Frequency-specific network changes in mesial temporal lobe epilepsy: Analysis of chronic and transient dysfunctions in the temporo-amygdala-orbitofrontal network using magnetoencephalography
Abstract
Objective
Mesial temporal lobe epilepsy (MTLE) is associated with disruptions in the temporo-amygdala-orbitofrontal (TAO) network, a key component of the limbic system. We aimed to investigate TAO network alterations in patients with MTLE using magnetoencephalography (MEG), which overcomes susceptibility artifacts that limit functional MRI analysis of the orbitofrontal cortex.
Methods
Nine seizure-free patients with MTLE post-temporal lobectomy and nine age- and sex-matched healthy controls were recruited. Preoperative MEG data were collected and segmented into frequency bands ranging from delta to ripple to assess functional connectivity (FC) between the bilateral hippocampi and TAO network.
Results
Patients with MTLE exhibited increased FC between the affected hippocampus and amygdala across all frequency bands. Additionally, FC between the affected hippocampus and the medial prefrontal cortex (mPFC), orbitofrontal gyrus (OFG), and amygdala was elevated in the gamma and ripple bands compared with healthy controls. Conversely, FC between the healthy hippocampus and mPFC decreased in the alpha and beta bands. Furthermore, FC within the TAO network fluctuated before and after epileptic spikes; there was a decrease in the delta band between the bilateral hippocampi and the amygdala, OFG, and thalamus, whereas FC between the hippocampus and mPFC increased in the alpha, beta, and ripple bands.
Significance
These findings suggest the formation of an abnormal network involving the affected hippocampus and the TAO network, particularly in the gamma–ripple bands, indicating epilepsy-induced network disruptions. Reduced FC in the healthy hippocampus and the TAO network may reflect frontal lobe dysfunction related to emotion and cognition. Additionally, both chronic and transient FC changes observed via MEG may contribute to the cognitive and psychiatric impairments experienced by patients with MTLE. This study highlights the significance of frequency-specific network alterations in understanding MTLE's pathophysiology and its impact on limbic system functions.
Plain language summary
In mesial temporal lobe epilepsy, there may be abnormal connectivity between the hippocampus and the limbic system, which is involved in memory, cognition, and emotion. The changes in connectivity observed using magnetoencephalography may be implicated in cognitive and psychiatric problems experienced by patients with mesial temporal lobe epilepsy. Examining disruptions in the connectivity across brain regions in relation to epileptic activity could further the understanding of the pathophysiology of this debilitating condition and its impact on behavioral and emotional functions, among others.
Key points
- Increased FC in the TAO network is linked to bilateral frontal lobe dysfunction in MTLE.
- Abnormal FC between the hippocampus and amygdala is found across all frequency bands.
- Reduced FC in the healthy hippocampus correlates with impaired cognitive function.
- Ripple band FC and cognitive scores in MTLE are negatively correlated.
- IEDs induce transient network changes, affecting hippocampal and mPFC FC.
1 INTRODUCTION
Mesial temporal lobe epilepsy (MTLE), the most common form of focal epilepsy, affects the limbic system. The limbic system forms an extensive network in the brain that comprises three major parts: the hippocampal-diencephalic and parahippocampal-retrosplenial network (Papez circuit), which is involved in memory and spatial orientation; the temporo-amygdala-orbitofrontal (TAO) network (Yakovlev circuit), which integrates visceral sensation and emotion with semantic memory and behavior; and the dorsomedial default mode network (DMN), which is involved in autobiographical memory and introspective self-directed thinking (Figure 1).1 When classifying neurological diseases based on functional neuroanatomy, temporal lobe epilepsy (TLE) is primarily attributed to disruptions in the TAO network.1
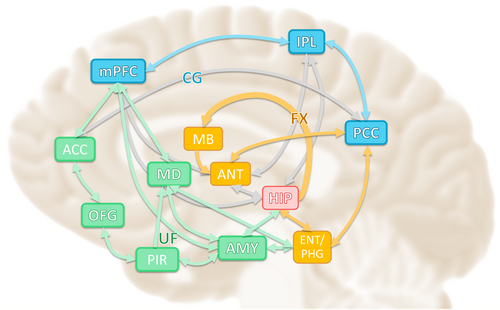
The hippocampus plays a crucial role in the abnormal epilepsy network in MTLE. Previous studies have reported changes in the hippocampal network and DMN in TLE2; however, the number of functional magnetic resonance imaging (fMRI)-based studies on the TAO network is limited because the blood oxygen level-dependent signal used in fMRI is adversely affected by magnetic susceptibility artifacts in the orbitofrontal cortex. These artifacts, resulting from inhomogeneities in the magnetic field between brain tissue and air-containing regions of the skull, complicate network analysis and limit its accuracy.3
The TAO network integrates visceral and emotional states into cognition and behavior4; therefore, chronic impairment of this network has been linked to mood disorders, psychosis, dementia, and TLE.1 In contrast, interictal epileptic discharges (IEDs) can induce transient cognitive dysfunction.5 Electroencephalography (EEG)-fMRI studies have shown that IEDs in patients with MTLE reduce functional connectivity (FC) in various parts of the DMN and alter neural activity before and after IEDs.6 These findings suggest that patients with TLE experience abnormal network changes both chronically and transiently, resulting in IEDs that cause various cognitive and mental impairments.
