Beneficial impact of a mix of dairy fat with rapeseed oil on n-6 and n-3 PUFA metabolism in the rat: A small enrichment in dietary alpha-linolenic acid greatly increases its conversion to DHA in the liver
Abstract
The impact of the amount of dietary α-linolenic acid (ALA) on its own tissue accumulation and conversion to longer n-3 polyunsaturated fatty acids (PUFAs) remains controversial and may depend on the other dietary fatty acids mixed with ALA. Whereas linoleic acid (LA) is well known to compete with ALA for its conversion to longer n-3 PUFAs, the concomitant presence of dietary ALA with dairy saturated fatty acids (C4:0–C14:0) that are highly susceptible to β-oxidation may inversely lead to its increased cellular storage and better conversion to long-chain n-3 PUFAs. The present study was therefore aimed at investigating further the putative beneficial effect of dietary dairy fat on n-3 PUFA tissue levels in the rat. Firstly, we showed that when combined with a well-defined dietary level of ALA (0.6% energy), substitution of olive oil for butterfat improved ALA storage in adipose tissue and liver, and had moderate effects on its conversion to n-3 long-chain PUFAs. Secondly, we showed that, when mixed with dairy fat, a small increase in dietary ALA (from 0.6 to 0.8% of energy) enhanced the ALA storage in adipose tissue only but conversely significantly increased its conversion to highly unsaturated n-3 PUFAs in the liver.
Practical applications: α-linolenic acid (ALA) is the most accessible source of n-3 PUFAs in the global diet. However, the intake of ALA is currently lower than dietary guidelines and the rate of ALA conversion to longer chain n-3 PUFAs is low. The results from this study showed that a small enrichment in dietary ALA combined with dairy fat increased adipose tissue ALA storage, which represents a slow releasable pool that may be utilized over time by other tissues and greatly increased the conversion of ALA to highly unsaturated n-3 PUFAs in the liver. This knowledge may possibly result in the development of new dietary strategies to increase the cellular level of n-3 PUFAs in animals and humans.
When mixed with dairy fat, a small increase in dietary α-linolenic acid (from 0.6 to 0.8% of energy) enhances the α-linolenic acid storage in adipose tissue and significantly increases its conversion to highly unsaturated n-3 polyunsaturated fatty acids in the liver.
Abbreviations
-
- ALA
-
- α-linolenic acid
-
- ARA
-
- arachidonic acid
-
- DHA
-
- docosahexaenoic acid
-
- DPA
-
- docosapentaenoic acid
-
- EPA
-
- eicosapentaenoic acid
-
- FA
-
- fatty acid
-
- LA
-
- linoleic acid
-
- MUFA
-
- monounsaturated fatty acid
-
- OLA
-
- oleic acid
-
- PUFA
-
- polyunsaturated fatty acid
-
- RBC
-
- red blood cell
-
- SFA
-
- saturated fatty acid
-
- TAG
-
- triacylglycerol
1 Introduction
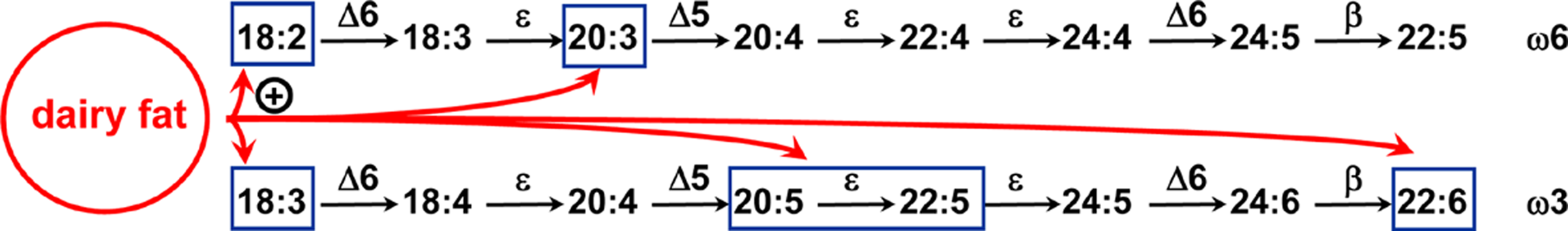
A high cellular and tissue level of n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs) has been shown to be beneficial throughout life for human health 1, 2. Among n-3 LC-PUFAs, the well-known eicosapentaenoic and docosahexaenoic acids (EPA, 20:5 n-3 and DHA, 22:6 n-3) are indeed required for many important positive physiological functions in humans and animals 3, 4. In addition to their structural role in maintaining cell membrane fluidity, EPA and DHA are precursors of eicosanoids and docosanoids involved in anti-inflammatory and anti-thrombotic signaling 2. In humans and animals, EPA/DHA can either be directly obtained from fish and seafood or synthesized from their essential α-linolenic acid (ALA) dietary plant precursor through a series of desaturation and elongation 