Skin derived branched-chain fatty acids in fetal horse gut
Abstract
Branched-chain fatty acids (BCFAs) are mostly saturated fatty acids with a methyl branch at the penultimate or antepenultimate position to the CH3 end. Very-long-chain BCFAs (C ≥ 20) are uniquely found in surface lipids. Human and California sea lion (Zalophus californianus) are the only two species that possess vernix at birth, and vernix particles carry loads of BCFA to the gut, potentially influencing the establishment of the first microbiota. Horse (Equus caballus) contains high levels of BCFA in its surface lipids, and the current study aims to investigate if BCFA are components of a neonatal foal's gut. Electron ionization tandem mass spectrometry was used to distinguish iso- and anteiso-form of BCFA from straight-chain fatty acids. Abundant very-long-chain iso-BCFA, mainly even-numbered iso-20:0, iso-22:0, iso-24:0, and iso-26:0 were found in horse meconium but not in their manure collected a few months later. There are little differences in BCFA types and concentrations between foal manure and adult manure. Moderate to high levels of very-long-chain anteiso-BCFA were also found. Comparisons of horse meconium BCFA to horse skin cholesterol ester BCFA content and to meconium from human and sea lion suggest its skin derived origin. The study expands our knowledge on the gut nutritional environment of a newborn horse, and further study is needed to establish the relationship between gut BCFA and microbiota composition in the first few hours of life.
Practical Applications: Branched-chain fatty acids (BCFA) are bioactive compounds found in vernix caseosa, the lipid-laden material covering the surface of a newborn baby. BCFA also reaches high concentrations from the third trimester of gestation until birth. However, the exact functionality and mechanisms of BCFA in the surface and gut of a fetus remain unclear. The current study aims to clarify the lipid conditions in the gut of newborn foals and to identify the associations between BCFA in the gut and on the skin at a critical point when the horse's microbiota establishes itself. The findings of the current study lay a path for studying the physiological functions of BCFA in horse models, potentially accelerating research related to BCFA.
1 INTRODUCTION
Branched-chain fatty acids (BCFA) usually refer to a group of fatty acids that bear one or more methyl branch on its acyl chain. The most common forms are characterized by either a terminal propan-2-yl (isopropyl) group (iso) or a butan-2-yl (sec-butyl) group (anteiso).[1] Although being a saturated fatty acid group, its bioactive properties have appealed to many researchers in the lipid field and other related areas.[2-4] Notably, BCFAs are a significant dietary component not only in regions where dairy consumption is high but also in some Asian countries where dairy consumption is low and habitual consumption of fermented food contributes to their BCFA intake.[5-8] Seafood is another source of BCFA including fish and edible invertebrates.[9-12] In addition, many plant sources also contain BCFA, for example, chia seeds, brussel sprouts, and some alpine plants.[13-15] The presence of BCFA in these food has prompted researchers especially in the food science and nutrition fields to investigate BCFA's connection to human health.
However, the detection and identification of BCFA are not an easy task for many, due to their low abundance in most food samples, the lack of authentic standards of some chain lengths, and the similarity of electron ionization mass spectrometry (EIMS) spectra of BCFA to their straight-chain counterparts. Traditionally, the identification of BCFA is mainly based on chemical derivatization of fatty acid methyl esters (FAME) into structures containing a charged head group, for example, picolinyl ester.[16] Our lab demonstrated an EIMS/mass spectrometry (MS) approach for the unambiguous identification of a methyl branch position in BCFA methyl ester,[17] and then established a chemical ionization (CI) MS/MS workflow that enables the simultaneous identification and quantification of low-level BCFA even when authentic standards are limited.[14, 18] In addition, a two-step method was established for the characterization of monounsaturated BCFA,[19] so far only known to be present in human sebum in significant concentrations.[20] Recently, a combination of liquid chromatography tandem MS and charge-switching chemistries was proved to be effective in distinguishing anteiso-BCFA from iso- form, however, it is not very robust in discriminating straight-chain from its iso-counterpart.[21] All these technical advances pave the way for the study of biological and physiological functionalities of BCFA.
