Strategies for Enzymatic Synthesis of Omega-3 Structured Triacylglycerols from Camelina sativa Oil Enriched in EPA and DHA
Abstract
Enzymatic production of enriched eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) structured triacylglycerols (STAGs) is done in a two-step reaction from a new source of omega-3 α-linolenic acid (ALA), Camelina sativa oil. A fast-pressurized liquid extraction method with ethyl acetate as green solvent is used to produce camelina oil with a 39.3% yield in less than 20 min. In a first stage, camelina oil with a 29.7% of ALA is used to enzymatically produce 2-monoacylglycerols (2-MAGs) with high ALA composition in position sn-2 (49.8% of ALA) using Lipozyme TL IM at 25 °C. For omega-3 incorporation into STAGs, several lipases are studied, with 2-MAG and omega-3 FAEEs at different ratios (1:5, 1:10, and 1:20 (w/w)), 10% (w/w) of biocatalyst at 35 °C. STAGs yields reached with EPA ethyl esters are notably higher than using DHA-EE. Lipase from Candida antarctica B (CAL-B) and Thermomyces lanuginosus (TLL) incorporate EPA into STAGs in the same degree, 95.1 and 95.5%, respectively. However, for enriched DHA STAGs, maximum reaction yield is reached with CAL-B (81.0%). The transesterification method of 2-MAGs from vegetable omega-3 sources like camelina oil, with marine EPA and DHA FAEEs, opens new approaches for developing VLC-PUFA fortified functional foods.
Practical Applications: Results provided in this research not only demonstrate the efficiency of this enzymatic method for enriching camelina oil in EPA and DHA, but offer a very effective strategy for the development of omega-3-enriched products from other vegetable oil sources like chia or echium as ingredients for functional foods with recognized health claims for applications in nutraceutical and food industries. Camelina oil proved to be an excellent source to enzymatically produce 2-MAGs enriched in omega-3 ALA. Moreover, lipase screening with commercial biocatalysts establish the most appropriate strategy to reach maximum STAGs yields with direct application in industry. Tested lipases exhibit different degree of substrate specificity toward EPA and DHA, omega-3 acids not present in natural seed oils. Thus, CAL-B seems to be the most suitable lipase to produce high content EPA and DHA enriched STAGs from camelina oil as new food ingredients for nutraceutical applications.
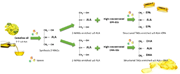
Enzymatic production of structured TAGs enriched in EPA and DHA is done in a two-step reaction from a natural source of omega-3 ALA, camelina oil, studying reaction kinetics and characterizing isolated products.
1 Introduction
Camelina sativa L. is an oilseed plant member of the Brassicaceae family with a renewed interest nowadays, due to the fact that the crop is adaptable to many different environmental conditions and require relatively low inputs.1-3 The main product of Camelina sativa is the oil extracted from the seeds, which contain about 35–40% in dry matter, and a very attractive composition in comparison to common edible oils. In addition, the European Commission and the Food and Drug Administration (FDA) allowed to use refined camelina oil as a food or food ingredient and consider it as an excellent source of omega-3. The composition of camelina seed oil is characterized for a high percentage of long chain fatty acids such as α-linolenic acid (ALA, C18:3n-3) (30%), gondoic acid (19%), oleic acid (17%), and linoleic acid (17%).4
The omega-3 ALA is considered an essential fatty acid with an important role in human nutrition.5, 6 Nevertheless, ALA is only one of the three major dietary omega-3 fatty acids recommended by health authorities in order to assure an optimal omega-6/omega-3 balance.7 In general, Western diets are deficient in omega-3 fatty acids and have excessive amounts of omega-6 fatty acids. Therefore, there is a need to promote the consumption of omega-3, and specially omega-3 very long chain polyunsaturated fatty acids (omega-3 VLC PUFAs) such us eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3). Numerous studies have reported the beneficial effects of EPA and DHA in human health related to maintenance normal blood pressure and triglyceride levels, prevention of cardiovascular diseases, and also, for the development of eyes and infant brain.8-11 However, EPA and DHA are not present in natural sources of vegetable oils, aside from genetically modified crops.12, 13
Most evidence indicates that omega-3 PUFAs can be digested more effectively in form of triacylglycerols (TAGs) as compared to those in form of free fatty acids (FFAs) and fatty acid ethyl esters (FAEEs).14, 15 Structured triacylglycerols (STAGs) are generally defined as TAGs that have been modified to change the fatty acid composition and/or their positional distribution in glycerol backbone by chemical or enzymatic reactions.16 Lipases are powerful tools for the syntheses of STAGs as alternative to chemical methods, because only mild reaction conditions are required, and the specificity of lipases allows better control over product characteristics.17 For the last few decades, designed STAGs with improved nutritional properties have received much attention as functional foods satisfying the current consumer demand.18 Based on the foregoing considerations, the incorporation of ALA, EPA, and DHA into STAGs could be relevant for a range of potential applications in human nutrition for food and nutraceutical industry.
