GC-MS Characterization of Cyclic Fatty Acid Monomers and Isomers of Unsaturated Fatty Acids Formed During the Soybean Oil Heating Process
Abstract
In this work, the possibility of formation and isolation of cyclic fatty acid monomers (CFAM) from polyunsaturated fatty acids via prolonged heat treatment of soybean oil are studied. The structures of CFAM are determined using Gas chromatography-Mass spectrometry (GC-MS) of their 4, 4-dimethyloxazoline (DMOX) derivatives, the yield of derivatization is 98%. Among CFAM, the cyclohexyl fatty acids with ring from C12 to C17 and the cyclohexneyl with ring from C10 to C15 formed acid are detected by GC-MS. The new cyclopentyl with ring at C14 position or between C13 to C17 are detected from linoleic acid. Also, the variation of major fatty acids composition of soybean oil during heated treatment at 150, 180, 210, and 240 °C for 60 h are analyzed. The results indicate that after heating, soybean oil contains four isomers of conjugated linoleic acid (CLA), four isomers of C18:1 and four isomers of C18:3. The heating process, the enrichment steps, and CFAM concentration can modify the profile of CFAM and permit the emergence of new compounds, which can be useful to know the edible oil structural changes during the phases of extraction and analysis.
Practical Applications: When heated at high temperature for a long time, soybean oil undergoes isomerization and cyclization reactions. In fact, CFAM structures with five and six membered carbon rings are identified from heated oil. A new mass spectrum of the CFAM derivative from the intramolecular C18:2 cyclization is also described. Although CFAM are present at very low concentrations in heated oils, these compounds are the most toxic metabolites produced in soybean oil heated at high temperature. In addition to the four isomers of conjugated linoleic acid, four isomers of C18:1 and four of C18:3 are detected during the heating process. The data clearly show that it is necessary to avoid heating the soybean oil to a temperature above 210 °C for a period of more than 60 h which can minimize the production of CFAM.
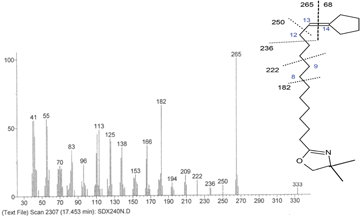
The gap of 68 amu between the molecular ion (333) and m/z 265 indicate the location of cyclopentyl ring at C14, which is first determined in heated soybean oil.
1 Introduction
The high temperature treatment has a negative impact on the integrity of fatty acid and tocopherol structure of edible oil.1, 2 According to Zahir et al.,3 the oxidation degree, and physicochemical and sensory properties of Corn and Mustard oils were remarkably altered after heating and frying.3 Other studies on edible oils have shown that the quality of the oil is decreased with a longer exposure to microwave heating, mainly due to the formation of primary and secondary oxidation products as well as the decrease in total contents of chlorophylls, carotenoids, phenols, and tocopherols.4
The Cyclic Fatty Acid Monomers (CFAM) are cyclic compounds produced during the vegetable oil heating. These compounds appear under the action of high temperatures (>200 °C). Although the presence of CFAM in processed oils is observed at very low concentrations (0.01–0.7%),5, 6 it has been demonstrated that these compounds can affect the fatty acids metabolism7, 8 and are therefore potentially toxic to human health.9, 10 Other studies showed that the free or esterified forms of CFAM have a great influence on the intestinal metabolism and their lymphatic recovery.11 The identification of the CFAM structures formed during the frying of vegetable oils was reported by Sebedio and Grandgirard, Dobson and Sebedio.5, 12 The origin of CFAM is made possible by unsaturated fatty acids transformation, particularly oleic, linoleic, and linolenic acids. 10, 12-14
Oleic acid, although its presence in a high proportion in vegetable oils, is the least exposed to the intramolecular cyclization process. The result of oleic acid cyclization is the formation of eight saturated isomers (four cycles of cyclopentyl type and four cycles of cyclohexyl type). Cyclopentyl CFAM are twice as abundant as cyclohexyl cycles.15 The linoleic acid heating leads to the formation of two types of cyclic compounds: CFAM and bicyclic fatty acid monomers (BFAM). Alpha linolenic acid is the most sensitive to heat treatment. In fact, the heating of this acid induces the formation of 16 cyclic dienes (eight cycles of cyclopentyl type and eight cycles of cyclohexyl type).16
Concerning the training mechanisms, three hypotheses have been proposed to explain the formation of the CFAM: the mechanism via thermal rearrangement,17 the mechanism involving intermediate allylic radicals,6 and the mechanism of concurrent cycloaddition reactions.18 CFAM are produced by the formation of new carbon-carbon bonds within fatty acid molecules.19
The current study aims to identify and characterize CFAM formed and isolated from soybean oil subjected to different heating temperatures and different treatment periods.