BCFAs are integral component of human sebum,[20, 22] hair,[23] and meibomian gland secretion,[24] and rich in vernix caseosa found in human[25, 26] and at least another species, California sea lion (Zalophus californianus).[27] The concentrations of BCFA from these origins, however, are especially high in the chain length range ≥C20, unlike dairy or seafood derived BCFA in the chain length range <C20. Besides, BCFAs are also surface lipid composition of a wide range of animals, including laboratory mouse (Mus musculus),[28] rat (Rattus spp.),[29, 30] monkey (e.g., Macaca fascicularis),[31] dog (Canis familiaris), [32] and horse (Equus caballus).[33] Vernix caseosa is a fat rich film on the skin of a newborn, established during the third trimester of pregnancy. Vernix serves as an innate antimicrobial barrier to the skin and its ingestion carries loads of BCFA to the gut, potentially reducing the incidence of necrotizing enterocolitis and influencing the establishment of the first microbiota.[34, 35] Due to the presence of BCFA-rich vernix particles in the meconium of human and California sea lion, it is speculated that BCFAs play a role in neonatal gut development, probably interacting with the establishing microbiota of human and sea lion.[25-27] However, it has not been demonstrated that if other species, without the apparent existence of vernix caseosa, also have a gut environment fulfilled with BCFA when they were born. According to Downing et al., horse surface lipid is mainly comprised of cholesterol, cholesteryl esters, and lactones. Notably, the fatty acid composition of cholesteryl esters contains about 45% BCFA w/w, with BCFA of even chain length 16–34 dominating (95%).[36] Adult horses were reported to produce milk containing iso-15:0 and iso-17:0,[37] and their meat was found to have a low level of C14–C18 BCFA at around 0.2%–0.4%, w/w.[38, 39] In addition, hindgut content of adult horses was reported to have moderate concentrations of C14–C18 BCFA, unlike horse surface fatty acids, which are mainly C ≥ 20 very-long-chain BCFA.[40] The current study aims to elucidate the gut lipid environment of newly-born foal and establish the relations between gut and skin BCFA around the critical point when horse microbiota settle in, taking advantage of a structure-elucidating MS method.
2 MATERIALS AND METHODS
2.1 Sample preparation
Meconium samples were collected from three newly born foals, and their regular manure together with manure of one of their mothers was collected on the same day 4–6 months after birth. All the procedures complied with routine operations at the horse barn near Cornell University. Samples were then transported to the lab and analyzed immediately according to a modified one-step fatty acid extraction, hydrolyzation, and methylation method, as previously described.[41] Briefly, about 150 mg samples were added to glass test tubes with 2 mL of heptane. Then, 1.4 mL of reagent A (made with 85:11:4, v/v/v, methanol/2,2-dimethoxypropane/concentrated sulfuric acid) and 1.6 mL of reagent O (heptane/toluene = 63:37, v/v) were added. The tubes were placed onto a heated dry bath at 80°C for 2 h with vigorous shaking. Lastly, the extracted FAME were dried under a gentle stream of nitrogen and reconstituted in heptane.
2.2 Instrument
An HP 5890 gas chromatograph (GC)—flame ionization detector was used to analyze the horse meconium and manure samples, with accurate quantification achieved by applying response factors (RF) to all the measure peak areas. An equal weight FAME standard mixture (GLC-462 from Nu-Chek Prep) was used for determining the RF. A GC (Varian Star 3400CX equipped with a 1078 split/splitless injector) coupled with a Varian Saturn 2000 3D ion trap MS (Varian) was used to identify and quantify coeluted FAME in the study. Both instruments were equipped with the same BPX-70 GC column (60 m × 0.32 mm × 0.25 µm; SGE). GC conditions were as follows: injector was kept at 250°C operated in splitless mode; oven temperature started at 60°C and held for 1 min, then increased to 170°C at 50°C min−1 and held for 6 min, then increased to 200°C at 2.5°C min−1 and held for 3 min, then increased to 222°C at 10°C min−1 and held for 8.7 min, and increased to a final temperature of 255°C at 50°C min−1 and held for 1 min. For tandem MS experiment, the excitation amplitude (collision energy) was set to 0.80 V for all branched-chain FAME (BCFAME) or straight-chain FAME.