Neural activity in specific networks, such as cognitive function and mental activity, occurs in specific frequency bands, which also applies to abnormal network formation during epilepsy. For example, in a rat model of kainic acid-induced epilepsy, abnormal ripple-band neural activity was observed in the prefrontal cortex (PFC), thalamus, contralateral hippocampus, and other remote areas, suggesting that epileptogenesis in this model resulted from the development of a large abnormal network, particularly linked to ripple-band neural activity.7
Therefore, brain networks in patients with epilepsy undergo chronic (at rest) and transient (during IEDs) changes in specific frequency bands, which may be reflected as changes in FC. Thus, analyzing FC changes in each frequency band between the hippocampal and TAO networks of patients with MTLE could reveal network-specific changes.
Previous studies have shown that in the early stages of epilepsy, FC increases on the affected side due to the formation of abnormal networks.8, 9 Gradually, as functional impairment progresses, FC on both sides decreases,10, 11 whereas compensatory changes increase FC on the healthy side.2, 12 We hypothesized that similar FC changes would also occur within the hippocampus –TAO network, which connects the temporal and frontal lobes.
In this study, we aimed to elucidate the network changes between the hippocampus and the TAO network in MTLE during the interictal period (resting state) and between before and after IEDs using FC analysis with magnetoencephalography (MEG). MEG is less affected by anatomical brain structures, such as the orbitofrontal cortex, and facilitates the evaluation of neural activity across various frequency bands; therefore, it is an appropriate method for this study.
2 METHODS
2.1 Participants
Between October 2014 and December 2019, a retrospective evaluation was conducted on 12 consecutive patients aged 18 years or older who underwent preoperative MEG and lesionectomy at our institution. The surgical procedures included standard temporal lobectomy and selective amygdalohippocampectomy. Patients were admitted for a 1-week hospital stay, during which they completed assessments using the Wechsler Memory Scale-Revised (WMS-R)13 and the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III).14 Preoperative evaluations of patients diagnosed with MTLE included psychiatric assessments, and postoperative monitoring for psychiatric disorders was performed for up to 1 year.
Among the 12 patients evaluated, three were excluded from the study due to unsatisfactory seizure outcomes within 2 years after surgery, classified as Engel class II, III, or IV.15 The final analysis included nine patients with Engel class I outcomes, who were free of disabling seizures, as well as nine healthy, age- and sex-matched controls. The control group was recruited from the Nagoya University Healthy Cohort.16 Cognitive impairments, including dementia (cutoff: 88/89) and mild cognitive impairment (cutoff: 82/83), were screened out using Addenbrooke's Cognitive Examination.17
This research received approval from the ethics committee of the Nagoya University Graduate School of Medicine (No. 1005-2). Informed consent was obtained in writing from all participants and their families. All study procedures complied with applicable guidelines and regulations. The findings were reported following the STROBE guidelines.
2.2 MEG
Interictal magnetic signals during sleep stages one and two were captured using a whole-head MEG system equipped with 160 axial gradiometers (PQ1160C, Ricoh Corp.). No pharmacological agents were used to induce sleep. Sleep staging was determined using simultaneously recorded EEG data, which were obtained at eight scalp locations (F3, F4, C3, C4, T3, T4, P3, and P4) in accordance with the international 10–20 system. The magnetic data were processed with an initial bandpass filter of 1–2000 Hz and digitized at a sampling rate of 5000 Hz. EEG recordings were conducted using a 1–100 Hz bandpass filter and the same sampling rate, with simultaneous electrocardiography. Participant head information was co-registered using standard anatomical reference points, head position indicator coils, and 3D digitization of the scalp surface. Each participant completed five recording sessions, each lasting 4 min. Real-time monitoring of MEG and EEG signals was conducted throughout the recording process. For individuals with MTLE, the recording duration was extended to 20 min if more than 10 IEDs were identified, as this was sufficient for subsequent analyses. If fewer than 10 IEDs were detected, the recordings were extended to 28 min (seven sessions) to ensure an adequate number of IEDs were captured.
