A perspective on the use of ammonia as a clean fuel: Challenges and solutions
1 INTRODUCTION
It has been crystal clear to everyone that energy is the most significant requirement for people and their existence. Energy consumption has always been ever increasing in the world due to rising energy demands. Everyday, new energy intensive technologies, vehicles, systems, and applications are entering into our daily routines, which drastically increase the fuel and power requirements for economic activities and societal developments. Such needs have impacted the energy equation with various constraints related to the environment, health, and sustainable development. It is now badly necessary to set up the energy equation without hydrocarbon fuels, and it is therefore fully recognized by many researchers, scientists, organizations, companies, etc. that it is time to move to renewables and clean fuels (particularly with hydrogen and ammonia) which have been advocated by Dincer1 for many years. In a recent perspective article,1 he has declared that COVID-19 coronavirus: closing carbon age, but opening hydrogen age, and he considered the year of 2020 a turning point. This age is even more necessary for human health and human welfare. In conjunction with this, there is a strong need to develop the technologies and economies to make a smooth transition as quickly as possible. That is why this perspective article takes ammonia into consideration for a fair evaluation and suggestions for better combustion practices.
It is a well-known fact that almost one-third of the total consumed energy in the world is used in the transportation sector where fossil fuels are primarily used to produce common transportation fuels, covering diesel, gasoline, jet fuel, etc. Their extensive use has been causing very high levels of greenhouse gases, ranging from 20% to 30% depending on the nation's development. Although there is a big attempt to transient to electrical and hybrid vehicles by manufacturer and governments, it seems that is not possible to complete this transition in a short time due to infrastructure, economical, and raw material issues. However, the current environmental indicators indicate the requirement for quick and effective actions. Moreover, the diesel- and gasoline-powered generators used in residential, commercial, utility sectors, and off-grid applications contribute to fossil-based fuel consumption and increase the CO2 emissions. The use of ammonia in combustion processes, such as internal combustion engines and gas turbines, can be a key solution in a faster transition to the hydrogen economy.
Recently, there have been numerous attempts to use ammonia in internal combustion engines and gas turbines. The California Public Utilities Commission has met with industry stakeholders on alternatives to diesel generators as part of an ongoing microgrid proceeding and is considering replacing the diesel generators with ammonia-driven ones for 2021. It is planning to change 350 MW of diesel generators used in 63 substations, to ammonia-fueled ones.2 Japan has launched a serious action plan for ammonia use to produce, especially in electricity production. It is expected that 1% of electricity consumption will be met by ammonia-driven systems in Japan.3 For this purpose, ammonia-fueled gas turbine program has been started for power generation in Japan.4 A Japanese marine company has announced starting a project on ammonia-fueled ships and fuel supply systems for it.5 Much more ammonia applications are introduced in the following sections in detail. Despite unique advantages of ammonia, there are some challenges related to its toxicity, flammability and combustion in traditional engines, turbines and power generators.
In this perspective article, clean ammonia is considered as a potential, carbon-free solution to processes and systems in various sectors and discussed from various viewpoints, such as potential sources, key methods for production, storage modes and distribution options, applications, advantages, disadvantages, combustions challenges and solutions, and future prospects. It is also evaluated as a clean fuel and proposed for combustion applications. Furthermore, some key solutions to potential challenges in the use of ammonia are discussed and addressed for practical applications.