In this work, an enzymatic production of STAGs enriched in EPA and DHA was done in a two-step reaction to obtain higher purities and yields. First, TAGs (camelina oil) were subjected to enzymatic ethanolysis rendering 2-monoacylglycerols (2-MAGs) rich in ALA. In a second step, enzymatic transesterification between 2-MAGs and EPA or DHA FAEEs was catalyzed by different commercial lipases. A two-step enzymatic reaction was also successfully used to obtain other STAGs.19-24 In addition, used camelina oil was extracted in our laboratory from camelina seeds with pressurized liquids using ethyl acetate at optimal conditions previously described for similar vegetable oilseeds.25, 26 Therefore, the aim of this work was to develop a highly efficient strategy for the enzymatic production of STAGs enriched in EPA and DHA as ingredients for functional foods with recognized health claims, from a natural source of omega-3 ALA like camelina oil.
2 Experimental Section
2.1 Materials
Camelina seeds (Camelina sativa L.) were provided by Camelina Company España (Madrid, Spain). Seeds were ground with a particle size less than 500 µm using a grinder (Moulinex-A320R1 700 W) and stored at 4 °C until their use.
Commercial lipases such us Novozym 435 (lipase from Candida antarctica B), Novozym 40086 (lipase from Rhizomucor miehei) and Lipozyme TL IM (lipase from Thermomyces lanuginosus) were kindly donated for Novozymes (Bagsvaerd, Denmark). EnerZona Omega 3 Rx – liquid (high concentrated EPA ethyl esters (EPA-EEs)) was purchased from Enervit S.p.A (Milano, Italy). FAEEs containing 80% DHA (high concentrated DHA ethyl esters (DHA-EEs)) were provided by Nutra Omega Biotecnológica Oleica, S.L. (NOBO) (A Coruña, Spain). Absolute ethanol (PRS grade), sodium hydrogen carbonate and potassium hydroxide were purchased from Panreac Quimica S.A (Barcelona, Spain). Molecular sieves pore size 4 Å, n-hexane and ethyl acetate were purchased from Scharlau (Barcelona, Spain). The solvents (2,2,4-trimethyl pentane, methyl tert-butyl ether, and 2-propanol) used for high-performance liquid chromatography (HPLC) analyses were HPLC-grade and purchased from LABSCAN (Dublin, Ireland). Fatty acid methyl esters standard (Supelco 37 FAME Mix) was from Supelco (Bellefonte, PA, USA). Glyceryl trilinoleate, dioleoylglycerol (mixture of 1,3- and 1,2-isomers), 1-oleoyl-rac-glycerol, oleic acid and ethyl linoleate used as HPLC standards was purchased from Sigma–Aldrich (St. Louis, MO, USA). All other reagents and solvents used were of analytical or HPLC grade.
2.2 Pressurized Liquid Extraction of Camelina Sativa Seed Oil
Pressurized liquid extraction was carried out with an ASE 350 DIONEX (Sunnyvale, California) extractor. Oil extraction was performed using 3.00 g of ground camelina seeds. Stainless steel extraction cells were used with a capacity of 10 mL. Extracts were collected under a nitrogen stream in different vials of 50 mL. Extraction conditions used were performed according to previous studies using ethyl acetate as solvent at 120 °C and 10 min of static time.21
The samples were evaporated in a rotary evaporator (Heidolph Hei-Vap Value HB/G3, Germany) under reduced pressure at 40 °C and dried under a nitrogen stream until constant weight. The oil content was determined gravimetrically and expressed as dry weight percentage. Oil obtained was stored in dark vessels with nitrogen atmosphere at 4 °C until their use.
2.3 Enzymatic Synthesis of 2-MAGs from Camelina Oil
Enzymatic synthesis of 2-MAGs was performed by ethanolysis reaction. In a typical experiment, camelina oil and ethanol (ratio 1:4 (w/w)) and 15% (w/w) of the biocatalyst Lipozyme TL IM were place in glass vials. The reaction was carried at 25 °C with constant stirring in an orbital shaker at 200 rpm (Unimax 1010, Heidolph, Germany) under dark conditions. A negative control without biocatalyst was carried out at the same conditions previously described. The reaction mixture was filtered to remove the biocatalyst and the excess of ethanol was removed under vacuum. The synthesis of 2-MAGs was followed by HPLC-ELSD. Reaction kinetics were done at least in duplicate. 2-MAGs obtained were stored in dark vessels with nitrogen atmosphere at 4 °C until their use.