5. However, in many countries, the intakes of both ALA and EPA/DHA are lower than the current dietary recommendations 6, 7. Because of the limited stock and declining nutritional quality of marine food sources 8, 9, ALA remains the most accessible source of n-3 PUFA in the global diet 10. However, the in vivo conversion rate of ALA to longer n-3 PUFAs and especially to DHA in mammalian tissues is low and suspected to be even lower in humans 11 than in rodents. It is therefore crucial to control the factors that can regulate the overall conversion of dietary ALA to cellular DHA, in order to manage the higher potential conversion level and meet n-3 LC-PUFA requirement in the absence of EPA/DHA in the diet.
Among these factors, the more obvious is the dietary intake level of ALA itself. But, the capacity of dietary ALA to maintain adequate tissue levels of n-3 LC-PUFA still remains controversial 1. Many studies in humans and animals have shown that the DHA content of most tissues does not respond linearly to dietary ALA 12-14. Increasing the ALA supply until approximately 0.8–1% energy is efficient to increase the synthesis of DHA 15, 16. However, diets containing ALA above this level result in the tissue accumulation of EPA and DPA (22:5 n-3) but no further increase in DHA is shown 14, 16. Moreover, there is increasing evidence that the other FAs mixed with ALA in the lipid fraction of the diet are crucial for its metabolic fate 16. Firstly, a high level of dietary linoleic acid (LA) 17 and/or imbalanced LA/ALA ratio 18 compromise the tissue n-3 LC-PUFA status because LA and ALA share the same enzymatic pathway for their conversion to longer n-6 and n-3 PUFA, leading to a competition between the two essential precursors. Secondly, because a high percentage (60–85%) of dietary ALA is rapidly directed toward β-oxidation 19, its concomitant presence with FAs that are highly susceptible to β-oxidation may lead to its sparing, increased cellular storage and better conversion to DHA 20-24. The dietary FAs known to be readily funneled into oxidative pathways include saturated FAs with short (C4:0), medium (C6:0–C10:0) and intermediate chain length (C12:0–C14:0) 25, 26. Indeed, dietary short and medium chain FAs are transported through the portal vein and rapidly taken up and oxidized by the liver 27. Dietary longer SFAs like C12:0 and C14:0 likely follow the chylomicrons pathway through the lymph and general circulation, but they have been shown to be more rapidly metabolized than C16:0 in cellular models, which may explain their small amounts in animal tissues 28, 29. In addition, studies have shown that myristic acid is an activator of the Δ6-desaturase, which catalyzes the rate-limiting step in the conversion of ALA to longer n-3 PUFAs 30.
In a previous study 22, we showed that a butterfat-enriched diet characterized by its high levels of short and medium chain SFAs, lauric acid and myristic acid 31, and containing 0.6% energy of ALA, increased the tissue (n-3) PUFA content in the rat. In this context, the purpose of the present study was to investigate further the putative beneficial effect of diets combining dairy fat and increasing levels of ALA on the (n-3) PUFA tissue levels in the rat. Two diets containing a similar level of ALA (2.5% of FAs representing 0.6% of energy) were designed to study first the effect of a substitution of olive oil (with low amounts of SFAs) for dairy fat. The third diet was designed to evaluate the effect of a significant increase in dietary ALA (from 2.5 to 3.4% of total dietary fatty acids, that is, from 0.6 to 0.8% of energy) on its storage and conversion to (n-3) LC-PUFA, when mixed with dairy fat.