2 Experimental Section
2.1 Samples and Reagents
The soybean oil was purchased from a local Tunisian market. All reagents were analytical grade: nhexane, diethyl ether, KOH, HCL, ethyl alcohol, methanol, 2-amino-2-dimethyl-propan-1-ol. All chemical reagents were supplied by Sigma–Aldrich–Supelco.
2.2 Heating of Oil
Soybean oil samples placed in flasks sealed under a stream of nitrogen were subjected to a temperature of 275 °C for 12 h. Further oil samples were subjected to 150, 180, 210, and 240 °C, the duration of treatment was 60 h for each.
2.3 Fatty Acids Methylation
The fatty acid methylation procedure was performed in a standardized way to ensure good accuracy and repeatability of fatty acid analyses. The derivation of the fatty acid methyl esters was the most used technique for lipid analysis by GC-FID. A 10 mg of oil sample was added to 1 mL of hexane and 500 μL of sodium methoxide, after vortexing, the reaction mixture was incubated at temperature between 40 and 50 °C for 15 min. Then, 4 mL of hexane were added to the mixture and 5 mL of water saturated with NaCl was used to wash the sample. Once the tube was vortexed and after allowing the layers to settle, the upper layer was transferred by using a containing delipidated cotton and ≈1 cm of dry magnesium sulfate, the filtrate was collected in a test tube.
2.4 The CFAM Isolation
The CFAM were prepared according to Sebedio and Grandgirard.5 Four steps are necessary in order to obtain the CFAM: saponification, esterification, column chromatography, and urea fractionation.
2.4.1 The Oil Saponification
In sealed glass ampoules and under nitrogen stream with a purity of 99.9999%, the soybean oil was submitted overnight at 275 °C. Ten gram of the heat-treated oil was mixed with 60 mL of ethyl alcohol (95%) and 10 mL of a KOH solution (50%, w/v). The mixture obtained was heated in the flask under reflux, with stirring, for 1 h, then neutralized by using 50 mL of HCl solution (10%). The contents of the flask were transferred into a separating funnel and 15 mL of hexane was added. After washing three times with saturated sodium chloride water, the organic phase was recovered, dried over anhydrous sodium sulfate and the solvent was evaporated.
2.4.2 Esterification of Free Fatty Acids
The recovered free fatty acids were converted to methyl esters by the addition of 4 volumes of 1% H2SO4 solution in methanol for 5 h under reflux. To remove all traces of acid, several washes with Na2CO3(2%) solution, water, and saturated aqueous NaCl solution were carried out. The organic phase was collected, dried, and the hexane evaporated.
2.4.3 The Fatty Acid Methyl Esters (FAME) Fractionation by Column Chromatography
Separation of the methyl esters (5 g) was carried out in a column chromatography of 2 cm in diameter containing 25 g of silica gel (70–200 mesh). The eluent used was a mixture of hexane and diethyl ether (95: 5 V/V). The methyl esters were recovered after eluent evaporation.
2.4.4 The Fractionation by Urea
The saturated urea solution was prepared by dissolving the urea crystals in methanol (1: 4, w/v). This mixture was heated until the urea was completely dissolved and the methyl esters were subsequently added with weight ratio of the mass urea /the mass of esters methyl (3:1; w/w). The resulting solution was cooled under nitrogen and stored at 4 °C overnight. The fraction retained by the urea was separated by filtration using a cooled Büchner funnel. A solution of HCl (3%) was added to the filtrate and the cyclic fatty acid methyl esters (CFAM) were extracted with hexane. The same volume of HCl (3%) solution was added to the urea crystals in order to recover the fraction retained by the latter. Again, the fatty acid esters were extracted with hexane. To remove all traces of water, the fractions obtained were dried over anhydrous sodium sulfate and then filtered and the hexane was evaporated.
