Nano-encapsulated flaxseed oil has the potential to enhance omega-3 fatty acid profile and modulate hepatic fatty acid–related gene expression in broiler chickens
Abstract
Chicken meat enrichment with n − 3 long-chain polyunsaturated fatty acid (LCPUFA) is a viable delivery route for these bioactive compounds to humans. This study was conducted to evaluate the effectiveness of nano-constructed flaxseed oil on growth performance, the deposition of n − 3 LCPUFA in meat, and the expression of some hepatic genes involved in lipid metabolism. One hundred and eighty-one-day-old Ross 308 chicks were randomly allocated to three treatments. Birds received either basal diet (control), FlxO (basal diet plus 1 mL kg−1 body weight [BW] oral flaxseed oil), or NanoFlxO (basal diet plus 1 mL kg−1 BW oral flaxseed oil nanoemulsion) treatments. In comparison to the control, both bulk and nano-form flaxseed oil resulted in increased BW but decreased feed conversion ratio (p < 0.05). Birds in the NanoFlxO group had a reduced saturated fatty acid content of breast meat compared to the control group (p > 0.05). The breast meat was more effective than thigh muscle in the accumulation of eicosapentaenoic acid and docosahexaenoic acid following NanoFlxo treatment. The ratio of n − 6/n − 3 PUFA was significantly lower in the breast and thigh meats following both flaxseed oil treatments. The n − 3 LCPUFA incorporation rate was two times higher in broilers that were given NanoFlxO in comparison to those fed FlaxO. The highest transcript levels of acyl coenzyme A (CoA) oxidase 1, lipoprotein lipase, and carnitine palmitoyltransferase 1 were observed in birds fed NanoFlxO. In conclusion, NanoFlxO has a promising potential to enrich the n − 3 LCPUFA content of chicken meat as well as improve lipid metabolism.
Practical Applications: This study offers further insights into the fortification of chicken meat with omega-3 fatty acids, essential nutrients that cannot be synthesized by human body but are crucial for overall health. In a wider perspective, this could serve as a safe and practical approach to produce omega-3 enriched meat rather than relying on supplements that may have potential side effects. Moreover, the findings of this research could pave the way to utilize nanocarriers in upcoming research in the fields of bioactive-enriched poultry products.
1 INTRODUCTION
Incorporating bioactive compounds and developing functional foods have received increasing attention in the food industry.[1] In line with this, n − 3 long-chain polyunsaturated fatty acids (LCPUFAs) have distinct bioactive properties because of their active role in cardiovascular diseases, brain functionality, immune response, and reproduction.[2] Comprising 40% of the world's meat production,[3] chicken meat is an excellent candidate to fortify with n − 3 LCPUFA and a vital delivery route of these nutrients to humans. In fact, unlike ruminants with complex stomachs, chickens are monogastric, and modifying their meat compositions through dietary supplementation would be a reliable, justified, and efficient method. Being the primary source of n − 3 LCPUFA, supplementing the poultry diet with more than 1%–2% fish oil is not recommended because of organoleptic problems in meat[4] and eggs.[5]
Flaxseed oil is an excellent source to increase meat n − 3 LCPUFA content because of the considerable amount of alpha-linolenic acid (ALA). ALA can be used as a substrate for ∆−5 and ∆−6 desaturase and elongase 2 enzymes to produce n − 3 LCPUFA in a bird's liver, accumulating in the eggs and meat. In laying hens, dietary flaxseed supplementation upregulated the transcript level of these enzymes and resulted in higher egg yolk docosahexaenoic acid (DHA) content.[6] Other studies also well documented the possibility of using flaxseed or its oil to enrich poultry meat with n − 3 LCPUFA.[7-9] On the other hand, long-term flaxseed feeding reduces broiler meat's functional properties (i.e., lower pH, more drip loss and cooking loss, and lighter color). Moreover, the oxidative stability of broiler meat is negatively affected by the duration of flaxseed supplementation.[10] Therefore, finding a strategy to tackle this problem would be worthwhile.