2 Materials and methods
2.1 Chemicals
Solvents and chemicals were obtained from Thermo Scientific (Elancourt, France), VWR (Fontenay-sous-Bois, France) or from Sigma (Saint-Quentin-Fallavier, France). Fractionated butterfat was provided by Lactalis (Retiers, France).
2.2 Animals and diets
All protocols complied with the European Union Guideline (2003/35/CEE) for animal care and use. Eighteen Sprague-Dawley male rats (60 g body weight, 3-weeks-old at the beginning of the experiment) were obtained from the breeding center R. Janvier (Le Genest-St-Isle, France). They had free access to water and food. They were fed with rodent chow (Special Diet Services, Witham, UK) for 1 wk until their random allotment into three groups (six rats per group). Each group was then fed during 8 wks with the experimental diets described below. The diets were prepared at the Unité de Production d'Aliments Expérimentaux (UPAE, INRA, Jouy-en-Josas, France). They were isoenergetic and isolipidic, and contained 100 g of fat (21% of energy), 198 g of casein, 426 g of corn starch, 213 g of sucrose, 36 g of mineral mix, 9 g of cellulose, 9 g of vitamin mix, and 9 g of agar-agar/kg. Lipid fractions of the three diets were made from a combination of natural fat sources, with particular respect to SFAs, monounsaturated fatty acids (MUFAs) and by carefully monitoring the ALA and LA levels. Olive oil, rapeseed oil, corn oil, fractionated butter, and commercial butter composition was 76:19:5:0:0 in the olive oil-enriched diet (OL with 0.6% energy of ALA), 0:19:12:69:0 in the fractionated butterfat-enriched diet (FBF with 0.6% energy of ALA) and 0:27:11.6:0:61.4 in the commercial butterfat-enriched diet (CBF with 0.8% energy of ALA). The fatty acid composition of each diet, analyzed using a GC method specifically dedicated to quantify short and medium chain SFAs (see below), is detailed in the Table 1. The olive oil-enriched diet contained mainly oleic acid (>70% of FAs), low level of SFAs (<12% of FAs), LA at about 12% of FAs (2.5% energy) and ALA at 2.5% of FAs (0.6% of energy). The fractionated butterfat-enriched diet contained two-times less oleic acid, higher level of SFAs (43%) including short and medium chain SFAs, lauric and myristic acids and similar amounts of both LA (12% of FAs) and ALA (2.5% of FAs) than the olive oil-enriched diet. The 3rd diet was designed to slightly increase the ALA level (3.4% of FAs, i.e., 0.8% of energy) in the dairy fat matrix. In this last diet, LA was also concomitantly increased (14.9% of FA i.e., 3.2% of energy) to maintain a similar and well-balanced LA/ALA ratio of 4–5, which corresponds to the current recommendations in human nutrition. In order to compensate for the increase in both ALA and LA without modifying the total SFA and MUFA levels compared with the FBF diet, we used commercial butterfat instead of fractionated butterfat to prepare this 3rd diet. As a consequence, total SFA is similar between the FBF and CBF diets but the distribution of each SFA is not totally identical.