2.4 Transesterification Between 2-MAGs and EPA/DHA-Ethyl Esters Catalyzed by Different Commercial Lipases
Transesterification between the synthesized 2-MAGs and omega-3 FAEEs was performed to produce STAGs. In a typical experiment, ethanolysis reaction product (2-MAGs) and omega-3 FAEEs at different ratios (1:5, 1:10, and 1:20 (w/w)), 10% (w/w) of biocatalyst and molecular sieves were place in glass vials. The reaction was carried out at 35 °C with constant stirring in an orbital shaker at 200 rpm (Unimax 1010, Heidolph, Germany) under dark conditions. A negative control without biocatalyst was carried out at the same conditions previously described. The synthesis of STAGs was followed by HPLC ELSD, and reaction yields were calculated relative to the concentration of starting acylglycerol reactants and products and quantifying each separated lipid species by HPLC method. Reaction kinetics were done at least in duplicate.
2.5 Lipid Fractionation by Solid Phase Extraction
Reaction products were fractionated using solid phase extraction (SPE) with ISOLUTE NH2 columns (aminopropyl bonded sorbent). SPE columns were preconditioned with 4 mL of hexane. The sample (50 mg reaction product/500 mL hexane) was allowed to adsorb to the matrix by percolation through the cartridge by gravity. SPE column was washed with 10 mL of solvent A (hexane), 4 mL of solvent B (hexane/isopropanol (10:1)), and 4 mL of solvent C (hexane/isopropanol (3:1)). The fractions eluted from the SPE column were dried down under nitrogen and re-dissolved to analysis by HPLC-ESLD. FAEEs were eluted in fraction 1, TAGs and diacylglycerols (DAGs) in fraction 2, and 2-MAGs in fraction 3.
2.6 HPLC-ELSD Analysis
The lipid composition as the enzymatic reaction time proceeded was kinetically controlled and analyzed by HPLC-ELSD. Time reaction samples were taken after 0, 0.3, 0.7, 1, 2, 3, 4, 6, 8, and 24 h. Samples were stored at −20 °C until their analysis.
HPLC-ELSD analyses were performed using an Agilent 1260 Infinity HPLC equipped with an Agilent 385 (Palo Alto, CA, USA) ELSD instrument. The chromatographic separation of FAEE, TAGs, DAGs, and MAGs was performed with a silica normal-phase ACE (250 × 4.6 mm2 i.d., 5 μm) column maintained at 30 °C using a ternary gradient as follows27: 0–2 min, 99.5% A and 0.5% B; at t = 6.5 min, 70% A and 30% B; at t = 11 min, 63% A, 27% B, and 10% C; at t = 18 min, 99.5% A and 0.5% B; and at t = 20 min, 99.5% A and 0.5% B. Eluent A consisted of 2,2,4 trimetilpentane, eluent B consisted of methyl tert-butyl ether, and eluent C consisted of 2-propanol. The flow rate was 2.0 mL min−1 except for minutes 13–16 which was 1.0 mL min−1. Optimal signal and resolution were achieved with the following ELSD conditions: evaporator temperature = 30 °C; nebulizer temperature = 30 °C; and evaporator gas N2 = 1.6 SLM.
2.7 Fatty Acids Composition by GC-MS
Fatty acids composition of camelina oil and reaction products were analyzed on an Agilent GC-MS series 5975 MSD (Palo Alto, Cal., USA) using a HP 88 capillary column (100 m × 0.25 mm, id 0.2 μm) (Agilent, CA, USA). Previous to analysis fatty acid methyl esters (FAMEs) were prepared by base-catalyzed methanolysis of the camelina glycerides (KOH in methanol). 1 μL sample was injected using a split ratio of 1:100. The column was held at 175 °C for 10 min after injection, the temperature programmed at 3 °C min−1 to 220 °C and held for 20 min more. Helium was used as gas carrier, at a constant column flow rate of 1.5 mL min−1. The injector temperature was 250 °C and the detector temperature was 230 °C. The mass spectrometer was operated at 70 eV with a mass range from 30 to 400 amu. Fatty acids were identified comparing their retention times and the mass spectra (NIST MassSpectral Library Version 2.0) with those obtained from the standards.
3 Results and Discussion
3.1 Oil Extraction Using Pressurized Liquids from Camelina Seeds
Camelina oil was extracted from camelina seeds using PLE in our laboratory. The best oil yield was obtained at 120 °C (39.3% ± 0.1) with ethyl acetate as solvent. PLE results were compared with an oil extraction reference method, Soxhlet extraction with hexane, achieving a similar oil yield (39.5% ± 0.6) (no significant differences at 1% level). Besides of using a green solvent as ethyl acetate, PLE showed a reduction in extraction time from 8 h of Soxhlet to less than 20 min, and a decrease in solvent consumption. Therefore, to follow the principles of green chemistry in relation to oil extraction and avoiding hazardous and toxic solvents,28 camelina oil for STAGs production was extracted using PLE with ethyl acetate.