Nanoemulsions are delivery systems that have the potential to increase the bioavailability of bioactive compounds via improving their bioaccessibility (i.e., releasing from food matrix and absorbing through the intestinal wall) and also bioactivity (i.e., reaching target tissue and generation of physiologic response).[1] Therefore, the nanoemulsion of bioactive compounds is an interesting emerging technology proposed for application in the poultry meat industry. Incorporating n − 3 fatty acids in nanoemulsions could overcome their low bioavailability, poor water solubility, and proneness to oxidation.[1] Moreover, this method would remove their undesirable flavors and odors. Compared to conventional soft gel capsules, the oral ingestion of emulsified fish oil increased the bioavailability of eicosapentaenoic acid (EPA) and DHA in healthy students.[11] Microencapsulation with plant spore exines was also used to enhance the bioaccessibility of EPA, as assessed by its serum concentration in healthy volunteers.[12]
Ultrasonic-assisted emulsification (UAE) has gained growing attention for the construction of nanoemulsions due to its potential to produce small droplets at good energy efficiency, relatively low cost, and particularly for efficient nutrient delivery systems.[13] The nanoemulsions produced using this method can have very fine droplets and narrow particle-size distributions, which makes them particularly suitable for many commercial applications[14] in comparison with conventional mechanical methods.[15] Moreover, UAE improves the stability and also the bioavailability of bioactive compounds in the digestive system.[16] Finally, bioactive compounds with submicron particle size have a large surface area, allowing rapid penetration through the intestinal walls.[1]
The liver plays a crucial role in whole-body lipid metabolism and adapts to changes in dietary fat compositions via altering the expression of genes involved in de novo fatty acid synthesis and β-oxidation. Diets enriched with unsaturated fatty acids increase liver fat content more than those with saturated fatty acids (SFAs).[17] Feeding a flaxseed-based diet upregulated genes involved in β-oxidation such as acyl coenzyme A (-CoA) oxidase and carnitine palmitoyltransferase, but it also simultaneously downregulated fatty acid synthase, such as fatty acid synthetase and acetyl-CoA carboxylase α pathway in broiler chicks.[18]
Although the effectiveness of ALA in modulating the meat composition of chicken was previously reported, enrichment of chicken meat via a nanoproduct, particularly concerning hepatic gene expression, requires further investigation. Thus, the present study was conducted to clarify the differential effects of ultrasound-assisted flaxseed oil–loaded nanoemulsion on productive performance, fatty acid profile, and hepatic gene expression in broiler chickens.
2 MATERIALS AND METHODS
2.1 Chemicals and ethical issues
The whey protein (WP) concentrate (80% protein) and maltodextrin (MD) used in this study were sourced from Arla Foods and Qinhuangdao Lihua Starch, respectively. All other materials were obtained from Sigma and Merck, unless stated otherwise. The research conducted in this study adhered to the guidelines approved by the Animal Care and Use Committee of the Gorgan University of Agricultural Sciences and Natural Resources (Iran).
2.2 Preparation and characterization of nanoemulsion
The preparation of sodium alginate–WP (SA/WP) stabilized nanoemulsion was conducted following the procedure outlined by Fioramonti et al.,[19] with minor adjustments. Initially, a 1% wt solution of SA was prepared by dissolving it in deionized water and stirring at 70°C for 35 min. Subsequently, a mixture of WP (2% wt) and MD (20% wt) was prepared by dissolving them in deionized water and shaking them for 2 h at room temperature. The pH values of all dispersions were adjusted to 7.0 using HCl and NaOH (0.1 mol L−1). The prepared solutions were then refrigerated at 4°C overnight. To create the primary emulsion, a mixture of 20 wt% oil phase and 80 wt% aqueous phase of WP + MD was subjected to ultrasonication using an ultrasonicator (Hielscher, UP400S) at a frequency of 24 kHz and 50% amplitude for 10 min. The resulting primary emulsion was diluted using the SA solution, and the pH was adjusted to 5.0, resulting in the formation of a secondary emulsion with the following composition: 10 wt% oil, 1 wt% WP, 0.25 wt% SA, and 10 wt% MD. To prevent overheating, the work time and rest time for all sonication experiments were set at 0.5 s. Additionally, during sonication, the tubes containing the samples were kept in crushed ice to maintain the temperature at 23 ± 2°C.
The size of particles in the freshly prepared nanoemulsion was determined using dynamic light scattering (DLS) (Zetasizer Nano ZS, Malvern Instruments). Additionally, the zeta potential of the nanoemulsion was measured using the same instrument, Zetasizer Nano ZS (Malvern Instruments).[20] The acquisition of images of the nanoemulsions was carried out using field emission scanning electron microscopy (FESEM; SU-70, Hitachi) at an accelerating voltage of 15 kV and with different magnifications.