2.3 Statistics
Fatty acid compositions were expressed as mean ± standard deviation, computed in Microsoft Excel software. Each analysis was repeated three times. Significant levels between meconium and manure were calculated by paired t test analyses in the same software.
3 RESULTS
3.1 Characterization of BCFAME by EIMS/MS
Identification of saturated BCFAME and straight-chain FAME was achieved by collision-induced dissociation of M+ ions, or an EIMS/MS method.[17] Using a prominent pair of C20 FAME as an example, Figure 1A presents the product ion chromatogram of m/z 326 (M+ ion for C20:0). Peaks #1 and #2 correspond to a pair of isomers of C20:0 and the peaks at much earlier retention time are ion residues from unknown substance, thanks to the complexity of horse feces. Interrogation of the MS/MS spectrum of peak #1 results in the discovery of a prominent ion m/z 283, corresponding to [M-43]+ ion (Figure 1B). [M-43]+ corresponds to loss of a propyl group and is characteristic of the iso- structural motif in saturated FAME. Thus, peak #1 is identified as iso-20:0. In comparison, the EIMS/MS spectrum of peak #2 does not have any outstanding ion and is characterized by an envelope of ions in low abundance distanced by 14 Da, representative of straight-chain FAME 20:0 (Figure 1C).
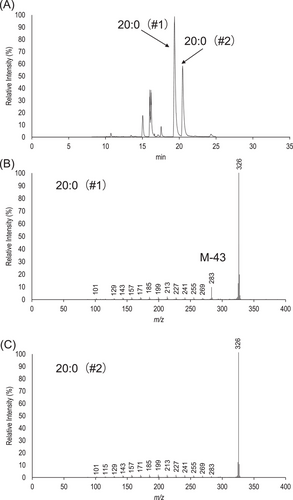
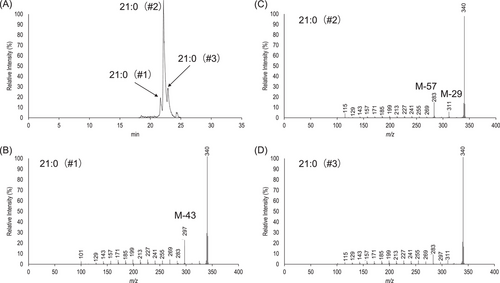
Figure 2A presents the product ion scan chromatogram of m/z 326, which corresponds to 21:0 and its isomeric forms. Under our current GC conditions, we observed three isomeric 21:0 only partially resolved, with the middle peak (#2) predominates in abundance. Analysis of the EIMS/MS spectrum of peak #1 unambiguously identifies it as iso-21:0 on the grounds of high [M-43]+ diagnostic ion (Figure 2B). Similarly, prominent fragments [M-57]+ and [M-29]+ as well as the nearly absence of [M-43]+ ion point to anteiso-21:0 for peak #2 (Figure 2C). As expected from the resolution of peak #2 and peak #3 seen in Figure 2A, spectrum of peak #3 is contaminated by coeluting major anteiso-21:0. However, the presence of m/z 297 (or [M-43]+ ion) and the reduction in abundance of m/z 283 (or [M-57]+ ion) and m/z 311 (or [M-29]+ ion) are noticeable, indicating that peak #3 is straight-chain 21:0 (Figure 2D).
