Exploring the effect of valproic acid levels on patients with epilepsy treated with ketogenic diet: An observational study
Abstract
Ketogenic diet (KD) therapy is an effective treatment for children with refractory epilepsy. Concurrent treatment with KD and valproic acid (VPA) has previously been shown to affect VPA blood levels. The aim of this study was to explore how VPA levels affect beta-hydroxybutyrate (BHB) levels of children treated with both. We identified 36 children <18 years old concurrently treated with VPA and classic KD between 2018 and 2022. Retrospective data collected from the medical record: date of birth, sex, feeding method, diet initiation and discontinuance dates, VPA initiation and discontinuance dates; and serial weights, KD ratios, levocarnitine dosages, VPA dosages, and BHB, free carnitine, and VPA levels. Repeated-measure data was assessed using univariate and multivariate linear mixed-effects regression analysis. Results showed a statistically significant negative association between VPA and BHB levels based on a univariate LME regression analysis. Conversely, for each 1 mmol/L increase in BHB level, VPA level decreased by 6.39 μg/mL (p < 0.001). Our study indicates that VPA and BHB levels should be monitored closely for children on both treatments and treatment plans may need adjusting if seizure control is not achieved.
Plain Language Summary
This study focuses on children with epilepsy treated with both the ketogenic diet and VPA. It shows that as levels of VPA increase, blood ketone levels decrease, and vice versa. These results suggest that doctors should closely monitor these levels and potentially adjust treatment plans for children on both therapies if seizures are not well controlled.
1 INTRODUCTION
Ketogenic diet (KD) therapy is an effective treatment for epilepsy in children whose seizures have been refractory to traditional pharmaceutical treatment.1 There are several forms of the diet with different macronutrient compositions, but all are high in fat and low in carbohydrate.2 Patients treated with most forms of KD produce ketones, which provide an alternative energy source to glucose.3 Ketones can be measured in the blood as beta-hydroxybutyrate (BHB) or in urine as acetoacetate.4 However, it has been shown that BHB levels correlate better with seizure reduction than urine ketones.5 Existing literature has shown mixed results with regards to the correlation of BHB levels and seizure reduction. Some studies have shown positive correlation,6, 7 and others have shown no8 or inconsistent statistical correlation.9 While the conflicting results of these studies may lead to the conclusion that there is no optimal level of BHB for all patients, they do not rule out the possibility that each individual patient may have their own optimal level of BHB necessary for seizure control.
Concurrent treatment with KD and valproic acid (VPA) has been shown to affect VPA blood levels, although with conflicting results. One study concluded that treatment with KD over a median duration of 153 days decreased VPA blood levels significantly.10 In contrast, 2 other studies concluded that there was no significant association between ketosis and VPA blood level after 1 month11 and 3 months of KD treatment.12 There are limited published studies exploring the impact of concurrent treatment with KD and VPA on ketone production as measured by BHB. Spilioti et al. observed a rise in BHB levels when VPA was discontinued. However, out of 75 patients treated with both, this was only observed in two cases.13 Another study conducted by Thurston et al. showed a statistically significant decrease in fasting BHB for 7 children treated with VPA vs. Eight children not treated with VPA, but these children were not concurrently treated with KD.14
One specific case stimulated our interest in how VPA might affect BHB levels: A patient with myoclonic-atonic epilepsy whose anti-seizure medication (ASM) regimen included VPA started the KD at age 2 years and 9 months. On achieving seizure freedom at a 2.5:1 ketogenic ratio with BHB levels ranging from 4.1–7.4 mmol/L, VPA and one other ASM were discontinued. The patient remained seizure-free on KD and ethosuximide (ESM) for 17 months except during illness. With an attempt at an ESM wean, seizures recurred and continued to increase despite full ESM dose. VPA was restarted, and patient's BHB level dropped to 2.2 mmol/L. KD ratio required an increase to 4:1 to achieve BHB levels that had been previously achieved at a much lower ratio. We have also observed other patients who are unable to achieve high BHB levels when concurrently treated with VPA.