2.3 Treatments and sampling
A total of 180 male broilers of the Ross 308 breed, aged 1 day, were allocated into three groups randomly. Each group consisted of 4 replicates, with 15 birds in each replicate. The birds in each group were given different treatments: control (basal diet, NRC 2001; Table 1), FlxO (basal diet plus oral flaxseed oil at a dosage of 1 mL kg−1 body weight [BW]), or NanoFlxO (basal diet plus oral nanoemulsion flaxseed oil at a dosage of 1 mL kg−1 BW). These treatments were initiated on Day 21 of the trial. A small tube was attached to a 1-mL syringe for administering the treatments via gavage. Birds were reared under controlled conditions with a 14:10 light:dark cycle, according to Ross 308 broiler recommendation. Water and feed were provided without any restriction. At the end of the study, the weekly weighing of both the birds and their total feed intake was used for the calculation of the feed conversion ratio (FCR) for each group. The FCR was calculated by dividing the grams of feed intake by the grams of live BW.
| Item | Starter (Days 1–21) | Grower (Days 22–42) |
|---|---|---|
| Corn | 56.00 | 62.26 |
| Soybean meal (CP = 44%) | 37.42 | 31.29 |
| Soybean oil | 2.82 | 3.13 |
| Dicalcium phosphate | 1.41 | 1.05 |
| Calcium carbonate | 1.28 | 1.38 |
| Vitamin permixa | 0.25 | 0.25 |
| Trace mineral permixb | 0.25 | 0.25 |
| DL-methionine | 0.15 | 0.07 |
| Salt | 0.42 | 0.32 |
| Chemical composition | ||
| ME (kcal kg−1) | 2950.00 | 3050.00 |
| CP (%) | 21.22 | 19.06 |
| Ca (%) | 0.92 | 0.86 |
| Available P (%) | 0.41 | 0.33 |
| Na (%) | 0.18 | 0.14 |
| Digestible amino acids (%) | ||
| Lysine | 1.15 | 1.00 |
| Methionine + Cysteine | 0.83 | 0.69 |
| Methionine | 0.48 | 0.37 |
| Threonine | 0.81 | 0.72 |
- a Supplied per kg diet: vit A, 3600 000 IU; vit D3, 800 000 IU; vit E, 9000 IU; vit K3, 1600 mg; vit B1, 720 mg; vit B2, 3300 mg; vit B3, 4000 mg; vit B5, 15 000 mg; vit B6, 150 mg; vit B9, 500 mg; vit B12, 600 mg; and biotin, 200 mg.
- b Supplied per kg diet: Mn, 50 000 mg; Fe, 25 000 mg; Zn, 50 000 mg; Cu, 5000; I, 500 mg; and choline chloride, 134 000 mg.
At the end of the experiment, a total of eight birds from each treatment group with BW close to the pen mean were selected and euthanized through cervical dislocation. Subsequently, small slices of liver tissue were promptly cut and immediately frozen in liquid nitrogen (N2). These frozen tissue samples were then stored at a temperature of −80°C until they could be analyzed for gene expression. To determine the fatty acid profile, samples from the breast and thigh muscles were carefully trimmed to remove any excess fat. These trimmed samples were then securely wrapped in plastic bags and stored at a temperature of −20°C until they could be analyzed.
2.4 Fatty acid profile
The basal diet samples, as well as the fat-free tissue fractions, were subjected to homogenization using a 2:1 mixture of chloroform and methanol, v/v, to extract total lipids. The fatty acids were then methylated following the method described by Eratte et al.[21] and subsequently analyzed using a gas chromatograph system (GC-4600). The GC operation conditions involved an initial temperature of 180°C for 9 min, followed by an increase to 200°C for 25 min. Helium was employed as the carrier gas at a flow rate of 0.95 mL min−1. Identification of individual fatty acids was accomplished by comparing their retention times with those of fatty acid methyl ester standards. The resulting data were expressed as percentages relative to the total fatty acids. Moreover, the synthesis and deposition efficiency of ALA and LCPUFAs in thigh and breast muscles of broilers fed two flaxseed oil forms during the grower period (Days 21–42) were determined using the following equation:
Fatty acid deposition efficiency in tissue = [fatty acid % in tissue/(fat% in diet × % fatty acid in diet)] × 100.