3.2 Comparison of horse meconium and manure
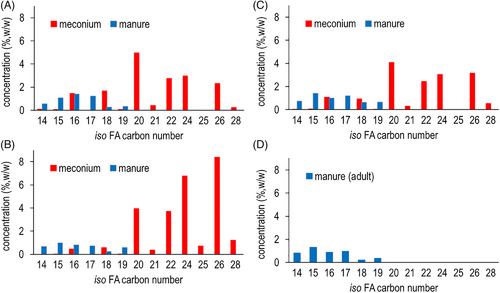
The fatty acid compositions of horse meconium collected at birth and manure collected after around 4–6 months from the same horses were compared, with special emphasis on the difference of BCFA. For iso-type BCFA (Figure 3), both meconium and manure have a small amount of C < 19 BCFA with meconium containing mainly iso-16:0 and iso-18:0, both of which constitute about 1% of total fatty acids w/w. Manure contains a complete list of C14–C19 iso-BCFA, each at around 1% or less. Manure does not contain any very-long-chain iso-BCFA (C ≥ 20). In sharp contrast, horse meconium is extremely high in very-long-chain iso-BCFA, with iso-20:0, iso-22:0, iso-24:0, and iso-26:0 constituting the majority. As we can see from Figure 3A–C, the variation of very-long-chain iso-BCFA is large. Meconium samples from foal No.1 (Figure 3A) and No.3 (Figure 3C) have about the same proportions of each individual very-long-chain iso-BCFA, with iso-20:0 the highest at around 4%–5% and C22, C24, and C26 iso-BCFA at around 2%–3% (w/w of total FA). On the other hand, meconium from foal No.2 (Figure 3B) has very high concentrations of iso-24:0 and iso-26:0 at around 7% and 9% of total FA, respectively. In addition, iso-28:0 consists of 1% of total FA in this sample (Figure 3B), while it is negligible in the other two meconium samples (Figure 3A–C). Manure from adult horse, in our particular case, the mother of one foal shows the same iso-BCFA distribution as that from the three foals (Figure 3D).
For anteiso-BCFA, C15 and C17 chain lengths were identified in foal manure and adult manure (Figure 4). Odd-numbered BCFA from C17 to C27 are present in meconium, and similarly to Figure 3A–C, meconium samples from foal No.1 (Figure 4A) and No.3 (Figure 4C) have about the same proportions of each individual anteiso-BCFA, all in low abundances (1% or less). In contrast, meconium from foal No.2 has skyrocketing levels of anteiso-25:0 and anteiso-27:0 at about 7% equally. In addition, anteiso-21:0 is also relatively high at almost 3%.
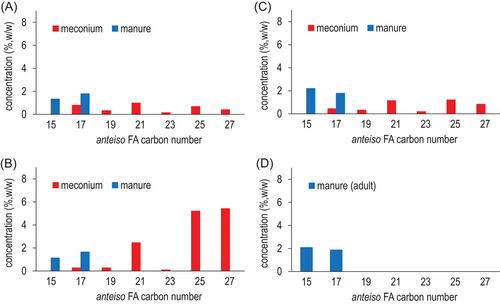
Table 1 presents the mean FA concentrations of meconium and manure of three foals along with one manure sample from one foal's mother. Significant differences across species of FA can be seen between meconium and their later defecation. Superscripted letters of a and b indicate that there are significant differences (p < 0.05). Manure from foal is significantly higher in various C < 20 saturated FA, including 14:0, iso-15:0, 15:0, anteiso-17:0, 17:0, 18:0, and iso-19:0. Notably, 14:0 and odd chain FA 15:0 and 17:0 consist of 4.23% ± 0.41%, 5.73% ± 1.36%, and 2.00% ± 0.54% of total manure FA, respectively, whereas