2.5 Preparation of 4,4-Dimethyloxazoline (DMOX) Derivatives
A 50 mg of 2-amino-2-dimethyl-propan-1-ol was added to 1 mg of CFAM in sealed flasks under a stream of nitrogen with a purity of 99.9999%, and then the mixture was placed in an oven at 150 °C overnight. After cooling, the 4,4-dimethyloxazoline derivatives were extracted with 5 mL of the hexane/diethylether mixture (1: 1 v/v) and then 5 mL of saturated water in NaCl was added. The recovered DMOX derivatives were dried over anhydrous sodium sulfate, the solvent was evaporated under a stream of nitrogen and then re-dissolved in hexane.
2.6 GC-MS Analysis of FAME and DMOX Derivatives
GC-MS analyses were performed using a Hewlett-Packard (Model HP 6890 Series II) chromatograph coupled to an Agilent model 5973N selective quadrupole mass detector; Palo Alto, CA, and connected to a computer with ChemStation software. The ionization voltage used was 70 eV at 250 °C. The samples in DMOX form were analyzed on a BPX-70 capillary column (60 m 0.25 mm di, 0.25 µm the film thickness of the stationary phase.) The injector and detector were maintained at 250 and 230 °C, respectively. Helium was used as the carrier gas at a constant flow of 20 mL min−1 in splitless mode. The inlet pressure of the carrier gas was 22.5 psi, the injection volume was 1 µL. Initially, the oven temperature was maintained at 60 °C for 1 min, programmed from 60 to 200 °C at 20 °C min−1 for 10 min, then ramping at a rate of 5 °C min−1 up to 240 °C for 20 min and finally, temperature was gradually increased up to 260 °C at a rate of 5 °C min−1 for 8 min. The identification of components was based on comparison of their mass spectra with those of NIST mass spectra.
3 Results and Discussion
The Gas-Chromatography (GC) analysis showed that untreated soybean oil is composed mainly of oleic acid (21.23%), linoleic acid (54.96%), and linolenic acid (6.25%) as major unsaturated fatty acids, whereas the major saturated fatty acids are palmitic acid (10.33%) and stearic acid (3.53%). After the heat treatment at 150, 180, 210, and 240 °C for 60 h, the saturated and monounsaturated fatty acid rates increased in parallel with temperature. However, the polyunsaturated fatty acid rates fell to less than 50% at 240 °C (Table 1). This is in agreement with the results reported by Blond et al.20 who mentioned a decrease of n-3 polyunsaturated fatty acids of heated linseed oil. It was also demonstrated that in the canola oil, both C18: 2n-6 and C18: 3n-3 diminished with frying due to the effects of oxidation and polymerization. However, the level of C14:0 increased fivefold. Both C16:0 and C16:1 also dropped for the same reason.21 This suggests that polyunsaturated compounds are more sensitive to heat treatment and are therefore capable of undergoing chemical transformation reactions including cyclization reactions. Polyunsaturated fatty acids are most susceptible to degradation when they are exposed to certain factors, such as oxygen, light, moisture, or high temperature. Under these conditions, both thermal and oxidative reactions occurred at an accelerating rate, causing the alteration of fatty acid composition.22
| Fatty acids [%]/temperature [°C] | C16:0 | C18:0 | C18:1△9 | C18:1△11 | C18:2△9,12 | C18:3△9,12,15 |
|---|---|---|---|---|---|---|
| Fresh oil (25 °C) | 10.33 | 3.53 | 21.23 | 1.1 | 54.96 | 6.25 |
| 150 | 11.08 | 3.51 | 21.16 | 1.08 | 54.86 | 5.73 |
| 180 | 10.72 | 3.51 | 21.44 | 1.05 | 53.37 | 5.75 |
| 210 | 12.28 | 4.08 | 23.64 | 1.15 | 51.25 | 3.74 |
| 240 | 21.41 | 7.06 | 30.75 | 1.63 | 25.15 | 00 |
Table 1 shows a thermal resistance of the heated oils at temperatures of 150 and 180 °C. However, this resistance decreased for polyunsaturated fatty acids when the temperature was raised to 210 and decreased further at 240 °C.
CFAM were absent in the unused soybean oil, but appeared after oil heating. It is estimated that the formation of CFAM begins only from the temperature of 210 °C, at which a significant decrease of C18:2 and C18:3 was recorded (Table 1).
The difficulty of GC-MS analysis lied in the thermal degradation of CFAM in GC column and the rearrangement which occurred in the mass spectrometer.23 In order to overcome this drawback, an adequate derivation method was selected, which could contribute to a better stabilization of the analyzed compounds.