The aim of this study was to examine how VPA affects the level of ketosis in children on the KD in our Epilepsy Clinic.
2 METHODS
The medical records of all children under 18 years of age who initiated a classic KD therapy under the guidance of Boston Children's Hospital's KD Program and were actively following the diet between 2018 and 2022 were reviewed. Those on concurrent treatment with VPA at any time during their KD course were included in the study. We defined a classic KD as having a precise ketogenic ratio and calorie goal.
Clinical data was collected retrospectively from the medical record and was stored in REDCap. Fixed data collected included date of birth, sex, feeding method (oral, tube, or both), diet initiation date, diet discontinuance date, VPA initiation date, and VPA discontinuance date. Serial data collected from each KD clinic visit included weight, KD ratio, levocarnitine daily dose, and VPA daily dosage. Laboratory testing information collected from each clinic visit included BHB and free carnitine levels. VPA levels tested within 3 months of the clinic visit were also collected, although the timing of administration of the medication in relation to the timing of laboratory collection of the VPA levels could not be determined.
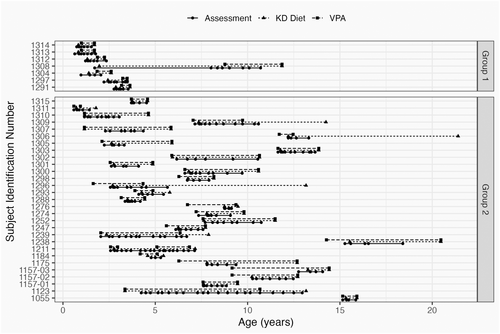
We divided the patients into two groups. Patients in group 1 were treated with the KD first, with VPA subsequently added during the course of KD treatment, while patients in group 2 were already being treated with VPA when the KD was initiated.
Mean, standard deviation (SD), and range were used for continuous variables and counts with percentages were used for categorical variables for summary statistics of demographic and time-invariant characteristics. We examined association using univariate and multivariate linear mixed-effects (LME) regression analysis with random intercept to account for repeated measures. Marginal and conditional R-squared statistics were reported to evaluate goodness-of-fit.15 Statistical significance was defined as p < 0.05. Data analysis was performed using R version 4.2.0.
3 RESULTS
We identified 36 patients concurrently treated with KD and VPA, with demographic data shown in Table 1. Most patients in the study (29) were being treated with VPA prior to starting KD therapy. One patient in this group started and stopped the diet 3 times, and we have counted that patient as 3 independent cases. Only seven patients started KD before VPA (Figure 1).
| Number of patients | 36 | |
|---|---|---|
| Sex | Female | 13 (36.1%) |
| Male | 23 (63.9%) | |
| Age at KD initiation | Mean (SD) | 5.51 (4.22) |
| Range | (0.73, 15.53) | |
| Duration of KD diet (years) | Mean (SD) | 3.24 (2.89) |
| Range | (0.59, 10.48) | |
| Feeding method | Oral | 21 (58.33%) |
| Tube | 12 (33.33%) | |
| Mixed | 3 (8.33%) | |
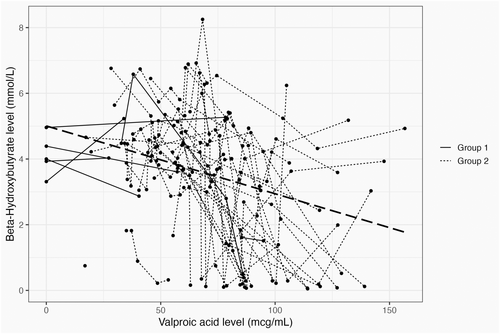
KD ratios ranged from 1:1 to 3.5:1, and the average VPA dosage was 40 mg/kg, ranging from 4 mg/kg to 128 mg/kg. Univariate LME regression analysis indicated that there was a statistically significant negative association between BHB and VPA blood levels (Figure 2; LME regression: p < 0.001; marginal R-squared statistic 0.15, conditional R-squared statistic 0.41). The R-squared statistic indicated that the goodness-of-fit of the LME regression model was moderate, suggesting that the model did not reveal a clear trend, but rather identified a population-level negative association BHB and VPA blood levels in this cohort. Additionally, Figure 2 demonstrated that the relationship between BHB and VPA blood levels may vary across individual patients.