they are all below 1% in meconium. Mean concentrations of all C12–C18 straight-chain FA are higher in manure than in meconium. In addition, phytanic acid with four methyl branches along its aliphatic chain was only detected in manure. In contrast, FA with chain length greater than 20 carbons is absent in manure but comprises a significant proportion of meconium FA. For example, very-long-chain BCFA including anteiso-21:0, iso-22:0, iso-24:0, anteiso-25:0, iso-26:0, and anteiso-27:0 each represent a few percent of total FA of meconium by weight, with iso-24:0 and iso-26:0 each exceeding 4%. Total very-long-chain BCFA (C ≥ 20) consists of 23.34% ± 12.65% of total FA, representing 88.54% of total BCFA in meconium. Very-long-chain straight FA are also of high levels in meconium, especially even chain 20:0, 22:0, 24:0, 26:0, and 28:0. Very-long-chain odd FA 21:0, 23:0, and 25:0 were detected in low levels at 0.29% ± 0.04%, 0.37% ± 0.43%, and 0.70% ± 0.14%, respectively. Summed iso-BCFA is 19.24% ± 5.72% and 4.75% ± 0.81% for meconium and manure, respectively. Summed anteiso-BCFA is 7.10% ± 5.78% and 3.31% ± 0.62% for meconium and manure, respectively. Total BCFA adds up to 26.36% ± 11.47% and 8.46% ± 1.51% for meconium and manure, respectively. Notably, manure from one adult horse, which in our case is one of the foals’ mothers, has a close overall FA profile compared to the foals’ manure.
| Meconium (n = 3) | Manure (n = 3) | Manure (one mother) | |
|---|---|---|---|
| 12:0 | 0.08 ± 0.06 | 0.21 ± 0.08 | 0.12 |
| 13:0 | – | 0.40 ± 0.15 | 0.46 |
| iso14:0 | 0.06 ± 0.02 | 0.64 ± 0.10 | 0.81 |
| 14:0 | 0.70 ± 0.33a | 4.23 ± 0.41b | 4.26 |
| 14:1 | 0.05 ± 0.01 | – | – |
| iso15:0 | 0.05 ± 0.02a | 1.14 ± 0.21b | 1.29 |
| ai-15:0 | – | 1.56 ± 0.56 | 2.09 |
| 15:0 | 0.29 ± 0.13a | 5.73 ± 1.36b | 6.67 |
| iso16:0 | 0.99 ± 0.49 | 1.05 ± 0.30 | 0.86 |
| 16:0 | 16.24 ± 5.90 | 28.63 ± 3.62 | 23.66 |
| 16:1n − 9 | 0.62 ± 0.27 | – | – |
| 16:1n − 7 | 3.52 ± 1.88 | 0.14 ± 0.11 | 0.20 |
| iso17:0 | – | 1.03 ± 0.27 | 0.97 |
| ai-17:0 | 0.51 ± 0.27a | 1.75 ± 0.08b | 1.88 |
| Phytanic acid | – | 0.40 ± 0.16 | 0.89 |
| 17:0 | 0.60 ± 0.23a | 2.00 ± 0.54b | 1.95 |
| 17:1 | 0.38 ± 0.21 | 0.27 ± 0.25 | 0.21 |
| iso18:0 | 1.06 ± 0.55 | 0.36 ± 0.21 | 0.20 |
| t18:1 | 0.13 ± 0.10 | – | – |
| 18:0 | 11.30 ± 2.02a | 34.00 ± 1.33b | 38.71 |
| 18:1isomers | 20.24 ± 8.07 | 9.96 ± 2.59 | 10.13 |
| iso19:0 | 0.04 ± 0.01a | 0.51 ± 0.17b | 0.34 |
| ai-19:0 | 0.31 ± 0.03 | – | – |
| 18:2n − 6+19:0 | 2.94 ± 0.90 | 2.54 ± 0.43 | 2.10 |
| 18:3n − 6 | 0.18 ± 0.09 | – | – |
| iso20:0 | 4.32 ± 0.56 | – | – |
| 18:3n − 3 | 0.15 ± 0.11 | 1.08 ± 0.48 | 0.74 |
| 20:0 | 2.07 ± 0.56 | 2.10 ± 0.24 | 1.33 |
| 20:1n − 9 | 0.67 ± 0.17a | 0.24 ± 0.11b | 0.14 |