2.5 Hepatic gene expression
The present study investigated the candidate genes lipoprotein lipase (LPL), acyl-CoA oxidase 1 (ACOX1), and carnitine palmitoyltransferase 1 (CPT1). Total ribonucleic acid (RNA) extraction and copy DNA (cDNA) synthesis were performed using an RNA extraction kit (BioFLUX) and a reverse transcription kit (Fermentase), respectively, following the manufacturer's instructions. The gene-specific primers were designed using primer3 software (Table 2), and the details were obtained from the National Center for Biotechnology Information gene bank. For the quantification of all transcripts, a 10-µL reaction buffer was prepared, consisting of 5 µL SYBR Green Mix, 0.2 µL cDNA, 0.6 µL of forward and reverse primers, 0.2 µL of master mix, and 2 µL of double-distilled H2O. To standardize the real-time polymerase chain reaction products, beta-actin was used as a housekeeping gene.
| Genea | Accession number | Sequence (5′ → 3′) | Annealing temp (°C) | Product length (bp) |
|---|---|---|---|---|
| LPL | NM-205282 |
F: TTGGTGACCTGCTTATGCTA R: ATTGCTGCCTCTTCTCCTTT |
58.15 | 187 |
| CPT1 | AY675193 |
F: GCCCTGATGCCTTCATTCAA R: ATTTTCCCATGTCTCGGTGA |
57.80 | 118 |
| ACOX1 | NM-001006205 |
F: CCAGTCAGCTTGGTAGAGGC R: AGTGACAGTGTGCCTCAGATG |
58.05 | 160 |
| -actin | NM-205518.1 |
F: AAGTTACTCGCCTCTGTGAAGG R: CACATCTATCACTGGGGAACAC |
59.00 | 198 |
- a LPL: lipoprotein lipase; ACOX1: acyl-CoA oxidase 1; CPT1: carnitine palmitoyltransferase 1; -actin: internal control.
2.6 Statistical analysis
In the present study, a completely randomized design was implemented, and the general linear model procedure of SAS software (SAS, 9.4) was employed to analyze the obtained data. To compare means, Tukey's honest significant difference test was utilized, with a significance level set at p < 0.05.
3 RESULTS
3.1 Fatty acid profiles of basal diet and flaxseed oil
Table 3 illustrates the fatty acid compositions in the basal diet and flaxseed oil. Linoleic acid (LA, C18:2 ω−6) was the predominant fatty acid in the basal diet, whereas ALA (C18:3 ω−3) was the primary constituent in flaxseed oil. The proportions of omega-3 PUFAs were 5.80% in the basal diet and 40.37% in flaxseed oil. The ratio of omega-6-to-omega-3 PUFAs in the basal diet and flaxseed oil indicated a balanced source of these essential fatty acids in flaxseed oil, whereas the basal diet contained higher levels of omega-6 PUFAs. This higher content of omega-6 PUFAs in the basal diet can be attributed to the increased use of grains, which are rich in omega-6 fatty acids, by the modern poultry industry.
| Item | Basal diet | Flaxseed oil |
|---|---|---|
| Myristic acid | 0.11 | ND |
| Palmitic acid | 14.56 | 7.05 |
| Margaric acid | 0.07 | ND |
| Stearic acid | 4.30 | 4.23 |
| Palmitoleic acid | 0.17 | ND |
| Oleic acid (ω9) | 16.90 | 26.85 |
| Linolenic acid (ω6) | 58.04 | 21.49 |
| Alpha-linolenic acid (ω3) (ALA) | 5.80 | 40.37 |
| ω6/ω3 | 10.00 | 0.53 |
| PUFA/SFA | 3.35 | 5.48 |
| MUFA/PUFA | 0.26 | 0.43 |
- Abbreviations: MUFA, monounsaturated fatty acids; ND, not detected.; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; ω3, omega-3; ω6, omega-6; ω9, omega-9.