2 FACTS ABOUT AMMONIA PRODUCTION AND UTILIZATION
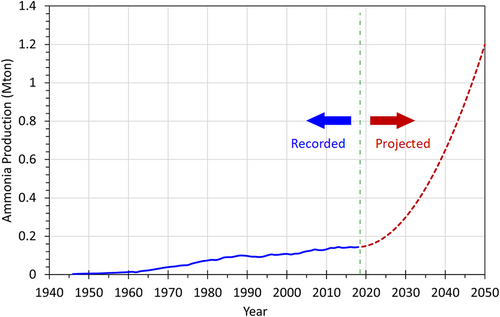
Ammonia (NH3) is known as a colorless and pungent-smelling gas at room temperature with a compound of nitrogen and hydrogen. Pure ammonia is hygroscopic and easily dissolved in water and humidity. Ammonia is, however, corrosive due to its alkaline properties. Having said that it is one of the most commonly produced industrial chemicals around the world. More than 75% of the ammonia produced is used in the agriculture sector as a fertilizer. Ammonia can also be used as a working fluid in a refrigeration cycle. The other common use of ammonia is in household cleaning solutions. Figure 1 illustrates the amount of ammonia produced around the world. The data in Figure 1 between 1945 and 2017 (given as blue line) have been acquired from Reference 6. Globally, about 146 million metric tonnes of ammonia was produced in 2019. Only a small portion of ammonia (approximately 4%) is used in direct applications; the remaining portion is used as a chemical in industrial applications or fertilizer in agriculture. It is expected to increase the ammonia consumption with the use of ammonia in the energy sector due to environmental concerns and attempts in reducing CO2 emissions. There are many attempts all around the world in order to use ammonia as a carbon-free fuel. Many countries have announced many ammonia-related projects for power generation, off-grid application, internal combustion engines, etc. Additionally, it will be expected to increase with the increasing hydrogen-powered systems since ammonia is a good hydrogen carrier. We foresee that the production and consumption of ammonia will be increasing exponentially. Our projection is illustrated in Figure 1 with a red-dotted line. In 2050, globally about 1.2 billion metric tonnes of ammonia is going to be produced and increase exponentially. It is nearly 8.2 times more than the amount of ammonia produced in 2019.
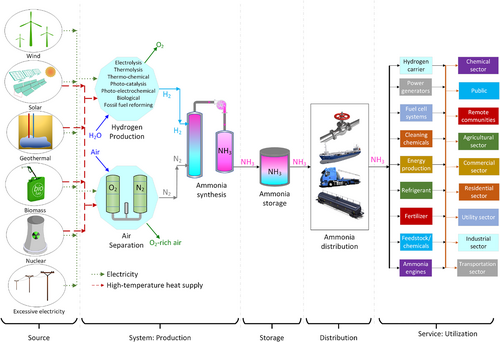
Here, we introduce the economic cycle of ammonia ranging from its production by using clean sources to final useful commodities for economic activities under services and is shown in Figure 2. Ammonia could be the key to finalize the increasing search for an alternative fuel as it can be produced with renewables. Ammonia is a chemical that can be used in many sectors and for many different purposes. It can serve as a fuel in energy production, a key chemical in feedstock and chemicals production, the main ingredient for cleaning materials, a fuel for engines, and a refrigerant for cooling systems. Its versatile and wide range of application potential makes ammonia an important, carbon-free alternative. Some key advantages of utilizing ammonia as a clean alternative are listed as follows:
- Ammonia has three hydrogen atoms and one nitrogen atom and can flexibly be produced with conventional or renewable resources.
- It is a potential hydrogen storage and carrier.
- Transportation of ammonia is much safer compared to hydrogen.
- When liquefied, it contains approximately 48% more hydrogen by volume than hydrogen.
- No carbon dioxide emissions are emitted during its use since it is carbon free.
- It can be utilized for a wide range of applications as a fuel, working fluid, refrigerant, hydrogen carrier, fertilizer, feedstock, chemical, cleaning agent, and many more.
- It can be easily detectable when any leakage occurs because of its distinctive smell.
- A strong fuel candidate for engines, gas turbines, power generators, and burners. The modifications needed for such engines are relatively small.
3 AMMONIA AS A CLEAN FUEL FOR COMBUSTION
Although ammonia has widely been used for many years in the refrigeration systems as a refrigerant and the production of fertilizers, household cleaning chemicals, and disinfectants, it has started regaining attention from researchers, scientists, engineers, and technologists due to its carbon-free nature which can be used as a potential fuel to reduce the CO2 emissions. It can play a critical role in solving various challenges related to hydrogen energy options and hydrogen economy as a unique hydrogen storage medium (with three atoms of hydrogen) and transportation and distribution, without changing the infrastructure, with the existing transportation and distribution options where industries currently deploy. In the last decade, the attempts have considerably increased to use ammonia in internal combustion engines and gas turbines. In Table 1, the common fuels used in internal combustion engines are given with their combustion properties. Numerous important advantages of ammonia as a potential fuel can be listed as follows:
- It is carbon free and environmentally benign.
- It has three atoms of hydrogen and may potentially be used as hydrogen carrier.
- Its production, storage, transportation, and distribution are much easier and less complicated than many other fuels.
- It is cost-effective and economically feasible for applications.
- It can be considered a potential replacement for gasoline, diesel, and kerosene.