| 20:1n − 7+iso21:0 | 0.36 ± 0.05 | ||
| ai-21:0 | 1.53 ± 0.81 | ||
| 21:0 | 0.29 ± 0.04 | ||
| 20:3n − 6 | 0.28 ± 0.19 | ||
| iso22:0 | 2.97 ± 0.67 | ||
| 20:4n − 6 | 0.37 ± 0.16 | ||
| 22:0 | 2.20 ± 1.16 | ||
| 22:1 | 0.27 ± 0.12 | ||
| ai-23:0 | 0.15 ± 0.05 | ||
| 23:0 | 0.37 ± 0.43 | ||
| iso24:0 | 4.26 ± 2.17 | ||
| 22:4n − 6 | 0.15 ± 0.10 | ||
| 24:0 | 4.29 ± 3.46 | ||
| 24:1 | 1.74 ± 0.92 | ||
| iso25:0 | 0.24 ± 0.41 | ||
| ai-25:0 | 2.37 ± 2.47 | ||
| 25:0 | 0.70 ± 0.14 | ||
| iso26:0 | 4.61 ± 3.30 | ||
| 26:0 | 1.00 ± 0.62 | ||
| ai-27:0 | 2.22 ± 2.78 | ||
| iso28:0 | 0.66 ± 0.50 | ||
| 28:0 + 28:1 | 1.50 ± 0.92 |
| Meconium | Manure | Manure (one mother) | |
|---|---|---|---|
| C ≥ 20 BCFA/total BCFA (%) | 88.54 | 0 | 0 |
| iso-BCFA | 19.24 ± 5.72 | 4.75 ± 0.81 | 4.47 |
| ai-BCFA | 7.10 ± 5.78 | 3.31 ± 0.62 | 3.97 |
| Total BCFA | 26.36 ± 11.47 | 8.46 ± 1.51 | 9.34 |
- Note: a Different letters denote significance level at p < 0.05 based on paired t test analysis.
- b The fatty acids are listed in the order of their retention time under our conditions.
- c Indicates that the specified fatty acid was below detection limit or not quantified due to coelution.
- d Trace levels of 22:5n − 3 and 22:6n − 3 coelute with anteiso-25:0 and 25:0, respectively.
- e ai- is used for anteiso.
- Abbreviation: BCFA, branched-chain fatty acid.
Monounsaturated FA oleic acid (and coeluted isomers) and linoleic acid are relatively high for both meconium and manure samples. Interestingly, meconium also has 3.52% ± 1.88% of 16:1n − 7, whereas manure has trace level. Highly unsaturated FAs are generally low in meconium, with trace levels of 18:3n − 6, 18:3n − 3, 20:3n − 6, 20:4n − 6, 22:4n − 6, 22:5n − 3, and 22:6n − 3 detected. Only about 1% of 18:3n − 3 was identified in manure.
3.3 Horse meconium and skin cholesterol esters BCFA
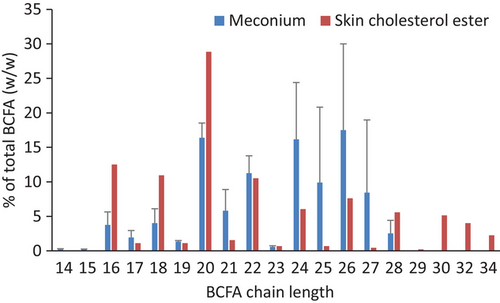
Very-long-chain BCFA are known to be a component of horse surface lipid, that is, the fatty acyl fractions of cholesterol esters.[36] Horse meconium BCFA is thus suspected to originate from horse skin, shed off from fetus, and suspended in horse amniotic fluid, swallowed by the fetus, and eventually ended up in its first defecation. Here, we compare the BCFA concentrations of each chain length as percentages of total BCFA (Figure 5 and Table S1). BCFA representing more than 10% of total BCFA include chain lengths C20, C22, C24, and C26 in meconium and C16, C18, C20, and C22 in skin cholesterol esters. Seemingly distinct differences in concentrations are seen with C25, C27, and C ≥ 30 BCFA; however, considering the very large variations in horse meconium measurement of different individuals and only one measurement for the reference, it could arise from individual variations.