3.2 Enzymatic Synthesis of 2-MAGs from Camelina Oil
In a first stage, synthesis of 2-MAGs from camelina oil by enzymatic ethanolysis was carried out. A selective hydrolysis of camelina oil using a 1,3-regiospecific lipase such as the lipase from Thermomyces lanuginosus (TLL)29 was used to produce 2-MAGs rich in ALA. Specifically, commercial TLL from Novozyme, Lipozyme TL IM, was applied.
To avoid the undesired acyl migration, which can be take place during hydrolysis of TAGs, reaction conditions were controlled. The reaction temperature is one of the major factors that favors acyl migration,30 therefore, ethanolysis reaction was done at low temperature (25 °C). Moreover, ethanolysis reaction was carried out in pure ethanol without the use of an additional solvent as reaction medium. Whereas, other authors described the production of 2-MAGs by ethanolysis using acetone31, 32 or hexane33 as reaction medium. The advantage of using pure ethanol, besides of being a green solvent allowed in food industry, lies in the fact that solvent is critical to acyl migration rate. Specifically, low solvent polarity was found to be the major factor implied on migration rate in the non-polar solvents.34
Figure 1 shows the lipid composition (TAGs, FAEEs, and 2-MAGs) analyzed by HPLC-ELSD as the reaction time proceed. Despite the low temperature, the reaction progressed quickly. After 40 min of reaction time, TAGs were completely hydrolyzed and DAGs were less than 1.5% (data not shown in Figure 1). The maximum theoretical percentage of 2-MAGs was reached in only 1 h of ethanolysis reaction, and 1-MAGs content was minimum (less than 2%). However, when increasing the reaction time, the percentage of 2-MAGs considerably decreased. The reduction of 2-MAGs percentage and the increase of FAEEs over reaction time suggest that 1-MAGs were produced due to acyl migration, and lipase continued its hydrolytic activity over the position sn-1-3 rendering more FAEEs. Thus, the optimal time for production of 2-MAGs from camelina oil was established at 1 h.
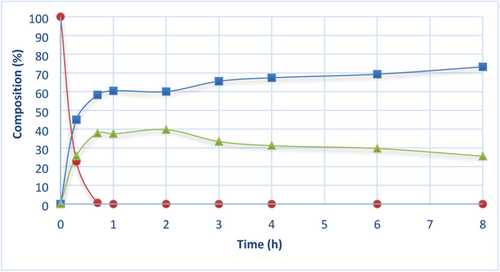
 ) TAGs, (
) TAGs, ( ) FAEEs, (
) FAEEs, ( ) 2-MAGs.
) 2-MAGs.After achieving the optimal time for the synthesis of 2-MAGs, ethanolysis reaction was scaled up 10 times respect to the initial reaction conditions. Similar 2-MAGs yields were produced after 1 h of reaction time. The scale facilitated the production of 2-MAGs for the transesterification reaction in the next step.
3.3 Characterization of Synthesized 2-MAGs by GC-MS
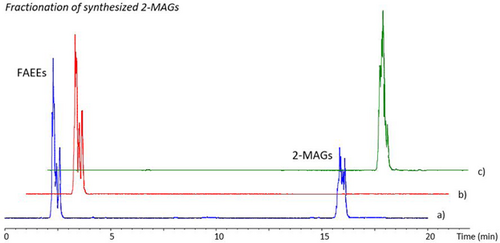
Once reaction conditions and optimal reaction time were determined for the 2-MAGs production from camelina oil, 2-MAGs were characterized by GC-MS. First, reaction mixture at optimal time to reach the maximum theoretical 2-MAGs (1 h) was fractionated by SPE using a polar stationary phase to purify 2-MAGs. Fractions obtained with the SPE were analyzed by HPLC-ELSD, an example chromatogram is shown in Figure 2. As can be seen, the fractionation procedure was successfully done by SPE. Original reaction products (Figure 2a) were composed by the mixture of 2-MAGs and FAEEs, and after the fractionation procedure a pure fraction of 2-MAGs was achieved (Figure 2c).
