in Sokujo-shubo (Iemura, Takahashi, et al., 1999; Iemura, Yamada, et al., 1999b; Taniguchi et al., 2020). LAB coexisting in Kimoto were found to gain more linoleic acid, which led to the higher enrichment of fatty acid content, including unsaturated double bonds in the phospholipid composition of yeast cell membranes, resulting in the acquisition of increased ethanol tolerance by the yeasts (Mizoguchi & Hara, 2010). Furthermore, bacteria found in arrested wine fermentations had a particularly strong prion-like element (designated [GAR+]) induction capacity (Jarosz et al., 2014), and it was presumed that something similar would be observed in sake brewing. In fact, LAB in Kimoto promoted the formation of [GAR+] in yeast cells during the brewing of sake and might be beneficial in making Yamahai-shubo sake (Watanabe et al., 2018). In addition, Takahashi et al. reported that the growth rate of Ll. sakei was influenced by the strain, pH, and temperature in kimoto preparation (Takahashi et al., 2021). It is assumed that there are various strains of Ll. sakei that grow predominantly in the Yamahai-shubo or Kimoto. These reports demonstrate that LAB in Yamahai-shubo significantly affect the properties of sake yeast as well as sake quality. The present study suggested that strain LP-2 in Yamahai-shubo has a more favorable effect on yeasts and Yamahai-shikomi sake quality with strains 61-02 and LM-1. The mechanisms are being elucidated, adding more characteristics of LAB strains. We are trying to clarify the mechanism by which the characteristics of yeast change depending on the LAB coexisting in the Yamahai-shubo so that we can improve the quality of Yamahai-shikomi sake. Then, we would like to contribute to the diversification of sake quality of Yamahai-shikomi sake by the differences in coexisting LAB (especially bacilli-LAB).
5 CONCLUSION
We found that the addition of the nitrate-reducing bacterium strain 61-02, the cocci-LAB strain LM-1, and the bacilli-LAB strain LP-2 to Yamahai-shubo produced Yamahai-shikomi sake with more favorable aromas and tastes than that of another bacilli-LAB strain, LS-4. Furthermore, it is likely that strain LP-2 in Yamahai-shubo played a significant role in improving sake quality by reducing diacetyl more than strain LS-4 in Yamahai-shikomi sake brewing.
ACKNOWLEDGMENTS
We thank the laboratory and sake brewing staff of Kizakura Co., Ltd. for sensory evaluation and preparation of raw materials.
FUNDING INFORMATION
There is no funding to report for this submission.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest associated with this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data are incorporated into the article and its online supplementary material.