| Fatty acid | OL with 0.6% en ALA | FBF with 0.6% en ALA | CBF with 0.8% en ALA |
|---|---|---|---|
| C4:0–C10:0 | 9.0 | 5.8 | |
| C12:0 | 2.6 | 1.9 | |
| C14:0 | 9.8 | 6.2 | |
| C16:0 | 7.8 | 15.3 | 18.5 |
| C18:0 | 3.1 | 4.8 | 6.7 |
| ΣSFA | 11.5 | 43.8 | 42.2 |
| C16:1 n-7 | 0.1 | 1.5 | 0.9 |
| C18:1 n-9 | 71.1 | 33.5 | 33.3 |
| C18:1 n-7 cis | 2.8 | 1.6 | 1.7 |
| C18:1 n-7 trans | ND | 1.7 | 1.5 |
| ΣMUFA | 74.2 | 40.3 | 39.2 |
| C18:2 n-6 | 11.8 | 12.6 | 14.9 |
| C18:2 cis-9 trans-11 | ND | 0.8 | 0.3 |
| Σ n-6 PUFA | 11.8 | 13.4 | 15.2 |
| C18:3 n-3 | 2.5 | 2.5 | 3.4 |
| Σ n-3 PUFA | 2.5 | 2.5 | 3.4 |
| C18:2 n-6/C18:3 n-3 | 4.7 | 5.0 | 4.4 |
- Bold values represent the sum of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and (n-6) and (n-3) polyunsaturated fatty acids (PUFA).
After an overnight fast, rats were finally anesthetized with two successive intraperitoneal injection of thiopental (37.5 mg/kg of body weight) (Nesdonal, Mérial, Lyon, France) 20. Blood samples were collected by cardiac puncture. The plasma was separated from the blood cells by centrifugation (2,500g, 10 min, 4°C). The tissues (epididymal adipose tissue and liver) were removed, weighted, snap-frozen in liquid nitrogen and stored at −80°C.
2.3 Lipid extraction and FA analysis
Lipids from liver and epididymal adipose tissue were extracted using a mixture of dimethoxymethane/methanol (4:1, v/v) 32. Lipids from plasma and red blood cells (RBC) were extracted with a mixture of hexane/isopropanol (3:2, v/v), after acidification with 1 mL HCl 3 mol/L 28. Total lipid extracts from liver, adipose tissue, plasma, and RBC were saponified for 30 min at 70°C with 1 mL of 0.5 mol/L NaOH in methanol and methylated with 1 mL BF3 (14% in methanol) for 15 min at 70°C. In order to protect the potential presence of volatile short and medium chain SFAs, FA methyl esters were extracted by adding 1 mL hexane and 5 mL NaCl 0.9% in deionized water. After shaking, the hexane phase was directly analyzed by gas chromatography using an Agilent 6890N (BiosAnalytic, Toulouse, France) with a split injector (40:1) at 250°C and a bonded silica capillary column (BPX70, 60 m × 0.25 mm, SGE, Villeneuve-St-Georges, France) with a polar stationary phase of 70% cyanopropylpolysilphenylene-siloxane (0.25 µm film thickness). The column temperature program started at 45°C and stayed at this temperature for 3 min in order to analyze short and medium chain SFAs. Then, the temperature ramped firstly at 10°C/min to 150°C and ramped secondly at 1.3°C/min to 220°C. The flame ionization detector temperature was 250°C (H2: 40 mL/min and air: 450 mL/min). Helium was used as the carrier gas (average velocity 24 cm/s). Response factors were determined for each fatty acid and corrections were made by using both authentic standards and the European certified reference material anhydrous milk fat (BCR164 obtained from Sigma).
2.4 Result expression and statistical analysis
The values (n = 6) reported are mean ± SD. Prior to analysis, data were assessed for normality assumptions. Then, statistical significance was determined by a one-way ANOVA. A Tukey post-hoc test was used to perform paired comparison. A p-value of 0.05 was assumed to represent statistical significance.
3 Results
3.1 Body weight and food intake
After 8 wks on the experimental diets, the mean body weight (536.8 ± 11.0 g) did not differ significantly amongst the groups. Mean food consumption was also similar with all diets.