2.3 MRI data preprocessing and head model construction
Sagittal T1-weighted MRI images were acquired using a 3T MRI scanner (Siemens; 3 Tesla system), with a slice thickness of 1.0 mm, an echo time of 2.5 ms, and a repetition time of 2500 ms, consisting of 192 slices. Brain structure extraction from these MRI images was performed using BrainSuite, an open-source software (Signal and Image Processing Institute, Department of Electrical Engineering Systems, University of Southern California).18 The individual MRI datasets were aligned and normalized to the Montreal Neurological Institute (MNI) space for further analysis.
The processed data were utilized to create a computational head model using Brainstorm, another open-source software from the same institute.19 For all participants, we adopted the ICBM152 standard template20 as the source space and employed overlapping spheres with the volume head model,21, 22 for forward modeling. The volume head model, commonly used to estimate three orthogonal dipoles at each point in the brain, was employed in this study to investigate subcortical function.23-25 Current sources were placed on a 5 mm isotropic grid within the brain parenchyma, resulting in ~13,478 grid locations for analysis.
2.4 MEG data preprocessing
The Brainstorm software facilitated the preprocessing of the MEG data recorded during sleep stages one and two. For patients diagnosed with MTLE, three epilepsy specialists (TI, SM, and YH) visually inspected the IEDs detected in the MEG recordings. The onset and offset times of the IEDs were marked within the MEG sensor space. Spikes deemed reproducible were selected for analysis based on unanimous agreement among the specialists. Conversely, spike-like events that appeared only once and were determined to be non-epileptic by consensus were excluded from further analysis. MEG data were separated into six frequency bands using a bandpass filter: delta (2–4 Hz), theta (4–8 Hz), alpha (8–14 Hz), beta (14–30 Hz), gamma (30–80 Hz), and ripple (80–250 Hz). Bandpass filtering was performed using Finite Impulse Response filters with a linear phase design. The filters were implemented with a Kaiser window, a stopband attenuation of 60 dB, and a passband ripple limited to 0.1%. The filter order ranged 18 128–36 254 depending on the frequency band, with narrower transition bands for lower frequencies. Filtering was performed symmetrically (zero-phase) to avoid phase distortion, using a sampling frequency of 5000 Hz. Standardized low-resolution brain electromagnetic tomography26 was employed as the inverse model, configured with an unconstrained dipole orientation, diagonal noise covariance regularization, and a regularization parameter of three for the signal-to-noise ratio. The output mode was set exclusively to the inverse kernel.
2.5 FC and seed regions
The MNI coordinates for the seed regions, encompassing the bilateral hippocampus and elements of the TAO network (amygdala, orbitofrontal gyrus [OFG], medial PFC [mPFC], and dorsomedial nucleus of the thalamus), were identified based on stereotactic atlases27, 28 and previously established methodologies.29, 30
Table 1 provides the specific MNI coordinates for these seed regions. For this analysis, the affected and healthy sides were analyzed separately. In cases where the affected side was on the right in patients with MTLE, as well as in their matched healthy controls, the results were standardized and displayed as if the affected side were on the left. This adjustment was made to ensure consistency in presenting the findings. Since we hypothesized that FC between the hippocampus and TAO network components might vary across frequency bands, we assessed FC using magnitude-squared coherence. This approach evaluates signal covariance in the frequency domain rather than depending solely on amplitude, as in conventional correlation analysis. Seed region configurations and FC computations were conducted using Brainstorm software.
| Regions | MNI coordinates (x, y, z, mm) | |||
|---|---|---|---|---|
| Hippocampus | Ipsi | −29 | −19 | −15 |
| Contra | 28 | −22 | −14 | |
| Amygdala | Ipsi | −23 | −4 | −20 |
| Contra | 22 | −4 | −20 | |
| Orbitofrontal gyrus | −1 | 15 | −22 | |
| Medial prefrontal cortex | −1 | 54 | 27 | |
| Mediodorsal nucleus of thalamus | Ipsi | −4 | −13 | 9 |
| Contra | 4 | −13 | 9 | |
- Note: The affected side is shown in uniformity with the ipsilateral side, and the healthy side as the contralateral side.