3.2 Characterization of nanoemulsions
The characterization of nano-encapsulated flaxseed oil was reported previously.[22] In brief, DLS analysis indicated a tight distribution of droplet sizes, with an average diameter of 464 nm. Additionally, the zeta-potential measurement and FESEM image confirmed a consistent and non-aggregated structure. The distinctive shell-like structure of these particles is anticipated to offer enhanced protection to the bioactive compounds in the acidic conditions of the stomach, making them well suited for precise delivery to chickens.[23]
3.3 Growth performance
Table 4 provides a summary of the influence of dietary treatments on growth performance. Control birds, FlxO, and NanoFlxO groups had nonsignificant BW on Days 1 and 21. On Day 42, however, BW in FlxO and NanoFlxO birds was significantly higher than control birds. Although there was no significant difference observed in the average daily gain and average daily feed intake among the treated birds, the FCR exhibited a significant improvement in the birds that were administered flaxseed oils.
| Traits | Body weight (g) | ADFI (g day−1) | ADG (g day−1) | FCR (g g−1) | ||
|---|---|---|---|---|---|---|
| 1 day | 21 days | 42 days | ||||
| Control | 45.20 | 494.50 | 2052.20b | 58.50 | 98.4 | 1.68a |
| FlxO | 45.50 | 498.40 | 2632.70a | 61.60 | 98.1 | 1.59b |
| NanoFlxO | 46.00 | 504.20 | 2658.40a | 62.2 | 97.7 | 1.57b |
| SEM | 0.56 | 13.40 | 36.50 | 0.67 | 1.65 | 0.05 |
- Note: Control: basal diet; FlxO: basal diet plus l mL kg−1 BW flaxseed oil; NanoFlxO: basal diet plus l mL kg−1 BW nanoflaxseed oil.
- Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio; SEM, means standard error of the mean.
- A,bDifferent superscripts within a column indicate a significant difference (p < 0.05).
3.4 Fatty acid profile of thigh and breast muscles
Although total SFA of thigh tissue was not significantly affected by treated groups, birds in NanoFlxO had significantly lower heptadecanoic acid (C17:0) than control and FlxO birds. Considering breast muscle, however, birds that received flaxseed oils compared to the control group had lower total SFA, heptadecanoic (C17:0), and hexadecenoic (C16:0) acids. The use of typical or nanoflaxseed oil did not significantly impact the total monounsaturated fatty acids and PUFA contents in the thigh and breast muscles. Nonetheless, an analysis of the fatty acids present in the thigh and breast muscles of birds that were given NanoFlxO showed a marked increase in n − 3 fatty acids, including ALA, eicosatrienoic acid (C20:3n − 3), docosapentaenoic acid, and DHA, when compared to the control group. A reversal in the trend was observed concerning n − 6 fatty acids, such as arachidonic (C20:4n − 6) and eicosatetraenoic (C22:4n − 6) acids. The use of nanoflaxseed oil led to a significant increase in long-chain n − 3 PUFA and PUFA/SFA ratio in both thigh and breast muscles compared to standard flaxseed oil and the control group of birds (Table 5).
| Fatty acids | Thigh muscle | SEM | Breast muscle | SEM | ||||
|---|---|---|---|---|---|---|---|---|
| Control | FlxO | NanoFlxO | Control | FlxO | NanoFlxO | |||
| C14:0 | 0.47 | 0.46 | 0.42 | 0.007 | 0.67A | 0.67A | 0.60B | 0.002 |