3.4 Comparison of meconium BCFA from human, sea lion, and horse
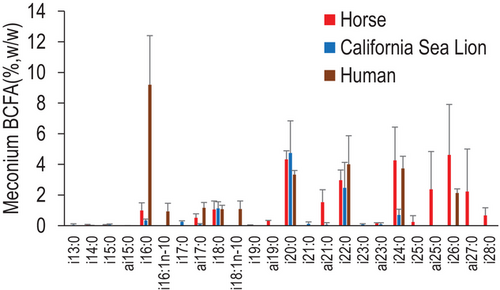
It has been found that human and sea lion fetus possess BCFA-rich vernix and these lipids go through their gut and can be found in their meconium when they were born.[25, 27] Although no apparent evidence shows that horse also possesses vernix, their skin lipid can similarly suspend in amniotic fluid in small droplet and enter fetus gut via swallowing. We here investigate if there is commonality of BCFA composition in human, sea lion, and horse meconium (Figure 6). Although horse and sea lion contain a small level of iso-16:0, it is the highest BCFA in human meconium at around 9%, w/w of total FA. This precursor also leads to the formation of two monounsaturated BCFA species, iso-16:1n − 10 and iso-18:1n − 10 each at around 1%. Notably, very-long-chain even numbered BCFA, that is, iso-20:0, iso-22:0, and iso-24:0, are relatively high among all three mammal species at relatively close levels. Specifically, mean iso-20:0 is around 4% and mean iso-22:0 around 3%–4% for meconium from horse, sea lion, and human. Mean iso-24:0 is around 1% for sea lion meconium and around 4% for both horse and human meconium. In addition, both horse and human meconium contain high levels of iso-26:0 at about 5% and 2%, respectively. Horse meconium is featured by the abundance of very-long-chain odd-numbered anteiso-BCFA, particularly anteiso-21:0, anteiso-25:0, and anteiso-27:0, each at about 2% of total FA.
4 DISCUSSION
The current study demonstrates that very-long-chain BCFA, a group of FA uniquely found in the skin lipids of some animals, for example, human,[25, 42] monkey,[31] dog,[32] and horse,[36] are naturally present in high concentrations in the gut content of a neonatal horse. A recent study reported that vernix and meconium contained BCFA with 12–24 and 14–22 carbon chain length, respectively. Consistent with results from out lab, iso-16:0 had the highest abundance among all BCFA.[25, 43] Although horse meconium also has abundant levels of BCFA, it does not contain high levels of iso-16:0, and it is more concentrated in very-long-chain BCFA, especially in the range of C24–C28, compared to human meconium. The key motif of a BCFA, which typically contains either a terminal propan-2-yl (isopropyl) group (iso) or a butan-2-yl (sec-butyl) group (anteiso), can be unambiguously identified by EI-MS/MS workflow. Specifically, a prominent [M-43]+ ion characterizes the iso-form, and prominent ion pairs [M-57]+ and [M-29]+ signifies the anteiso-form. The EI-MS/MS approach has been successfully employed to characterize BCFA in bovine milk, dairy products, fermented food, and vernix caseosa of human newborns and sea lions.[5, 6, 25, 27, 44] Although there is some close spectral resemblance between EI-MS/MS and CI-MS/MS, namely, to identify the iso-form by [M-43]+ ion abundance, there is also one nuance between the two MS methods, that is, the anteiso-form characterized only by the [M-57]+ ion in the CI-MS/MS methodology.[14, 45] Although both the EI-MS/MS and CI-MS/MS allow de novo assignment of a branch position, the sensitivity and quantitative capability of CI-MS are superior to EI-MS, making CI the better choice for the characterization of low-abundance BCFA.[18] Horse meconium and manure defecated later in its life have a distinctly different BCFA profile, reminiscent of human (and sea lion) meconium and manure FA compositional differences.