- Abbreviations: Contra, contralateral (right, healthy side); Ipsi, ipsilateral (left, affected side); MNI, Montreal Neurological Institute.
2.6 Interictal FC of patients with MTLE compared with that of healthy controls
MEG recordings were used to extract 3-second interictal periods during sleep stages one and two, excluding intervals from 3 seconds before and 6 seconds after an IED in patients with MTLE. This procedure was uniformly applied to recordings from both MTLE patients and healthy controls, with a total of 50 time points analyzed. FC between the hippocampus on the affected side and TAO network components was evaluated across six frequency bands: delta, theta, alpha, beta, gamma, and ripple. Average FC values for each individual, both patients and controls, were computed using Brainstorm software.
Two-sample t-tests were performed to identify differences in FC between patients with MTLE and healthy controls, followed by multiple comparisons using Tukey's test to detect significant changes in FC. All statistical analyses were performed using IBM SPSS Statistics (IBM Corp.), with the significance threshold set at p < 0.05.
2.7 Correlations between interictal FC and WMS-R, WAIS-III subscores
Spearman's correlation was used to assess the relationships between interictal FC in patients with MTLE and their subscores on the WMS-R and WAIS-III.31 The strength of the correlation coefficients was categorized as follows: |r| = 1.0–0.7 (strong), |r| = 0.7–0.4 (moderate), |r| = 0.4–0.2 (weak), and |r| < 0.2 (none).32 Statistical analyses were performed using IBM SPSS Statistics (IBM Corp.), with the significance threshold set at p < 0.05.
2.8 IED-related FC changes in patients with MTLE
Four distinct 3-second periods were defined relative to the onset of the IED spike (0 s): rest (−6 to −3 s), pre-spike (−3 to 0 s), spike (0–3 s), and post-spike (3–6 s). These segments were extracted from MEG recordings of patients with MTLE, ensuring no preceding spikes or noise were present in the 12 s prior. A total of 53 IEDs, with an average of 6.6 IEDs per patient, were analyzed across nine patients with MTLE. FC between the affected hippocampus and the TAO network components was assessed within six frequency bands and averaged for each patient using the Brainstorm software.
FC during the pre-spike, spike, and post-spike periods was compared against the resting period. Significant changes in FC were identified using paired t-tests and further examined with multiple comparisons using Tukey's test. Statistical analyses were conducted using IBM SPSS Statistics (IBM Corp.), with a significance level set at p < 0.05.
3 RESULTS
3.1 Clinical profiles
A total of nine individuals were included in the analysis; their clinical characteristics are detailed in Table 2, and the WMS-R and WAIS-III scores are presented in Table 3. All patients underwent postoperative follow-ups for >4 years (mean, 79 months; range, 50–111 months) and achieved a favorable surgical outcome classified as Engel class I. The left hemisphere was dominant in all MTLE cases, with the epileptogenic zone identified on the left side in six cases. None of the patients with MTLE developed depression or other psychiatric disorders before or after the surgery. The WMS-R subscores were generally lower for verbal memory in patients with MTLE; five patients had verbal memory scores below 80, and four had scores below 90. However, the visual memory, attention, and concentration subscores remained higher than the verbal memory scores in all patients with MTLE.
| Case # | Sex/age (years) | Epilepsy type | Seizure type and semiology | Dominant side | Surgery | Resection area | Pathology | Engel class | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/27 | L-MTLE | Olfactory aura, R-hand dystonic posture, LOC | L | L-SAH | L-Hip, Amy, PHG, Un | HS | Ia | 133 |
| 2 | M/62 | L-MTLE | R-arm tonic seizure, LOC | L | L-ATL | L-Hip, Amy, PHG, FuG, Un, TP, aSTG, aMTG, aITG | HS | Ia | 121 |
| 3 | M/44 | L-MTLE | Oral and hand automatism, head and trunk version, LOC, FBTCS | L | L-SAH | L-Hip, Amy, PHG, Un | HS | Ic | 123 |
| 4 | M/40 | R-MTLE | L-facial spasm, head and trunk version, LOC, FBTCS | L | R-ATL | R-Hip, Amy, PHG, FuG, Un, TP, aSTG, aMTG, aITG | Gliosis | Ic | 119 |
| 5 | F/44 | R-MTLE | LOC, tonic posture | L | R-SAH | R-Hip, Amy, PHG, Un | FCD type IIb | Ia | 92 |
| 6 | M/30 | L-MTLE | LOC, FBTCS | L | L-SAH | L-Hip, Amy, PHG, Un | FCD type IIb | Ia | 91 |
| 7 | M/19 | L-MTLE | Oral and hand automatism, LOC | L | L-ATL | L-Hip, Amy, PHG, FuG, Un, TP, aSTG, aMTG, aITG | HS | Ia | 89 |
| 8 | F/19 | L-MTLE | Hand automatism, LOC | L | L-SAH | L-Hip, Amy, PHG, Un | FCD type Iib | Ia | 72 |
| 9 | M/56 | R-MTLE | LOC, FBTCS | L | R-ATL | R-Hip, Amy, PHG, FuG, Un, TP, aSTG, aMTG, aITG | HS | Ia | 72 |
- Abbreviations: a-, anterior; Amy, amygdala; ATL, anterior temporal lobectomy; F, female; FBTCS, focal to bilateral tonic–clonic seizure; FCD, focal cortical dysplasia; FuG, fusiform gyrus; Hip, hippocampus; HS, hippocampal sclerosis; ITG, inferior temporal gyrus; LOC, loss of consciousness; M, male; MTG, middle temporal gyrus; MTLE, mesial temporal lobe epilepsy; PHG, parahippocampal gyrus; SAH, selective amygdalohippocampectomy; STG, superior temporal gyrus; TP, temporal pole; Un, uncus.