3.2 Saturated and MUFA profiles of the tissues
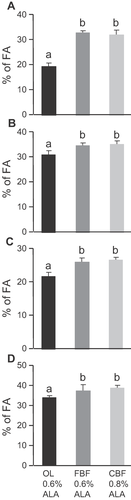
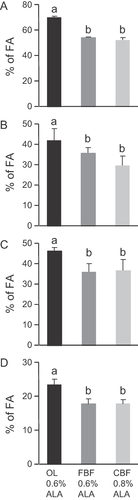
The study was focused on four tissues (adipose tissue, liver, plasma, and RBC). As expected, total SFAs in all the tissues were similar in rats fed with the two diets enriched with butterfat and significantly higher than in rats fed with the diet enriched with olive oil (Fig. 1). Inside the SFA group, we looked at short and medium chain SFAs (C4:0–C10:0) coming from the two diets enriched with dairy fat (FBF and CBF) but none of them was found whatever the tissue analyzed (Supplementary Tables S1–S4), except non-quantifiable trace amounts of C10:0 in adipose tissue. Lauric acid was the shortest SFA detected and it was stored only in adipose tissue (Supplementary Table S1). Daily intakes of the two dairy fat diets also resulted in the tissue accumulation of myristic acid, related to its dietary level (Supplementary Tables S1–S4). Finally, total MUFAs (including especially oleic acid) were significantly higher in rats fed with the OL diet (Fig. 2) compared with the two butterfat-enriched diets.
3.3 Essential FA (ALA and LA) storage in the tissues
The OL- and FBF-enriched diets were designed to contain equal amounts of ALA (2.5% of FA and 0.6% of energy) and no n-3 long chain derivatives (Table 1). Interestingly, even receiving equal daily ALA intake levels, significantly higher amounts of ALA were found in the adipose tissue and liver of the rats fed with the FBF-enriched diet compared to the rats fed with the OL diet (Fig. 3A and B). A similar trend to ALA increase also appeared in the plasma but not in RBC. These results are in agreement with the hypothesis that the presence of ALA in the liver and its storage in adipose tissue are higher when mixed with dairy fat compared with olive oil. The 3rd diet was slightly enriched with ALA (3.4% of fatty acids, i.e., 0.8% of energy). As a consequence, ALA was significantly higher in adipose tissue of the rats fed with the CBF diet than in rats fed with the two other diets (Fig. 3A). However, surprisingly, ALA was significantly lower in CBF rat RBC than in OL and FBF rats (Fig. 3D) and similar in the liver and plasma of CBF rats compared with FBF rats (Fig. 3B and C). Therefore, the diet with the highest level of ALA (0.8% energy) led to a significant higher level of ALA in adipose tissue only.
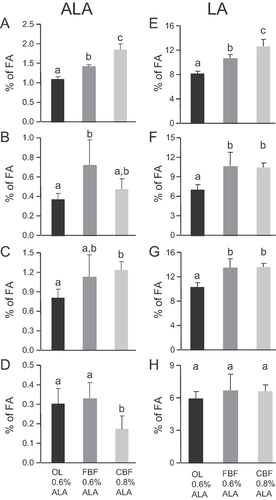
The OL- and FBF-enriched diets were also designed to contain equal amounts of LA (about 12% of FA and 2.5% of energy) and no n-6 long chain derivatives (Table 1). However, as already shown for ALA (Fig. 3), significantly higher amounts of LA were stored in adipose tissue, liver and plasma of the FBF group compared to OL animals (Fig. 3E and G). Again also, the increase in dietary LA in the CBF diet (3.2% of energy) enhanced the LA level only in adipose tissue (Fig. 3E) when compared with the FBF diet (2.5% energy of LA).