- It can be considered for all combustion systems, ranging from engines to gas turbines.
- It can be a potential fuel solution for clean power generation in remote areas.
| Properties | Units | Gasoline | Diesel | LPG | CNG | Gaseous hydrogen | Liquid hydrogen | Ammonia |
|---|---|---|---|---|---|---|---|---|
| Formula | C8H18 | C12H23 | C3H8 | CH4 | H2 | H2 | NH3 | |
| Lower heating value | MJ/kg | 44.5 | 43.5 | 45.7 | 38.1 | 120.1 | 120.1 | 18.8 |
| Flammability limits, gas in air | Vol. % | 1.4-7.6 | 0.6-5.5 | 1.81-8.86 | 5.0-15.0 | 4-75 | 4-75 | 16.25 |
| Flame speed | m/s | 0.58 | 0.87 | 0.83 | 8.45 | 3.51 | 3.51 | 0.15 |
| Autoignition temperature | °C | 300 | 230 | 470 | 450 | 571 | 571 | 651 |
| Minimum ignition energy | MJ | 0.14 | N/A | N/A | N/A | 0.018 | N/A | 8 |
| Flash point | °C | −42.7 | 73.8 | −87.7 | −184.4 | N/A | N/A | −33.4 |
| Octane | 90-98 | N/A | 112 | 107 | >130 | >130 | 110 | |
| Fuel density | kg/m3 | 698.3 | 838.8 | 1898 | 187.2 | 17.5 | 71.1 | 602.8 |
| Energy density | MJ/m3 | 31 074 | 36 403 | 86 487 | 7132 | 2101 | 8539 | 11 333 |
| Latent heat of vaporization | kJ/kg | 71.78 | 47.86 | 44.4 | 104.8 | 0 | N/A | 1369 |
| Storage method | Liquid | Liquid | Comp. liquid | Comp. gas | Comp. gas | Comp. liquid | Comp. liquid | |
| Storage temperature | °C | 25 | 25 | 25 | 25 | 25 | −253 | 25 |
| Storage pressure | kPa | 101.3 | 101.3 | 850 | 24 821 | 24 821 | 102 | 1030 |
| Costa | $/liter | 0.58 | 0.65 | 0.72 | 0.57 | 0.14 | 0.18 | 0.24 |
- a Cost data for April 2020.
A comparison of various types of fuels with ammonia is given in Table 1. Although energy density of ammonia in pressurized tank is 2.5 times lower than gasoline, it has a big advantage according to traditional fuels in terms of the specific energetic cost. Also, when ammonia is produced with renewables, the life cycle costs of ammonia-fuel blend–driven systems can be reduced substantially.
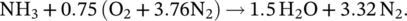 (1)
(1)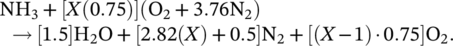 (2)
(2)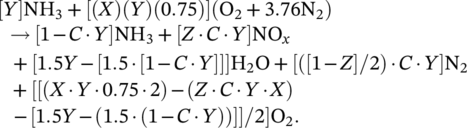 (3)
(3)The partial oxidation of ammonia reduces the energy output of the reaction by about 30% according to the ideal stoichiometric combustion reaction. Also, as the ignition temperature of ammonia is high, a portion of ammonia does not combust, and it exhausts in the gas form. Therefore, it is critical to provide complete combustion for ammonia. As given in Table 1, the autoignition temperature of ammonia is approximately 200°C higher than gasoline and diesel. Therefore, ignition difficulty is seen in both spark ignition and compression engines. High ignition temperature causes low combustion temperature and power reduction in the engine.
4 CHALLENGES WITH AMMONIA COMBUSTION
It is important to point out that ammonia, when used for combustion, has some side effects, just like the way all drugs have some side effects. Such side effects (so-called: challenges) may be listed as follows:
- High ignition temperature
- Low flame velocity
- Slow chemical kinetics
Since there are increasing research efforts to minimize the impacts and improve the combustion performance, it is necessary to provide some key remedies to overcome the issues. These will be discussed in the next subsections.
4.1 Solutions to the challenges
Three above-listed side effects of ammonia combustion are discussed and possible technical solutions are provided as follows.