The major methods for derivatizing fatty acids are 4,4-dimethyloxazoline (DMOX) derivatives and picolinyl esters formed by reacting the carboxyl moiety with two amino-2-methyl-1-propanol (AMP) or 3-hydroxymethyl pyridine (HMP), respectively. The derivation into 4,4-dimethyloxazoline (DMOX) allows the stability of the ions as the nitrogen atom present on this fragment carries the charge upon ionization of the sample in GC-MS.24 Moreover, the DMOX derivatives spectra were more easily interpretable than other ones. DMOX Derivatives of fatty acids provide characteristic ion fragments that allow an accurate location of the ethylenic double bonds 25 and in polyunsaturated fatty acids, the use of DMOX derivatives in combination with FAMEs can be very useful for the identification of fatty acids in unknown samples.26
Thus, CFAM were identified by analysis of nonpolar fraction obtained from heated regular soybean oil at 240 and 275 °C. The CFAM fractions were converted into 4,4- dimethyloxazoline derivatives and characterized by mass spectrometry.
The mechanisms of CFAM and BFAM formation during the heating of edible oils are widely studied in literature.27
3.1 C18:1 Isomers
Due to its high proportion in vegetable oils with an improved oxidative stability at high temperature,28 oleic acid is the least exposed to the process of cyclization. Although oleic CFAM was not detected in this work; it is probably present at a very low amount. The mass spectrum of the DMOX derivatives of C18:1 displayed prominent peaks characteristic of the DMOX moiety at m/z = 113 and 126. The peak at m/z 126 was the base peak, and the molecular peak was at m/z 335 (Figure 1).
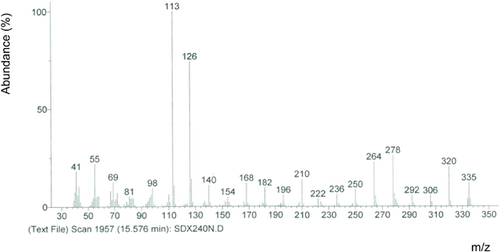
The gap of 12 atomic mass unit (amu) between m/z 196 and 208, 210 and 222, 224 and 236 locates the double bonds in positions 9, 10, and 11 of aliphatic chain, respectively. Regular intervals of 14 mass units occurring from fragments 236, 250, 264, 278, 292, 306, and 320 indicated saturated character of the aliphatic chain between C12 and C18 carbons (Table 2).
| C18:1isomers | RT [min] | m/z | Common fragments |
|---|---|---|---|
| △9t | 15.233 | 196–208 | 236–250–264–278–292–306–320 |
| △9c | 15.426 | 196–208 | |
| △10 | 15.576 | 210–222 | |
| △11 | 15.582 | 224–236 |
3.2 Conjugated Linoleic Acid (CLA)
At high temperature (275 °C), conjugated double bond systems were detected, which is in agreement with the results found by Lu et al.29 The CLA is formed from linoleic acid during heating oil in small amounts. CLA contains two conjugated double bonds, which can be found between positions 8–14 in the carbon chain and can show (trans, trans), (cis, trans), (trans, cis), and (cis, cis) configuration (Table 3).
| Isomers CLA [%] | RT [min] | % TFA | % CLA |
|---|---|---|---|
| 1. tt | 15.871 | 0.78 | 4.17 |
| 2. ct | 16.182 | 4.96 | 26.51 |
| 3. tc | 16.375 | 3.74 | 19.99 |
| 4. cc | 16.483 | 9.23 | 49.33 |
- RT, retention time; TFA, total fatty acids; CLA, conjugated linoleic acids.
Considering the fact that trans isomers elute before cis isomers, Figure 2 obtained from GC-MS should correspond to the order of elution of trans-trans, cis-trans, trans-cis, and cis-cis isomers of linoleic acid, respectively.
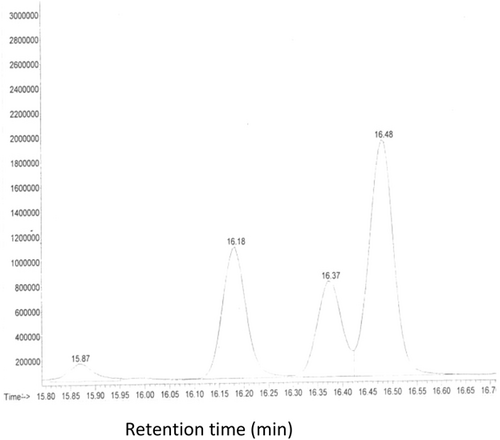
The result given in Table 3 shows that isomer (cc) remained the major form of CLA (50%) and ct isomer was in the second position. Based on this result and comparing the percentage of total linoleic acid at 240 °C (25.65%: 25. 15 (C18:2cc) and 0.5 (C18:2tt) (not shown) to the total linoleic acid at 27 5°C (18.71%) (Table 1), the difference (6.94%) will be represented CFAM and other compounds derived from heated C18:2.