| Case # | WMS-R | Verbal memory | Visual memory | General memory | Attention/ concentration | Delayed recall | WAIS-III | Verbal IQ | Performance IQ | Full-scale IQ | Verbal comprehension | Perceptual organization | Working memory | Processing speed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | 99 | 85 | 89 | 85 | 82 | 101 | 89 | 88 | 99 | 81 | 100 | ||
| 2 | 84 | 99 | 87 | 106 | 64 | 97 | 94 | 95 | 93 | 95 | 105 | 97 | ||
| 3 | 75 | 106 | 83 | 110 | 81 | 73 | 94 | 81 | 76 | 101 | 85 | 75 | ||
| 4 | 58 | 92 | 63 | 78 | 74 | 54 | 59 | 52 | 54 | 63 | 50 | 60 | ||
| 5 | 87 | 108 | 92 | 92 | 91 | 92 | 90 | 90 | 100 | 99 | 90 | 70 | ||
| 6 | 76 | 111 | 98 | 124 | 84 | 84 | 83 | 82 | 66 | 95 | 119 | 94 | ||
| 7 | 50 | 91 | 50 | 90 | 50 | 77 | 65 | 69 | 88 | 70 | 88 | 63 | ||
| 8 | 65 | 109 | 72 | 118 | 73 | 80 | 90 | 83 | 78 | 91 | 103 | 110 | ||
| 9 | 85 | 95 | 86 | 90 | 86 | 83 | 74 | 76 | 92 | 89 | 81 | 54 |
- Abbreviations: IQ, intelligence quotient; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition; WMS-R, Wechsler Memory Scale-Revised.
3.2 Interictal FC of patients with MTLE compared with that of healthy controls
Figure 2 indicates significant increases (red lines) and decreases (blue lines) in FC. Table S1 lists interictal FC changes in patients with MTLE compared with those in healthy controls, showing significant differences.
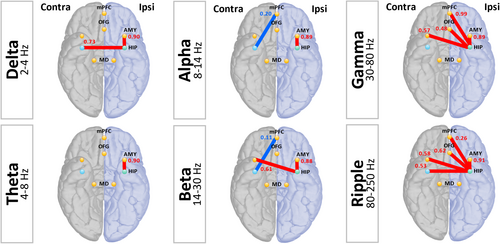
In patients with MTLE, FC in the affected (ipsilateral) hippocampus and amygdala increased across all bands (delta: t = 7.318, df = 16, p < 0.001; theta: t = 7.799, df = 16, p < 0.001; alpha: t = 7.561, df = 16, p < 0.001; beta: t = 9.314, df = 16, p < 0.001; gamma: t = 8.725, df = 16, p < 0.001; ripple: t = 14.489, df = 16, p < 0.001).
In the gamma and ripple bands, FC between the affected hippocampus and mPFC (gamma: t = 2.234, df = 16, p = 0.04; ripple: t = 2.611, df = 16, p = 0.02), OFG (gamma: t = 2.341, df = 16, p = 0.03; ripple: t = 5.799, df = 16, p < 0.001), and bilateral amygdala (ipsilateral amygdala: as previously mentioned; contralateral amygdala: gamma: t = 3.525, df = 16, p = 0.003; ripple: t = 4.157, df = 16, p = 0.001) increased in patients with MTLE.