The purified 2-MAGs were analyzed by GC-MS to determine the fatty acids content and compare with the original camelina oil. Results of the GC-MS analysis are shown in Table 1. Camelina oil was characterized by a high percentage of polyunsaturated fatty acids (PUFA) (48.84%), followed by monounsaturated fatty acids (MUFA) (41.68%) and a very low content of saturated fatty acids (SFA) (9.48%). The major fatty acid (% of total fatty acids) was ALA, which accounted for 29.42%. ALA was followed by gondoic acid (20:1 n-9) 19.57%, oleic (18:1 n-9), and linoleic acid (18:2 n-6), 17.54 and 17.40% respectively, palmitic acid (16:0) 5.23%, erucic acid (22:1 n-9) 4.06%, and other minority fatty acids. Results were in accordance with other authors.35-37 In addition, erucic acid level was found to be lower than the maximum limit of 5% permitted in cooking oils.38
| % Fatty acidsa) | |||
|---|---|---|---|
| Fatty acid | RT (min) | Original oil | 2-MAGs |
| 16:0 | 12.9 | 5.23 ± 0.10 | – |
| 18:0 | 16.4 | 2.41 ± 0.01 | – |
| 18:1 n-9 | 17.5 | 17.54 ± 0.07 | 19.26 ± 0.29 |
| 18: 2 n-6c | 19.2 | 17.40 ± 0.03 | 30.96 ± 0.10 |
| 20:0 | 20.3 | 1.57 ± 0.10 | – |
| 18:3 n-3 | 21.3 | 29.42 ± 0.22 | 49.77 ± 0.39 |
| 20:1 n-9 | 21.5 | 19.57 ± 0.52 | – |
| 20:2 n-6 | 23.2 | 1.73 ± 0.19 | – |
| 22:0 | 24.3 | 0.27 ± 0.01 | – |
| 20:3 n-3 | 25.2 | 0.30 ± 0.01 | – |
| 22:1 n-9 | 25.4 | 4.06 ± 0.02 | – |
| 24:1 n-9 | 29.6 | 0.51 ± 0.01 | – |
| – | |||
| SFA | 9.48 | – | |
| MUFA | 41.68 | 19.26 | |
| PUFA | 48.84 | 80.74 | |
| n-6 | 19.13 | 30.96 | |
| n-3 | 29.72 | 49.77 | |
| n-6/n-3 ratio | 0.64 | 0.62 | |
- RT, retention time; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
- a) Results expressed as percentage over the total content (relative content). Values are the mean ± SD of two determinations.
On the other hand, the synthesized 2-MAG was composed by only three fatty acids: oleic acid, linoleic acid, and ALA. Specifically, the omega-3 ALA was the major fatty acid with a percentage of 49.77% ± 0.39. Thus, 2-MAGs was enriched in the omega-3 ALA 1.7 times compared with the original oil. Therefore, camelina oil was demonstrated to be an excellent source to produce 2-MAGs enriched in omega-3 with interest for food and nutrition applications.
3.4 Enzymatic Transesterification of 2-MAG with EPA/DHA Ethyl Esters
In a second stage, transesterification between the synthesized 2-MAGs from camelina oil with omega-3 FAEEs was done. The reaction was carried out in a solvent-free system acting FAEEs themselves as reaction medium. Solvents such us acetone, ethyl acetate, isooctane or hexane have been reported to improve lipase catalyzed reactions yields.39-41 However, recent trends imply the reduction of organic solvents consumption and promote the development of solvent-free alternatives. Other authors described that under solvent-free conditions reaction yields dramatically decrease.40, 42 Thus, to assure a high STAG yield in a solvent-free reaction medium, different 2-MAGs to FAEEs ratios were evaluated (see Section 3.4.1).
In addition, depending on the desired nutritional characteristics of the final product and therefore, the upcoming application of synthetized STAGs as functional foods, the impact of fatty acid chain length and the degree of unsaturation on esterification rate into STAGs was investigated. For this purpose, two different high omega-3 concentrated FAEEs were used: (1) high concentrated EPA ethyl esters (EPA-EEs), and (2) high concentrated DHA ethyl esters (DHA-EEs) (see Figure 3). After the GC-MS analysis, results determinate that high concentrated EPA-EEs were composed by 56.7% of EPA and 21.7% of DHA, and high concentrated DHA-EEs were composed by 4.8% of EPA and 87.4% of DHA.