3.3 CFAM Linoleic
Figure 3 shows the important mass spectra with peaks at m/z = 113 and 126; the latter remains the base peak of the spectrum. These ions are characteristic for the dimethyloxazoline rings, the peak at m/z = 333 is the molecular peak. The 26-unit gap between m/z 196 and 222 suggests that the double bond remained at its original position (C9). The characteristic peak of the ring presence is the one at m/z = 277 (333–56) formed via the McLafferty rearrangement (Figure 3. 1); whereas the 80 unit gap between m/z 182 and 262 (277–15) indicates ring position. The cyclohexyl ring identified in soybean oil is located between C12 and C17.
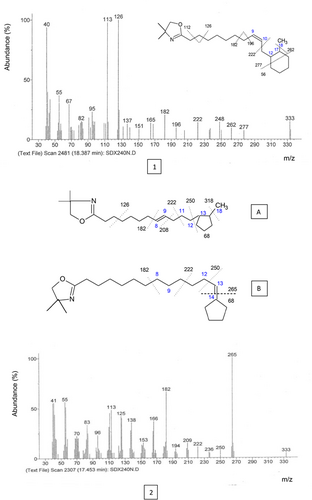
When the double bond is near either end of the molecule, interpretation of spectra can be more difficult. Indeed, the gap of 68 amu between m/z = 333 and 265 indicates the terminal cylopentenyl ring. Moreover, the absence of ions in the region can discard any ambiguity (Figure 3. 2). The ion at m/z = 250 results from the loss of methyl group from the fragment m/z 265. The gaps of 14 amu between m/z = 250 and 236, and between 236 and 222 correspond to the loss of methylene groups.
Knowing that the mechanism of cyclization involved the loss of one of the double bonds, we suggested two hypotheses to explain the formation of a new compound: 1. The gap of 12 amu between m/z = 182 and 194 suggests a double bond in position 8 rather than 9 (Figure 3. 2.A). 2. The double bond was in position 13 rather than 12 of the chain, which can be explained by the reaction of isomerisation before the cleavage reaction (Figure 3. 2.B).
Despite the high number of CFAM identified by Sebedio et al.30, this new compound was not reported in their study. This can be explained by the method of enrichment steps and concentrate CFAM which could modify the profile of CFAM. In fact, the research reported by Gere et al.31 showed a loss of 20% of CFAM during the complexation step by using urea.
According to Christie et al.,31 the linoleic CFAM are formed predominantly by two cyclopentenyl acids having cycles between C10 and C14 and C8 and C12 carbons. In most cyclopentenyl rings, the double bond within the ring remained at its original position (C9 and C12). However, it is possible to encounter other structures having double bonds delocalized to one of the two substituted carbon atoms (between C8 and C12 and C10 and C14). The isomers possessing a double bond cis on the linear chain in the C9 or C12 position are composed either of the cyclic fatty acids with cyclopentyl rings located between the carbons C13 and C17; C5 and C9 or by those having cycles of cyclohexyl type between carbon atoms C5 and C10 and C12 and C17/C13 and C18. The isomers possessing a double trans bond at C9 or C12 positions on the linear chain were represented only by the cyclic monoenes characterized by the 6-carbon saturated rings between C5-C10 and C12-C17 carbons. Based on the previous data, our study shows that C18:2 CFAM with ring position between C12 and C17 was formed from isomers having a double cis or trans bond in the linear chain at position C9. However, the mechanism of formation of molecular structures A and B remains unknown.
Predicting the molecular structure of the mass spectrum in Figure 3 was very difficult. In fact, the three mechanisms of explaining the formation of CFAM are thermal rearrangement, intermediate allylic radicals, and cycloaddition accompanied by prototropic migrations 1, 6 and 1, 7 established, respectively by Christie and Dobson, Destaillat and Angers, Gast et al.31 were incapable to explain the formation of compounds A and B CFAM from linoleic acid.