4.1.1 High ignition temperature
In order to solve the difficult ignition problem of ammonia, it is a common way to mix ammonia with traditional fuels using in internal combustion engines such as gasoline, diesel, LPG, CNG, ethanol, methanol, hydrogen, etc. Figure 3 illustrates the use of ammonia-fuel blends in internal combustion engines. Ammonia can be taken into the engine either with air in its gas form from the air intake manifold or injecting to the cylinder in its liquid form separately from accompanying fuel. Since the traditional fuels will ignite at a lower temperature, it will increase the temperature of the cylinder and it helps to ignite ammonia.
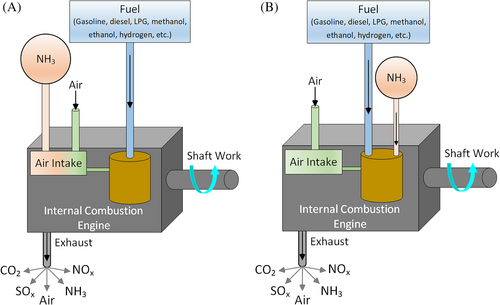
Ammonia-fuel blends can reduce the need for additional devices or modifications in engines. Thus, the transition to the hydrogen economy can be achieved cost-effectively in internal combustion engines. The common way to send the ammonia into the engine is to send with air in gas form from the air intake manifold (Figure 3A). In order to achieve as possible as maximum power and as possible as the lowest emissions, the ammonia, fuel, and air mixture should adjust carefully. This requires a comprehensive blender design and automatic control system. Therefore, an optimum blender should be designed for each conventional fuel to be mixed with ammonia to adjust the optimum mixing ratio for ammonia, fuel, and air. Ammonia can also be taken in by injecting ammonia and fuel separately into the intake manifold in the liquid phase, as seen in Figure 3B. In this method, the flow rates of ammonia and fuel should be well adjusted. Also, effective ammonia injectors should be developed to prevent ammonia slip.
On the other hand, ammonia-fuel blends cause power reduction due to ammonia combustion. Yapicioglu and Dincer13 have performed a comprehensive study to investigate the effective ammonia-fuel blends in a power generator engine. Figure 4 demonstrates the effect of changing the ratio of ammonia fuel blends on power output. As expected, the power output from the generator has reduced with increasing the ratio of ammonia in the fuel mixture. Also, the highest power output has been obtained by ammonia-hydrogen blend. Additionally, the exhaust temperature tends to decrease with increasing the ratio of ammonia in the fuel mixture. While the ratio of ammonia in fuel blend is changing from 0.20 to 0.80, the exhaust temperature has reduced about 60°C in diesel, 150°C in hydrogen, and 100°C in propane and natural gas blends.13
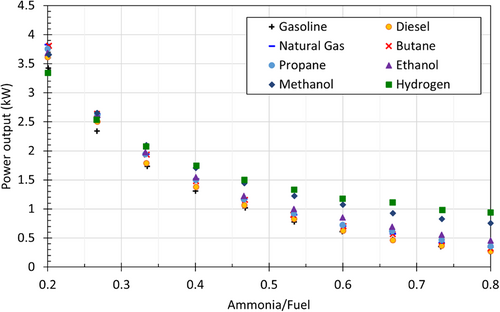
In order to enhance the combustion processes and power output, a supercharger can be integrated into the engine. The engine can be more excessively supercharged than traditional engines due to high octane rating of ammonia. Higher compression ratios can also help to solve difficult ignition problem. Increasing pressure will increase the temperature of the fuel mixture in the cylinder. Thus, the combustion can occur more easily. Preheating for ammonia can also help the autoignition. Higher temperature ammonia intake can help enhance the initiation and progression of combustion.
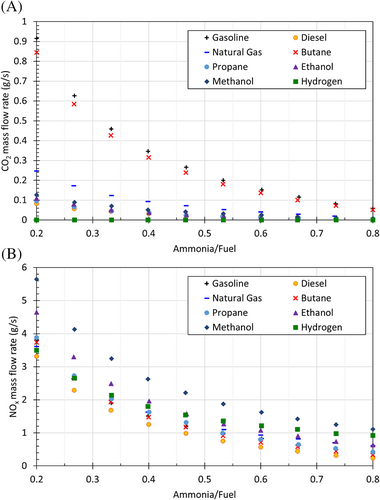
On the other hand, the accompanying fuel causes CO2 and NOx emissions. When emissions for the engines fueled by traditional fuels and ammonia-fuel blends are compared, ammonia provides a significant advance to reduce emissions. Here, the use of hydrogen in the ammonia-fuel blend suppresses the carbon emissions since the fuel mixture does not contain carbon compounds. Ammonia-hydrogen blend is also significant for reducing carbon emissions as there is no carbon in the mixture. The effect of ammonia-fuel blends on CO2 and NOx emissions is shown in Figure 5, as expressed in the next subsection.