| C16:0 | 20.67 | 20.63 | 20.38 | 0.141 | 23.55A | 23.19B | 23.00B | 0.112 |
| C17:0 | 0.27a | 0.26a | 0.22b | 0.009 | 0.30A | 0.25B | 0.24C | 0.003 |
| C18:0 | 8.28 | 8.25 | 8.20 | 0.091 | 8.96 | 8.88 | 8.64 | 0.129 |
| ∑SFA | 29.67 | 29.61 | 29.22 | 0.206 | 33.49A | 32.99B | 32.49B | 0.218 |
| C16:1 | 4.10 | 4.03 | 4.00 | 0.058 | 3.42 | 3.41 | 3.31 | 0.144 |
| C18:1 | 36.45 | 36.41 | 36.28 | 0.165 | 30.69 | 30.67 | 30.50 | 0.194 |
| ∑MUFA | 40.56 | 40.44 | 40.28 | 0.181 | 34.11 | 34.08 | 33.81 | 0.330 |
| C18:2 n − 6 (LA) | 25.86 | 25.83 | 25.63 | 0.248 | 24.95 | 24.84 | 24.71 | 0.357 |
| C18:3 n − 3 (ALA) | 1.66b | 1.92b | 2.53a | 0.115 | 1.47C | 1.75B | 1.92A | 0.032 |
| C20:3 n − 6 | 0.26a | 0.22b | 0.20c | 0.007 | 0.36 | 0.36 | 0.34 | 0.021 |
| C20:3 n − 3 | 0.10c | 0.14b | 0.18a | 0.005 | 0.28B | 0.30B | 0.36A | 0.007 |
| C20:4 n − 6 (AA) | 1.38a | 1.33b | 1.30b | 0.013 | 4.04A | 3.86B | 3.72B | 0.079 |
| C20:5 n − 3 (EPA) | 0.00 | 0.00 | 0.00 | 0.000 | 0.00 | 0.07B | 0.19A | 0.001 |
| C22:4 n − 6 | 0.22a | 0.21a | 0.19b | 0.004 | 0.64A | 0.60B | 0.59B | 0.008 |
| C22:5 n − 3 (DPA) | 0.11b | 0.13b | 0.29a | 0.008 | 0.35C | 0.56B | 1.21A | 0.019 |
| C22:6 n − 3 (DHA) | 0.13 | 0.14 | 0.14 | 0.003 | 0.24C | 0.55B | 0.63A | 0.012 |
| ∑PUFA | 29.73 | 29.94 | 30.50 | 0.442 | 32.39 | 32.91 | 33.67 | 0.586 |
| ∑PUFA n − 3 | 2.01b | 2.34b | 3.18a | 0.127 | 2.40C | 3.24B | 4.32A | 0.047 |
| ∑PUFA n − 6 | 27.72 | 27.60 | 27.32 | 0.338 | 29.99 | 29.67 | 29.37 | 0.541 |
| PUFA/SFA | 1.00b | 1.01b | 1.04a | 0.009 | 0.96B | 0.99B | 1.03A | 0.011 |
| MUFA/PUFA | 1.36 | 1.35 | 1.32 | 0.173 | 1.05A | 1.03A | 1.00B | 0.009 |
| LA/ALA | 15.57 | 13.45 | 10.13 | 0.045 | 16.97A | 14.19A | 12.86B | 0.045 |
| n − 6 LC-PUFA | 1.86 | 1.76 | 1.69 | 0.012 | 5.04 | 4.82 | 4.65 | 0.015 |
| n − 3 LC-PUFA | 0.34b | 0.41b | 0.64a | 0.006 | 0.91C | 1.48B | 2.39A | 0.015 |
| PUFA n − 6/n − 3 | 13.78a | 11.81b | 8.59c | 0.256 | 12.49A | 9.15B | 6.79C | 0.154 |
- Note: Control: basal diet, FlxO: basal diet plus 1 mL kg−1 BW flaxseed oil; NanoFlxO: basal diet plus 1 mL kg−1 BW nanoflaxseed oil. Means bearing non-similar superscripts (a–c, A–C) in each row differ significantly (p < 0.05).
- Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; LCPUFA, long-chain polyunsaturated fatty acids; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
- A–CDifferent superscript capital letters within the same row of breast muscle indicate significant differences (p < 0.05).
- a–cDifferent superscript lowercase letters within the same raw of thigh muscle indicate significant differences (p < 0.05).
3.5 The efficiency in synthesizing omega-3 fatty acids
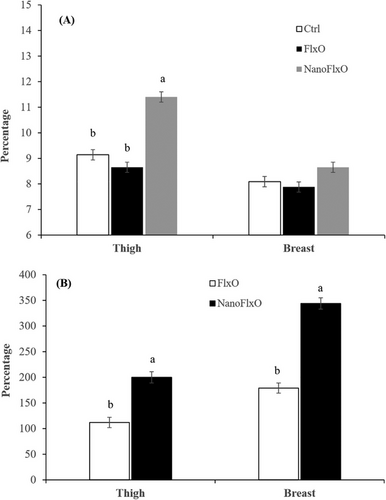
Figure 1 illustrates the efficiency of the synthesis and deposition of ALA and LCPUFAs in the thigh and breast muscles of broilers fed two flaxseed oil forms during the grower period (Days 21–42). Broilers that were fed NanoFlxO exhibited a higher ALA deposition in the thigh muscle compared to those fed basal or FlxO diets. However, there was no significant difference in ALA deposition in the breast muscle among the different treatments. Furthermore, the consumption of NanoFlxO resulted in increased levels of LCPUFA in both thigh and breast muscles compared to FlxO diet.