FC between the healthy (contralateral) hippocampus and mPFC decreased in patients with MTLE in the alpha and beta bands (alpha: t = −3.238, df = 16, p = 0.005; beta: t = −3.133, df = 16, p = 0.006).
3.3 Correlations between interictal FC and WMS-R, WAIS-III subscores
Correlation coefficients between FC and the WMS-R and WAIS-III subscores for the affected (ipsilateral) hippocampus (Figure 3A), healthy (contralateral) hippocampus (Figure 3B), and the TAO network were color-coded.
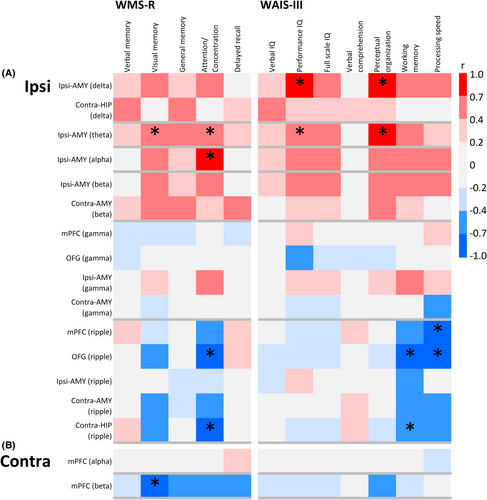
FC between the affected hippocampus and TAO network, which increased relative to that in healthy controls, was negatively correlated in the ripple band with attention and concentration subscores of the WMS-R and with working memory and processing speed subscores of the WAIS-III (Figure 3).
The FC between the healthy hippocampus and the TAO network, which was decreased relative to that in healthy controls, tended to correlate negatively in the beta band with the visual memory subscore of the WMS-R (Figure 3).
3.4 IED-related FC changes in patients with MTLE
Figure 4 shows significant increases (red lines) and decreases (blue lines) in FC. Table S2 lists interictal epileptic discharge-related FC changes with significant differences.
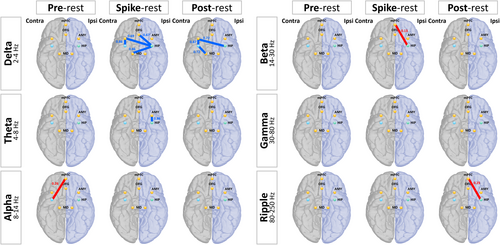
The FC between the hippocampus and TAO network varied across different frequency bands before and after the spike. In the delta band, FC decreased between the bilateral hippocampus and amygdala (spike: ipsilateral hippocampus–contra amygdala: t = −3.138, df = 16, p = 0.01; contra hippocampus–contra amygdala: t = −2.503, df = 16, p = 0.04; post-spike: ipsilateral hippocampus–contralateral amygdala: t = −2.687, df = 16, p = 0.03; contralateral hippocampus–contralateral amygdala: t = −2.654, df = 16, p = 0.03), OFG (spike: ipsilateral hippocampus–OFG: t = −2.509, df = 16, p = 0.04), and healthy thalamus (spike: ipsilateral hippocampus-thalamus: t = −3.091, df = 16, p = 0.02; post-spike: contralateral hippocampus-thalamus: t = −2.336, df = 16, p = 0.048) in the spike and post-spike time windows.
Conversely, FC between the hippocampus and mPFC increased before and after the spike in the alpha, beta, and ripple bands (pre-spike: alpha: t = 2.395, df = 16, p = 0.04; spike: beta: t = 3.03, df = 16, p = 0.02; post-spike: ripple: t = 2.453, df = 16, p = 0.04).
4 DISCUSSION
4.1 Interictal FC of patients with MTLE compared with that of healthy controls
Here, we investigated whether network changes resulting from the long-term adverse effects of chronic TLE on brain networks occur between different brain lobes connected by the TAO network, such as the hippocampus in the temporal lobe and the frontal lobes, and whether these effects vary across frequency bands.