3.4 n-3 LC-PUFA profiles of the tissues
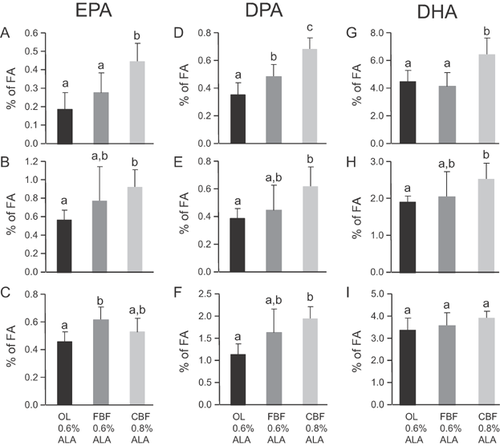
When comparing the OL and FBF diets containing the same amount of dietary ALA, EPA was significantly higher in RBC (Fig. 4C) and DPA was significantly higher in the liver of FBF rats (Fig. 4D). No difference was shown for DHA, whatever the tissue analyzed (Fig. 4G–I). When comparing now the n-3 LC-PUFA levels of the rats fed the CBF diet containing 0.8% energy of ALA with the two groups fed the diets containing 0.6% energy, EPA (Fig. 4A), DPA (Fig. 4D), and DHA (Fig. 4G) were significantly higher in the liver. In the plasma of the CBF group, EPA (Fig. 4B), DPA (Fig. 4E) and DHA (Fig. 4H) were significantly higher than in the OL animals. In RBC, DPA was also higher in the CBF group compared to the OL animals (Fig. 4F). Altogether, when comparing total n-3 PUFA level amongst groups, a significantly higher level appeared in adipose tissue between FBF and OL diets containing 0.6% energy of ALA (Supplementary Table S1). Total n-3 PUFAs were also significantly increased in the adipose tissue and liver of the CBF animals compared with the FBF animals (Supplementary Tables S1–S2). Finally total n-3 PUFAs were significantly higher in all the tissues of the CBF group compared to the OL group (Supplementary Tables S1–S4).
3.5 n-6 LC-PUFA profiles of the tissues
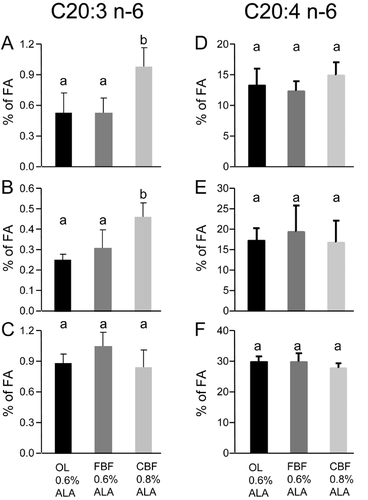
Among the n-6 LC-PUFAs, an increased level of eicosatrienoic acid (C20:3 n-6) was found in the liver and plasma of the rat fed with the CBF diet compared with the two other diets (Fig. 5A and B). No effect of the diets was shown on arachidonic acid (C20:4 n-6) (Fig. 5D and F). Altogether, an increasing significant level of total n-6 PUFAs was shown in adipose tissue of OL, FBF, and CBF rats (Supplementary Table S1). A single significant difference in total n-6 PUFAs was shown in the liver between CBF and OL rats (Supplementary Table S2).
4 Discussion
This study aimed at describing n-3 PUFA tissue composition in rats when: (i) dietary olive oil was substituted for dairy fat with the same ALA dietary level corresponding to 0.6% of energy; and (ii) ALA was slightly increased from 0.6 to 0.8% of energy with a similar dairy fat basis. In this study, we indeed used moderately enriched diets, with ALA and LA composition set to values achievable in a human diet when expressed in energy %. In the experimental diets, ALA and LA were carefully monitored to represent respectively 0.6 or 0.8% and 2.5 or 3.2% of energy with a well-balanced LA/ALA ratio of 4–5, which fits with current recommendations in human nutrition 6. When dietary ALA was increased from 0.6 to 0.8%, the LA/ALA ratio was kept constant by a concomitant increase in dietary LA (from 2.5 to 3.2%). Therefore, competition between the two precursors for their conversion to longer n-3 and n-6 PUFAs would not be implied in the results shown.