[25, 27, 46] Although both human and sea lion have been confirmed to biosynthesize vernix caseosa, which translates into the high BCFA concentrations observed in human and sea lion fetuses’ gut content, consultation of horse physiology experts resulted in negative responses over the presence of horse vernix. We speculate that horse skin lipids enter the gut of horse fetus in another form different from vernix caseosa, and the exact particle form loaded with very-long-chain BCFA needs to be further characterized. BCFAs, although with a shorter chain length, are components of many bacteria species that inoculate the gut of either a human, a sea lion, or a horse early in their life. Horse is another mammal, after human and California sea lion, that has meconium FA similar to skin FA composition, loaded with a wide range of very-long-chain BCFA. Horse, sea lion, and human meconium have significant and comparable levels of iso-20:0 and iso-22:0. They differ greatly in the concentrations of iso-16:0, monounsaturated BCFA, very-long-chain odd-numbered anteiso-BCFA (e.g., anteiso-25:0), and C > 24 iso-BCFA. These differences could be attributed to their skin lipid biosynthesis, for example, different functionality and expression of FADS2 and various ELOVL genes. We found large variation of a wide range of iso- and anteiso-BCFA in the meconium obtained from foal No. 2 compared to the other two foals. This might be attributed to the gestational ages of the newborn horses. Indeed, meconium and vernix caseosa from termed human infants were reported to contain a much higher level of BCFA.[43] The molecular mechanisms of BCFA as a driver to impact bacteria growth may relate to the membrane compositions of various bacteria, with Bacillus and many other bacterial species incorporate up to >90% BCFA into their membrane. BCFA can significantly increase the fluidity of bacterial membrane compared to straight-chain saturated FA.[35, 47] While highly possible, the interaction between skin derived BCFA present in the gut and the establishment of microbiota remains largely unknown. Further research should be directed to study the modulating effects of very-long-chain BCFA on microbiota composition of a newborn.
This study has some limitations that should be acknowledged. First, the number of horse meconium and manure samples collected is relatively small. Second, the gestational ages of the three foals are not available, thus making it difficult to assess the possible causes of high individual variation in both very-long-chain iso- and anteiso-BCFA concentrations. Lastly, we attempted several times but failed to acquire horse amniotic fluid samples, which could further demonstrate the biosynthetic origin of meconium BCFA. Further research should establish the correlation between the concentrations of various horse meconium/amniotic fluid BCFA and gestational age.
5 CONCLUSION
The current study presents a third animal that has a gut environment filled with BCFA at the critical time when the first microbiota is established, reminiscent of human and sea lion, which have BCFA-rich vernix caseosa. The BCFA composition of foal meconium is comparable with those of human and sea lion, with abundant very long-chain BCFA (C ≥ 20). However, no evidence indicates the presence of vernix on the skin of a newborn horse, suggesting a different gut infiltration manner of skin derived lipids for horse.
AUTHOR CONTRIBUTION
Dong Hao Wang and Zhen Wang: Conceptualization, design, and drafting of the manuscript. Dong Hao Wang: Did the experiment. Dong Hao Wang, Lerong Qi, Tingxiang Yang, and Yachen Ren: Generating figures/tables and editing. All authors approved the final version for submission.
ACKNOWLEDGMENTS
The authors would like to thank Douglas Antczak and his lab members for their generous help with sampling, and J. Thomas Brenna and Kumar Kothapalli for their support for the study.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