FC between the affected hippocampus and amygdala was elevated across all frequency bands. According to our hypothesis, the findings indicate the formation of an epilepsy-induced abnormal network between the affected hippocampus and amygdala. The hippocampus and amygdala are strongly connected in the TAO network1 and likely function as hubs within it. The formation of an abnormal pathological network by this hub is demonstrated by resting-state fMRI as increased FC in the amygdala region of the hippocampus.33
FC between the affected hippocampus and the bilateral TAO network increased in the gamma and ripple bands, indicating an abnormal temporal lobe-to-frontal lobe network formation due to epilepsy. This suggests that these changes spread bilaterally from the affected hippocampus to the frontal lobe. Disturbances in the limbic white matter tracts, such as the uncinate fasciculus in TLE, occur diffusely and can spread to the brain's unaffected side.34
Additionally, the severity of depressive symptoms in patients with TLE correlates with a stronger increase in FC in the affected hippocampus and PFC. Regression analysis indicated that the hippocampus and PFC significantly contribute to FC.35 The hippocampus is connected to the uncinate fasciculus via the parahippocampal gyrus and amygdala. The uncinate fasciculus terminates as a fiber bundle at the parahippocampal gyrus, amygdala, temporal pole, and uncus and projects via the extreme capsule to the anterior insular gyrus, the OFG, or to the cingulate gyrus and mPFC.1 We previously reported a case of MTLE in which epileptic discharges propagated from the hippocampus and amygdala via the uncinate fasciculus to the OFG, resulting in hypermotor seizures.36
The effectiveness of selective amygdalohippocampectomy, in that case, was possibly due to the disconnection of the abnormal network formed in the TAO network connecting the hippocampus to the OFG and mPFC. The neural activity of such abnormal epilepsy networks may occur in the gamma and ripple bands.
FC between the healthy hippocampus and mPFC decreased in the alpha and beta bands.
The TAO network, involved in emotion and cognition, with increased and decreased FCs, may contribute to various cognitive and psychological dysfunctions associated with epilepsy. Since higher brain functions, such as memory and attention, as well as psychological functions contributing to depression and anxiety, may be highly dependent on the coordinated activity of multiple resting-state networks, the TAO network may be a key factor in the development of epilepsy-related cognitive and psychological dysfunctions. Furthermore, when the resting-state networks of patients with MTLE were compared with those of healthy controls, a generalized lack of FC with the mPFC across all resting-state networks was observed.6 Neuroticism, depression, and anxiety in patients with TLE are significantly associated with decreased FC between the hippocampus and mPFC and between the hippocampus and OFG in resting-state fMRI.37, 38
Previous research suggests that network, cognitive, and psychiatric changes in TLE vary with the disease stage. Pathological driving connections are proposed to initially form in areas remote from the focus, including the contralateral side. The driving region then evolves into a secondary focus, with an increasingly complex network that alters seizure semiology, impairs cognitive and psychiatric functioning, and leads to poor seizure prognosis.11, 33 Based on these findings, we speculate that the abnormal network formed by the affected hippocampus with the TAO network contributes to dysfunction in the bilateral frontal lobes, thereby impairing the normal network and manifesting as decreased FC between the healthy hippocampus and the TAO network.
4.2 Correlations between interictal FC and subscores of WMS-R and WAIS-III
The question of whether there is an association between cognitive and psychological functions and changes in FC in the patients with MTLE in this study is addressed in this section.
Since none of the patients with MTLE in this study experienced psychiatric disorders before or after surgery, psychological effects could not be directly evaluated. The functional implications of FC changes were examined based on the correlation between preoperative higher brain function test scores (WMS-R and WAIS-III) and FC in patients with MTLE. The FC between the affected hippocampus and the TAO network showed a positive correlation with all scores across the delta– beta bands; however, this observation has no clear clinical implications. This may be due to a combination of various network changes between the affected hippocampus and the TAO network, including the formation of an abnormal network, impairment of normal function, and compensatory mechanisms, which may complicate the trend (upper part of Figure 3A).
Meanwhile, the increased FC between the affected hippocampus and TAO network within the ripple band (compared with that in healthy controls) likely indicates an abnormal epileptic network, showing a trend toward a negative correlation with attention and concentration scores on the WMS-R and with the working memory and processing speed indices on the WAIS-III. This suggests that the affected hippocampus may play a role in frontal lobe dysfunction (lower part of Figure 3A).
The correlation between FC (decreased relative to that in healthy controls and indicative of functional impairment) between the healthy hippocampus and mPFC and WMS-R showed that FC tended to correlate negatively with visual memory, attention, and concentration (Figure 3B). This finding suggests a possible compensatory effect of visual memory, attention, and concentration for verbal memory impairment. Visual memory and attentional focus scores exceeded verbal memory scores on the WMS-R in all patients with MTLE (Table 3). Similarly, the correlation between the FC of the healthy hippocampus, which was reduced compared with that of healthy controls, and the WAIS-III showed that FC tended to be negatively correlated with perceptual organization function, suggesting a possible compensatory effect (Figure 3B).