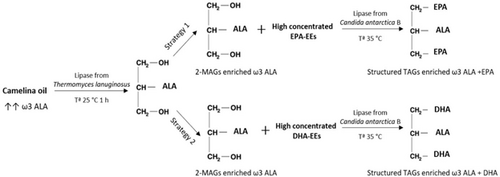
3.4.1 Optimization of Ratio 2-MAGs to Omega-3 FAEEs for STAGs Production
Different 2-MAGs to omega-3 FAEEs ratios (1:5, 1:10, and 1:20) were investigated to optimize the reaction conditions in solvent-free medium to produce the maximum efficiency yield in the shorter reaction time (Figure 4). One of the most well-studied lipase in a wide range of reactions was applied, lipase from Candida antarctica. Novozym 435 is the commercial isoform B of the lipase originating from Candida antarctica (CAL-B) immobilised on a hydrophobic carrier.43
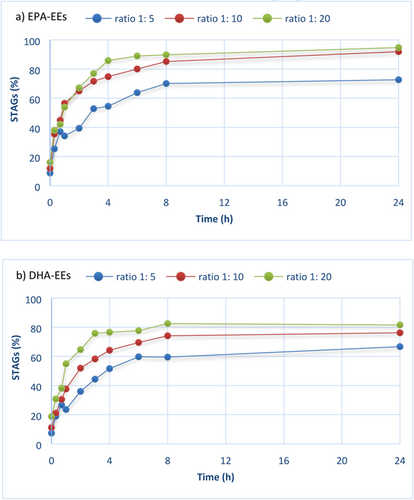
Figure 4a shows the effect of the different ratios investigated on the incorporation of high concentrated EPA-EEs into STAGs. As expected, CAL-B using the ratio 1:5 catalyzed the lowest reaction velocity in comparison with the other ratios tested. STAGs yields were 39.4, 54.5, and 70.1% after 2, 4, and 8 h of reaction time, respectively. Moreover, STAGs yield did not increase with the progress of transesterification reaction, achieving 72.6% of STAGs after 24 h. Hence, ratio 1:5 was not the best option to produce high purity STAGs in short reaction times. For ratios 1:10 and 1:20, the initial velocity of transesterification reaction was similar. After 2 h, STAGs yields were 65.0 and 67.1% (no significant differences at 5% level) in this case, respectively. However, it should be noted that significant differences were found after the first 4 h of reaction time, since STAGs yields were 85.8 and 74.8% for ratios 1:20 and 1:10, respectively, implying an increase of 12.8%. As the reaction time proceeded, CAL-B exhibited similar STAGs yields after 8 h, and finally, reaction was almost complete with a high purity of STAGs enriched in EPA after 24 h, with 94.75 and 91.9% for both 1:20 and 1:10 ratios, respectively. Therefore, results suggest that at short reaction times, such us 4 h, the best STAGs yield was achieved with the highest 2-MAGs to EPA-EEs ratio tested; however, no differences were found between ratios 1:20 and 1:10 for longer reaction times.
Production of STAGs enriched with DHA is considered an interesting lipid with application in developing new functional foods. So, the reaction conditions with DHA esters were studied to optimize the synthesis of TAGs with DHA in positions sn-1 and sn-3, and ALA in sn-2. Figure 4b shows the STAGs yield using high concentrated DHA-EEs. The maximal incorporation degree for DHA-EEs was reached with the ratio 1:20 and 1:10, followed by 1:5 ratio which is the same pattern as observed for high concentrated EPA-EEs. However, in this case remarkable differences between proportions 1:20 and 1:10 were found, especially in the initial velocity that was higher for esterification with relation 1:20. The highest DHA incorporation was achieved at the ratio 1:20 in 8 h of reaction time, 82.5% STAGs, with no increase at reaction time of 24 h. Thus, the use of 1:20 ratio implied a high initial velocity for the esterification of DHA-EEs into STAGs and was the optimal ratio for the esterification of DHA-EEs.
Comparing the incorporation degree of the two omega-3 FAEE investigated, STAGs yields achieved with DHA-EEs were significant minor than using EPA-EEs for all the tested ratios. For instance, after 4 h of reaction time and ratio 1:20, STAGs yield was 85.8% for high concentrated EPA-EEs and 76.5% for high concentrated DHA-EEs. Thus, EPA-EEs were more easily esterified than DHA-EEs. These results suggest the impact of omega-3 FAEEs type in the incorporation into STAGs. Most microbial lipases have been found EPA as better substrate than DHA.44, 45 This type of substrate specificity owing to the fact that lipases are quite sensitive to the double bond positions within fatty acid groups. Specifically, lipases show less catalytic rate when double bonds are located nearest the carboxyl group, such as DHA.46-48 The chemical structure of DHA implies a difficulty for lipases into the FAEEs incorporation, and therefore transesterification reaction rendered minor product yields in comparison with EPA-EEs. Moreover, taking into consideration that the DHA-EEs used in the present study contain a high purity of 87% DHA, the production of STAGs become a difficult task. Even though, a high STAGs yield of more than 80% was reached.
As a result of the optimal ratios investigated, CAL-B showed that relations 1:20 and 1:10 could either be used in the production of enriched EPA and DHA STAGs. However, to evaluate different commercial lipases (see Section 3.4.2.), the ratio 1:20 was selected to carry out the transesterification reaction. An excess of omega-3 FAEEs was preferred to assure that the investigated lipases catalysed the STAGs production and showed differences in short reaction times. Moreover, recycling and reuse of the FAEEs could be done after the purification of the STAGs to reduce process costs for possible applications in the food industry.