3.4 CFAM Linolenic
The heating of soybean oil results in the formation of cyclohexenyl dienes originated from the two basic structures having cycles between C10-C15 carbons and an acyclic double bond at C16 or C8. The double bond within cyclohexenyl rings also remained at position C12.
Mass spectra for each of main structures of six-carbon rings fatty acid is presented in Figure 4.
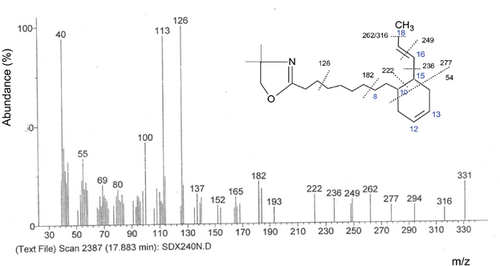
The molecular ion at m/z = 331 corresponds to the mass of the DMOX derivatives of linolenic acid, thus confirming the octadecatrienoic structure of the fatty acid, the base pic was ion at the m/z = 126.
The molecular ion at m/z 277 suggests the presence of a cyclic diene structure, whereas gaps of 54 between m/z 331 and 277 correspond to the loss of a monounsaturated Diels-Alder retro-type fragment composed of four carbons (C11 to C14), indicating that the 6-carbon ring contains just one double bond at C12.31 While the m/z 113 fragment was formed via the McLafferty rearrangement.
In fact, the intervals of 13, 26, and 40 amu are characteristic for unsaturation. Indeed, the gap of 13 amu between m/z = 236 and 249 indicates the location of double bond at C16. However, the gap of 40 amu between m/z = 182 and 222 refers to the location of a double bond rather at C8 (not reported). Without going into the detailed cyclization process via the mechanism of free intermediate allylic radicals of formation of this molecule, it seems that the formed cyclic radical loses hydrogen radical, thus forming a new double bond, which explains the presence of three double bonds in the final structure, but such a structure has not been reported in the literature.
Among the cyclohexenyl dienes, the isomers possessing trans double bonds have been more abundant, which is probably due to the displacement of the double bond of its original position, C9 to C8 and C15 to C16.
Because linolenic acid was present in very low amounts in soybean oil, there were a few unsaturated CFAM that can come from this oil.
3.5 C18:3 Isomers
Besides the CFAM formation as a product of heated treatment, some isomers of non cyclic linolenic acid were also detected: C18:3Δ10, 12, 15; C18:3Δ10, 13, 15; C18:3Δ10, 13, 16 and C18:3Δ9, 12, 15. The characteristics of the double bond localization of these isomers are provided in the Table 4.
| Position of double bond [△] | △9 | △10 | △12 | △13 | △15 | △16 |
|---|---|---|---|---|---|---|
| Specific peaks (m/z) | 196–222 | 210–236 | 236–262 | 250–276 | 276–302 | 290–316 |
| Gap (amu) | 26 | 26 | 26 | 26 | 26 | 26 |
- amu, atomic mass units.
The C18:3 isomers were formed by the displacement of the double bond of its original position from C9 to C10, C12 to C13 and C15 to C16.
4 Conclusion
This study shows that soybean oil submitted to high temperatures gives rise to the formation of thermal and alteration products, especially CFAM and CLA. CFAM formation represents an excellent chemical marker of oil degradation at high temperature.
The DMOX derivatives, obtained from CFAM formation, require high reaction temperature (≥210 °C) and long reaction time (60 h) that may result in partial degradation of PUFA. Despite the low number of CFAM identified from the processed oil, the CFAM structure can be affected by the concentration procedure or another additional measure.
In the present study, we were able to elucidate the hypothesis explaining the formation of new compound (CFAM) derivates from intramolecular cyclization of linoleic acid. CFAM formed from linoleic acid were a mixture of five and six carbon- member ring isomers; however, those formed from linolenic acid were only 6-membered ring isomers.
Although the trans-fatty acids and conjugated linoleic acid isomers may be responsible for lipid composition changes and not cyclic fatty acids monomers which remained minors compounds during heated of soybean oil, CFAM represented the most toxic compounds produced in heated soybean oil at high temperature. The data clearly show that it was necessary to avoid heating the soybean oil more than 210 °C for a period of 60 h which can minimize the production of CFAM.
Acknowledgment
The authors are thankful for the technical support of Mr. Ronan Corcuff. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.