While previous studies reported compensatory increases in FC in the affected hippocampus for such dysfunction,2, 12 we did not observe increased FC between the healthy hippocampus and the TAO network. We hypothesize that as MTLE progresses and FC between the healthy hippocampus and TAO network becomes more complex, these compensatory changes become more subtle.
4.3 IED-related FC changes in patients with MTLE
Tong et al. examined the FC between the hippocampus and OFG in the time window before and after a spike in TLE using EEG-fMRI. They observed decreased FC between the hippocampus and OFG and increased FC between the hippocampus and mPFC, which is consistent with our results.39 Consistent with our original hypothesis, our results also showed that compensatory network changes resisting the abnormal epilepsy network occurred in the alpha band during the 3 s before the spike, as indicated by increased FC between the hippocampus and mPFC. Following spike onset, abnormal network changes associated with epilepsy were observed in the beta and ripple bands.
EEG-fMRI studies have observed FC changes between the temporal and frontal lobes and the mediodorsal nucleus of the thalamus in TLE, with FC increasing 4–5 s before the spike in the alpha band and then decreasing.40 Our results may reflect similar neural activity changes before and after spike onset. These abnormal epileptic network changes between the hippocampus and TAO network before and after spike onset resulted in impaired function, reflected as decreased FC in the delta band. This suggests a network disturbance resulting from abnormal network changes induced by the transient effects of MTLE-generated IEDs. Therefore, IEDs may cause transient impairments in psychological function. While IEDs can transiently impair cognitive function,5 examining transient impacts on psychological function is more complex than assessing cognitive impairments.
4.4 Limitations and future research
First, in the present study, we analyzed the spike period, including the spike itself. The effects of spikes, including the ringing artifact caused by filtering, could not be excluded from the FC during the spike period. Since we aimed to observe the FC during the spike, the analysis in this study was conducted without processing the spike itself. The treatment of spikes in the analysis process requires further study.
Second, the magnitude-squared coherence used in this research is an index indicating the phase consistency between signals. However, because it is based on cross-spectra and auto-spectra, variations in signal power may affect the outcome. In light of this, the following measures were taken: FC was assessed separately in six frequency bands (delta, theta, alpha, beta, gamma, and ripple) to suppress the effects of wide-band power. In addition, differences in FC between the patient and healthy control groups and between the IED and resting groups were assessed using t-tests and multiple comparison tests to reduce the effects of random power variations. However, due to the profile of magnitude-squared coherence, it cannot be concluded that the effects of signal power were completely excluded. This point is considered in the interpretation of the outcomes.
Third, since the study focused only on patients who became completely seizure-free immediately following epilepsy surgery and were confirmed to have MTLE, the sample size analyzed was small. This may explain the limited significant correlations between neuropsychological tests and FC. Additionally, the psychological effects related to the physiological involvement of the TAO network were not objectively evaluated using psychiatric tests because no psychiatric disorders were identified during preoperative evaluations, limiting the analysis of the clinical significance of FC changes in psychological aspects.
However, through comparative analysis of FC between patients with MTLE and healthy participants using MEG, specific network changes in each frequency band, which could not be obtained in previous fMRI studies, were clarified. These changes are believed to reflect the effects of MTLE. Although the MTLE patients included in this study did not exhibit psychiatric disorders as clinical symptoms, it is possible that network changes had already begun before the onset of psychiatric symptoms, and the present findings may reflect such changes.
Fourth, we first normalized the MRI and then estimated the current source of the MEG based on that space. Accordingly, inaccuracies in standardizing the patient's brain to the MRI may have affected the outcome.
In future research, we aim to include a larger sample size to assess the association with the dominant hemisphere and conduct a large-scale statistical evaluation of neuropsychological and psychiatric tests. Additionally, we plan to analyze network changes before and after surgery to further clarify the relationship between psychological function and FC changes between the hippocampus and the TAO network.
AUTHOR CONTRIBUTIONS
T.I.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology (equal), project administration (equal), resources (lead), visualization, writing –original draft. S. M.: Project administration (equal), resources (lead), supervision (equal), validation. T.S.: Resources (supporting). M.H: Resources (supporting). Y.I.: Resources (supporting). H.Y.: Resources (supporting). T.T.: Resources (supporting). J.N.: Resources (supporting). M.H.: Methodology (equal), resources (lead). R.S.: Supervision (equal). All authors reviewed the manuscript.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Number 23K15663 (Principal Investigator: Tomotaka Ishizaki).
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study include patients' personal information and are available from the corresponding author upon reasonable request.