3.6 Hepatic gene expression
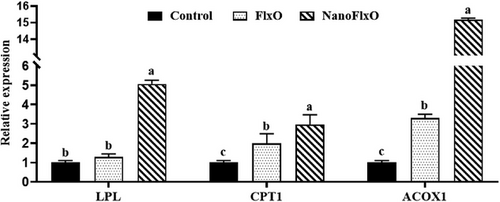
Relative expressions of candidate genes are presented in Figure 2. The transcript levels of ACOX1 and CPT1 were significantly higher (p < 0.05) in the NanoFlxO group compared to both the control and FlxO groups. A more than 15-fold increase in the ACOX1 transcript was noted in the hepatic tissue of NanoFlxO compared to control birds. Although LPL gene expression was not significantly affected by flaxseed oil treatment, a ≈5-fold increase was observed in NanoFlxO birds.
4 DISCUSSION
Functional foods are becoming increasingly popular as people are more concerned about their diet's health potential and nutrient composition. The present study evaluated the efficacy of two types of flaxseed oil to enhance the n − 3 fatty acid content in poultry meat. Irrespective of bulk- or nano-form, broilers’ BW and FCR improved after oral flaxseed oil administration. Duarte et al.[24] obtained similar results when fed broilers with isoenergetic diets containing 3.3%, 6.6%, or 9.9% flaxseed at Days 21–42.
In contrast, broilers fed flaxseed for 28, 36, 42, or 46 days notably decreased BW and weight gain. However, their feed intake and FCR were higher compared to broilers that were given corn-soybean, rapeseed, chia seed, or chai meal.[25] In another study, BW, weight gain, feed intake, and FCR of broilers were not significantly affected following dietary supplementation of flaxseed oil for various periods of 35–42, 28–42, 21–42, 14–42, 7–42, or 1–42 days.[9] These contradicting results would arise from the presence of anti-nutrients, such as non-starch polysaccharides, mucilage, cyanogenic glycosides, and vitamin B6 antagonists, in the flaxseed or resulting from various levels, sources, and administration routes.[18, 26] A significant reduction in the total SFA content of breast meat in flaxseed oil treatments suggests the incorporation of PUFA in chicken meat at the expense of SFA.[9] In addition, the suppressive effect of n − 3 fatty acids on lipogenic enzymes (e.g., acetyl-CoA carboxylase and fatty acid synthase) was previously reported.[27] The probable reason for the reduction of C17:0 content in both thigh and breast meat and C14:0 and C16:0 in the breast muscle might be their role as substrates of elongase and desaturase enzymes to synthesize long-chain unsaturated fatty acids.[28] Total n − 3 LCPUFA was almost doubled in NanoFlxO in both thigh and breast muscles. In accordance with this, the incorporation rate of n − 3 LCPUFA was twice as high in broilers fed NanoFlxO compared to FlaxO, suggesting the promising ability of nanostructured flaxseed oil in transferring ALA into the cellular milieu. This finding aligns with the hypothesis of converting dietary ALA into LCPUFA in broilers.[29] Desaturation and elongation are steps in converting LA and ALA to their receptive liquid chromatography n − 6 and n − 3 PUFAs. Even though Δ−6 desaturase has a greater affinity for ALA, a higher concentration of LA in the cytoplasm could lead to a higher conversion of LA to n − 6 LCPUFA.[30] Thus, providing more ALA as a substrate would favor the production of n − 3 LCPUFA. Further, dietary flaxseed or its oil supplementation upregulated ∆−5 desaturase, ∆−6 desaturase, and elongase 2 transcript levels, resulting in higher LCPUFA in egg yolk[6] or poultry meat.[31] During the production period of broilers (Days 1–37), incorporating 12% flaxseed into their diet led to a notable enhancement in the EPA content (3-fold) of breast or thigh meat and the DHA content (1.8-fold) of the breast.[32] Hence, the inclusion of ALA in the present study could serve as a method to offer a precursor for n − 3 LCPUFA and to diminish the transformation of medium-chain n − 6 fatty acids into their long-chain forms.