3.4.2 Screening of Commercial Lipases for the Production of STAGs with the Optimal 2-MAGs to Omega-3 FAEEs Ratio
The ability of different lipases to catalyse the transesterification reaction of 2-MAGs from camelina oil with EPA and DHA FAEEs was investigated. The screening of commercial lipases included the lipases originating from Rhizomucor miehei (RML) (Novozym 40086), TLL (Lipozyme TL IM), and CAL-B (Novozym 435). Investigated lipases catalysed the incorporation of high concentrated EPA-EEs and DHA-EEs into STAGs at various ranges and different rates. Figure 5a shows the effect of lipase type on incorporation of high concentrated EPA-EEs into STAGs during different reaction times (2, 4, 8, and 24 h). STAGs percentage exponentially increased with the time course of transesterification reaction for all tested lipases up to a maximum FAEEs incorporation degree achieved at 24 h. CAL-B and TLL gave the highest degree of incorporation (no significant differences at 1% level), 95.1% ± 0.45 and 95.51% ± 1.72, respectively, however, RML exhibited a slightly minor STAGs yield (92.28% ± 0.01) (significant differences with CAL-B and TLL at 5% level). To optimize reaction, conditions need to be a compromise between STAG yield and the time taken to produce it. For instance, CAL-B reached a STAGs percentage yield of 86.59% ± 1.09, 88.95% ± 1.18, and 95.07% ± 0.45, after 4, 8, and 24 h, respectively. STAGs yield only increased an 8.9% from 4 to 24 h of reaction time. Thus, depending on the objective of the reaction and the purity of the final product, a shorter reaction time could be enough to obtain an important content of STAGs without reaching the maximum yield. Comparing results with the investigated lipases, CAL-B showed the highest STAGs yield in short reaction time, i.e., 4 h, achieving an increase of 6.3% in comparison with TLL. Therefore, CAL-B seems to be the lipase with more potential in the incorporation of high concentrated EPA-EEs into STAGs at short reaction time, while TLL and CAL-B could be equally either used for longer reaction times.
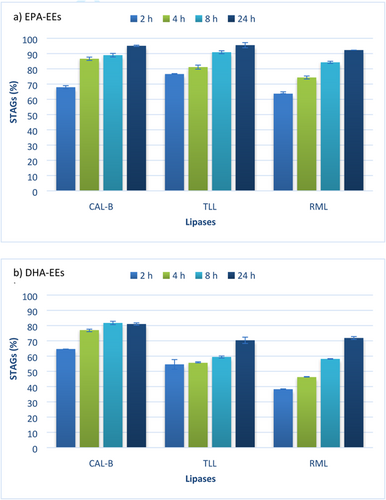
A more interesting finding was the impact of screened lipases in the incorporation of high concentrated DHA-EEs into STAGs (Figure 5b). After 24 h, the maximum incorporation degree was achieved with CAL-B (81.01 ± 0.78), followed by RML (71.86 ± 0.94) and TLL (70.33 ± 2.06). CAL-B exhibited the highest efficiency of esterification with the highest STAGs yields in comparison with other tested biocatalysts (significant differences at 1% level). However, these remarkable differences using the evaluated lipases were not found in the incorporation of high concentrated EPA-EEs into STAGs. Whereas STAGs yield was nearly to 100% after 24 h using EPA-EEs, significant lower yields using high concentrated DHA-EEs were found even when the reaction time continued up to 48 h (data not shown in Figure 5). TLL and RML were the lipases which showed less DHA-EEs incorporation mostly in shorter reaction times. For example, after 2 h of reaction time, RML reached a percentage yield of 38.25% ± 0.26 using DHA-EEs and 63.71% ± 1.25 using EPA-EEs. These results suggest the remarkable effect of the selectivity of the different lipases tested towards EPA-EEs and against DHA-EEs. Lipase selectivity results in this work were supported by other authors that have described the specific esterification ability toward EPA and DHA of different lipases. Hamam and Shahidi49 investigated the incorporation of EPA and DHA in high-laurate canola oil reporting no significant differences in the EPA incorporation using commercial CAL-B, TLL and AP-12 from Aspergillus niger, however, CAL-B incorporated higher DHA amount in comparison with TLL. Moreover, Moreno-Perez et al.50 described the synthesis of DHA TAGs catalyzed by different immobilized lipases achieving the best production yield with CAL-B. Wongsakul et al. found that CAL-B discriminates DHA from EPA and can therefore be used for the selective enrichment of the more desirable DHA.51, 52 Zhang et al.53 investigated the production of TAGs enriched in EPA and DHA from fish oil using the same lipases than in the present work, and also described the same incorporation degree order of CAL-B > TLL > RML.