4.1.2 Low flame velocity
The second challenge for the use of ammonia in an internal combustion engine is the lower flame speed of ammonia according to other traditional fuels. The lower flame speed restrains temperature diffusion in the cylinder during the combustion stroke and causes the power reduction. This problem is seen in both spark ignition and compression engines. Consequently, the use of ammonia in a conventional engine is not possible to use without power losses. However, when it is taken into consideration that being carbon-free fuel and its potential to reduce carbon emissions, ammonia is still a worthwhile and significant alternative fuel for internal combustion engines. All the solutions described above can also help in resolving low flame velocity. Namely, ammonia-fuel blend, preheated ammonia, and higher compression ratios can improve flame velocity.
Ammonia can be used in spark ignition engines by injecting ammonia and gasoline separately into the intake manifold in liquid phase. As ammonia combusts one-fifth time slower than gasoline, the spark timing requires special arrangement of the crank angle/piston position. When 70% of gasoline is substituted into ammonia, same amount of carbon dioxide is reduced. Ammonia should not be injected into the cylinder unless pressure is higher that cylinder compression pressure. In the compression ignition engines, ammonia is premixed with the air and introduced through the intake manifold and small quantity of diesel fuel or another promoter is injected inside the cylinder to have the ammonia-air mixture ignited. As mention before, here, it is important to adjust and autocontrol mixture ratios of ammonia, fuel, and air is a significant issue.
4.1.3 Slow chemical kinetics
The chemical reaction rate is the speed at which a chemical reaction occurs. It is also defined as the speed at which the reactant converted into the products. When ammonia is used in internal combustion engines as a fuel, the chemical reaction rate is slow than traditional fuels due to its high ignition temperature and low flame velocity. This slow chemical reaction rate causes ammonia to be discharged from the exhaust without burning. The common way to enhance the chemical reaction rate of ammonia combustion is to use the promoter in ammonia-air mixture. Traditional fuels and hydrogen are commonly used as a promoter in ammonia-fueled engines, as expressed in the previous section. Ammonia-fuel blends can increase the chemical reaction rate of ammonia combustion. Also, NaCl, BaCL2, and NaF are used for a catalyst to enhance chemical kinetics of ammonia combustion.
5 CO2 AND NOx EMISSIONS
Figure 5 shows the CO2 and NOx emissions for different ammonia-fuel mixtures with respect to the mixture ratio. It is clear that increasing the amount of ammonia in the mixture substantially reduces CO2 and NOx emissions. In pure ammonia and ammonia-fuel blends, especially ammonia-hydrogen mixture–driven combustion engines, NOx emissions are a critical problem. In order to prevent this problem, ammonia oxidation catalyst and catalytic reduction can be used. Thus, both NOx and NH3 can be reduced. The use of ammonia-hydrogen blend can eliminate carbon emissions. Also, enhancement of ammonia combustion can reduce NOx emissions.
6 CLOSING REMARKS
Hydrogen-fueled internal combustion engines, using ammonia as the carrier, are an immediate, viable way to convert to a carbon emission free. Ammonia can provide a cost- and environmentally effective transient to hydrogen economy. Many attempts have been conducted to use ammonia as a fuel in combustion engines since it does not contain carbon with the environmental concerns. Beside its advantages provided environmentally, there are a few significant problems in use of it in combustion engines such as difficult ignition, low flame speed, required higher compression, etc. Following key directions can help to solve these problems seen in ammonia use and spread its use:
- Mixing ammonia with traditional fuels can help to solve the difficult ignition problem.
- Ammonia-fuel blends also increase the performance of the engine than the engines fueled by pure ammonia.
- An effective and autocontrolled ammonia-fuel blender should be developed.
- Preheating for ammonia can be performed by easy ignition and to reach high combustion temperature.
- Higher compression ration in the cylinder can help in autoignition.
- Ammonia oxidation catalyst and catalytic reduction can be applied in order to reduce NOx emissions.