Higher EPA and DHA contents of breast muscle compared to thigh muscle are related to the source in which they accumulate. EPA and DHA are preferentially incorporated in phospholipid, being in higher proportion in the breast muscle, but ALA mainly participates in triglyceride (TG), being in higher proportion in the thigh muscle.[33] The n − 6/n − 3 ratio in breast and thigh meat decreased in both oil treatments, with the reduction primarily attributed to the significant increase in n − 3 PUFA, particularly notable in the NanoFlxO group.
The modulation of transcript levels of genes associated with fatty acid metabolism was observed when utilizing flaxseed oil, specifically in its nano-form.[34] De novo lipogenesis in birds primarily takes place in the liver and is subsequently stored in the adipose tissue.[34] Specifically, the enzyme LPL facilitates the conversion of the TG core of very-low-density lipoprotein (VLDL) into fatty acids and glycerol. These fatty acids are then transported to the neighboring tissues for either β-oxidation or storage, such as in the adipose tissue. In the adipose tissue, they undergo re-esterification and are stored as TGs. In the present study, a fivefold increase in the LPL gene expression reveals the effectiveness of reducing serum TG and VLDL contents. This impact is intricately linked to atherosclerosis and cardiovascular ailments in humans. This effect was confirmed by Shahid et al.,[6] who suggested that higher hepatic LPL mRNA is associated with reduced blood TG, VLDL, and low-density lipoprotein levels in laying hens.
CPT1, an enzyme found in the outer membrane of mitochondria, facilitates the transformation of long-chain acyl-CoA into their respective long-chain acyl-carnitine. This enzymatic process serves as a crucial step in the beta-oxidation of fatty acids, acting as a rate-limiting factor.[35] As a consequence, the highest expression of this enzyme in the liver reflects increased oxidation of fatty acids in this group. It is worth noting that dietary EPA has the intriguing ability to enhance both mitochondrial and peroxisomal β-oxidation, whereas DHA primarily stimulates peroxisomal β-oxidation. EPA, but not DHA, is effective in stimulating mitochondrial CPT1 activity, an indicator of fatty acid β-oxidation.[36] In line with our results, feeding flaxseed oil upregulated hepatic CPT1 mRNA after a 12-week feeding period in Hyline laying hens.[6] Flaxseed oil did not show any notable impact on the hepatic CPT1 of laying hens when compared to our data.[37]
Beta-oxidation is a primary pathway for fatty acid degradation in mitochondria and peroxisomes. Mitochondria play a crucial role in the degradation of short-, medium-, and long-chain fatty acids, whereas peroxisomes are responsible for processing very long-chain fatty acids. Acyl-CoA oxidase serves as the primary and controlling enzyme in the peroxisomal β-oxidation pathway.[38] In consistency with the findings of Abbasi et al.,[39] the current study demonstrates that utilizing the nanoemulsion form of flaxseed oil led to elevated ALA levels in the liver, consequently enhancing the expression of the ACOX1 gene. These data support the hypothesis that submicron bioactive components have the potential to enhance cellular ALA bioavailability. Likewise, supplementing a 10% flaxseed diet with carbohydrate enzymes to reduce the antinutritive effects was more effective in stimulating the β-oxidation pathway by enhancing CPT1 and ACOX1 transcript levels.[18]
5 CONCLUSION
The results of our study demonstrated a favorable increase in the n − 3 LCPUFA levels in poultry meat within real-world or business settings. The inclusion of significant amounts of EPA and DHA in animal-derived foods could be advantageous for individuals who prioritize their health. Additional investigation is necessary to elucidate the fundamental processes, ideal levels, and feeding schedules required to ensure consistent output while reducing expenses and mitigating any potential negative impacts on nutrition.
AUTHOR CONTRIBUTIONS
Seyedeh Fatemeh Miraeez: Investigation; methodology; formal analysis. Fatemeh Abbasi: Investigation; methodology; data curation. Mahdi Ansari: Writing—original draft. Firooz Samadi: Investigation; conceptualization; resources; methodology; validation; formal analysis; supervision; project administration. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We express our gratitude to Gorgan University of Agricultural Sciences and Natural Resources for valuable assistance in providing the necessary facilities.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All the data produced or examined throughout this investigation have been incorporated within this manuscript.