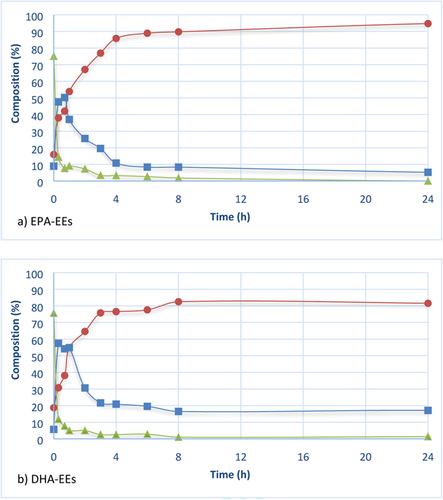
Since CAL-B was capable to efficiently catalyse the esterification of EPA and DHA, it seems to be the most suitable lipase for the synthesis of these STAGs. Thus, a complete reaction kinetic to better understanding the transesterification course with CAL-B is shown in Figure 6. As can be seen in Figure 6a, DAGs were synthesized from the incorporation of EPA-EE into 2-MAGs achieving a maximum percentage of 50.3% in only 40 min of reaction time. From this point, DAGs considerably decrease with the time course of transesterification due to the production of STAGs, and after 4 h DAGs were less than 10%. In contrast, for high concentrated DHA-EEs (Figure 6b) a different trend was observed. The reaction was not totally complete suggesting that the final mixtures contained intermediate reaction products, such as DAGs. Almost 20% of DAGs were in the final reaction products. Therefore, production of STAGs from camelina oil enriched with EPA and DHA, is considered an interesting approach to develop new functional foods with healthy lipids.
 ) STAGs, (
) STAGs, ( ) DAGs, (
) DAGs, ( ) 2-MAGs.
) 2-MAGs.4 Conclusions
In conclusion, this study shows the potential interest of camelina oil as a new source of omega-3 α-linolenic acid for the synthesis of STAGs enriched in EPA and DHA using commercial lipases in short reaction times. Camelina oil demonstrated to be an excellent source to enzymatically produce 2-MAGs enriched in omega-3 ALA (up to 49.8%). In transesterification reaction for EPA and DHA incorporation into STAGs, the number of double bonds and the chain length had a marked effect on the incorporation of omega-3 FAEEs into STAGs. High concentrated EPA-EEs was swiftly esterified into STAGs rendering higher yields than using high concentrated DHA-EEs. The 2-MAGs to FAEEs ratio 1:20 and 1:10 could either be used in the production of enriched EPA and DHA STAGs with yield higher than 90% at optimal conditions, while 1:5 ratio achieved the lower STAGs yields. Products were purified and completely characterized by HPLC and GC/MS to determine their composition in ALA, EPA, and DHA. Moreover, lipase screening established the most appropriate strategy to reach maximum STAGs yields. Tested lipases exhibited different degree of substrate specificity towards EPA and DHA. CAL-B and TLL incorporate EPA-EEs into STAGs in the same degree, while for enriched DHA STAGs, the maximum yield was achieved with CAL-B.
Overall, CAL-B seems to be the most suitable lipase to produce high purity EPA and DHA enriched STAGs as ingredients for functional foods with recognized health claims, from a natural source of omega-3 ALA like camelina oil. Results not only demonstrate the efficiency of this enzymatic method for enriching camelina oil in EPA and DHA, but offer a highly efficient strategy for the development of omega-3 enriched products from other vege table oil sources like chia or echium as functional foods for applications in nutraceutical and food industries.
5 Abbreviations
2-MAGs, 2-monoacylglycerols; ALA, α-linolenic acid; CAL-B, lipase from Candida antarctica B; DAGs, diacylglycerols; DHA, docosahexaenoic acid; DHA-EEs, high concentrated DHA ethyl esters; EPA, eicosapentaenoic acid; EPA-EEs, high concentrated EPA ethyl esters; FAEEs, fatty acid ethyl esters; FAMEs, fatty acid methyl esters; FDA, Food and Drug Administration; FFAs, free fatty acids; GC-MS, gas chromatography-mass spectrometry; HPLC-ELSD, high performance liquid chromatography-evaporative light scattering detector; MUFA, monounsaturated fatty acids; omega 3 VLC PUFAs, omega-3 very long chain polyunsaturated fatty acids; PUFAs, polyunsaturated fatty acids; RML, lipase from Rhizomucor miehei; SFA, saturated fatty acids; SPE, solid phase extraction; STAGs, structured triacylglycerols; TAGs, triacyclglycerols; TLL, lipase from Thermomyces lanuginosus.
Acknowledgments
The authors thank the Spanish Ministry of Education, Culture and Sport for the pre-doctoral contract (FPU 2013-01796) granted to N.C. The authors also thank Camelina Company España for kindly providing camelina seeds and Novozymes for donating commercial lipases.
Conflict of Interest
The authors declare no conflict of interest.