Out of 36 patients, 30 received carnitine supplementation. The average free carnitine blood level was 36.3 μmol/L (SD 16.1, range 7.8–109), with a normal range of 26.0–60.0 μmol/L. A multivariate linear mixed-effect regression controlling for carnitine level showed that carnitine level did not change the association between BHB and VPA blood levels (LME regression: p < 0.001, indicating significant negative association between BHB and VPA blood levels; marginal r-squared statistic 0.19, conditional r-squared statistic 0.49, indicating moderate goodness-of-fit).
4 DISCUSSION
This retrospective cohort study extends the work of Thurston et al.14 in showing a statistically significant negative correlation between BHB and VPA blood level in a population of patients on KD and brings this interaction back into clinical focus. The magnitude of this association was estimated at the population level, based on a cohort of 36 patients. It is clear from Figure 2 that individual patient responses may vary. The regression line displayed in the graph is derived from the linear mixed-effects model and accounts for individual-level deviations from the population average. For one of our patients, the VPA blood level rose to 102.5 μg/mL after 3 months of treatment, and BHB blood level decreased from 6.92 to 2.17 mmol/L despite a stable KD ratio, with no observed change in seizures.
These results are in concordance with reports of VPA effect to reduce ketones in animals and humans in the 1980s,14, 16 as well as more recent clinical reports.13, 17 The exact mechanism by which VPA affects ketone levels is unclear though there are many possibilities. Because ketone bodies are primarily synthesized in the liver from fatty acids,18 one hypothesis suggests that VPA may affect fatty acid oxidation by impairing mitochondrial fatty acid metabolism in the liver.19 Impaired biosynthesis of carnitine via reduction of alpha-ketoglutarate, or its depletion by formation of valproylcarnitine metabolites could also be implicated.19 However, we monitored free carnitine levels for diet patients and supplemented when needed. Our statistical analysis demonstrated that the carnitine level did not impact the association of BHB and VPA.
As a secondary outcome, our study corroborates the earlier investigation by Heo et al.10 showing a statistically significant decrease in VPA levels as BHB levels increase for patients on longer term KD treatment.
Limitations of this study include: (1) Small sample size of 36 patients, (2) the retrospective design preventing control of VPA dosing, frequency and timing of assays, and impact of protein binding, and (3) lack of a control group to study natural fluctuations in ketone levels in diet-treated individuals. A larger sample size would improve the statistical power of the findings as well as allow us to analyze other confounding factors, such as the ketogenic ratio, impact of changes in BMI z-scores on BHB, and simultaneous treatment with additional ASMs. Future studies should also include consideration of the pharmacokinetics of VPA and the resulting variability in individual metabolism and effectiveness for seizure control.
5 CONCLUSION
While combining VPA and KD has been shown to be safe,20 the interaction between VPA and ketosis needs to be emphasized. Our study indicates that clinicians should consider adjusting treatment plans for children on both treatments as some patients may experience reduced ketosis when taking VPA. Introduction of VPA to a diet-treated patient or weaning VPA when on diet may result in unwanted reduction or escalation in ketosis, respectively, with clinical consequences. Any adjustments in VPA dosages should be carefully managed with monitoring of VPA and BHB levels in an individualized manner. Future prospective study to include control groups and consideration of more facets of VPA and ketone metabolism will be helpful.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.