By comparing first the olive oil and fractionated butterfat-enriched diets containing identical amounts of ALA (0.6% energy) and LA (2.5% energy), the results showed a higher accumulation of both ALA and LA in adipose tissue and the liver (Fig. 3) when these essential fatty acids were mixed with dairy fat. ALA storage in adipose epididymal tissue is physiologically important as it represents a slow releasable pool that may be utilized over time by other tissues. ALA storage in the liver is also crucial, since it is the major organ in which conversion of dietary ALA to longer n-3 PUFAs occurs. Indeed, in the liver of rats fed the fractionated butterfat-enriched diet, a significant increase in DPA was shown (Fig. 4D). However, DHA level was not modified (Fig. 4G). By contrast, no effect of the increased storage of LA was shown on the hepatic level of longer n-6 PUFAs like C20:3 n-6 and C20:4 n-6 (Fig. 5). These results are therefore in agreement with the hypothesis that, in the liver and adipose tissue, the storage of dietary ALA is enhanced when mixed with dairy fat instead of olive oil, leading to a moderate hepatic increase in n-3 intermediates like DPA, which does not reach DHA. The storage of dietary LA is also increased when mixed with dairy fat but no effect on n-6 LC-PUFA is shown, which may be related to the higher affinity of desaturases and elongases for the n-3 PUFA and/or to specific incorporation mechanisms, which could occur at the tissue level.
In previous studies 20-22, we have already shown that saturated fatty acids (SFAs) mixed with ALA in the lipid fraction of the diet are crucial for the metabolic fate of this specific FA. Firstly, when myristic acid was supplied at physiologically relevant levels for 2 months in the diet of rats (from 0.2 to 1.2% of dietary energy), with a concomitant decrease in stearic acid (C18:0) and a constant level of ALA in each diet (0.3% of energy), a dose–response accumulation of EPA was exhibited in the liver and plasma 20. In a 2nd study, the rats were fed with higher amounts of myristic acid (from 1 to 6% of energy) and again a constant level of ALA (0.3% of energy). The result showed that the concentration of ALA was dose-dependently increased in each tissue. PUFA derivatives of the n-3 family (EPA, DHA) also increased in the brain and RBC 21. In humans, compared with a diet containing 0.6% of myristic acid, a diet containing 1.2% of myristic acid for a 5 wk consumption period significantly enhanced EPA and DHA levels in the plasma phospholipids and DHA level in the plasma cholesteryl esters 33. All these results suggest that the overall conversion of the n-3 precursor to derivatives increases because of the presence of myristic acid in the diet. In addition, the fact that myristic acid has a specific and dose-dependent increasing effect on Δ6-desaturase (FADS2) activity in cultured rat hepatocytes 34 was demonstrated.
More recently, the substitution of olive oil for butterfat containing myristic acid but also short and medium chain fatty acids 22 was shown to increase the storage of ALA in adipose tissue, plasma and liver, the concentration of EPA in adipose tissue and RBC and the level of DPA in the liver, heart and retina in the rat. Moreover, in ALA-deficient post-weaning rats, it has been shown that a dairy fat-based diet (with myristic acid at 7% and short and medium chain FAs at 6.1% of FAs) providing 1.5% of FAs as ALA (0.3% of energy) is more efficient than a palm oil blend (with no myristic acid and no short and medium chain FAs, but with 36.2% of palmitic acid) containing a similar level of ALA for increasing DHA levels in the brain 23, 24. Finally, in the plasma of male offspring rats from mother rats supplemented with butter, DHA was significantly higher than in pups from mothers supplemented with olive oil and margarine 35. These studies suggested therefore that some effects can be attributed not only to myristic acid but also to short and medium chain fatty acids from dairy origin. Dietary short and medium-chain saturated fatty acids (MCFAs) including C4:0 (butyric acid), C6:0 (caproic acid), C8:0 (caprylic acid), and C10:0 (capric acid) have indeed physical and metabolic properties that are distinct from those of long-chain saturated fatty acids (LCFAs ≥ 12 carbons), leading therefore to distinct physiological effects, as well. Intakes of equal-caloric diets rich in MCFAs have been shown to decrease adiposity and increase energy expenditure compared to similar diets rich in LCFAs in overweight humans 36, 37. A diminished storage of fat has also been reported in rats overfed with a MCT diet compared with a LCT diet 27, 38. These data suggest that dietary MCT are readily hydrolyzed to MCFAs, transported through the portal vein and rapidly taken up and oxidized by the liver, leaving no MCT for fat deposition in adipose. In the liver, MCFAs may therefore compete with ALA for the β-oxidation pathway and increase its storage. In the present study, because no short and medium chain SFAs (C4:0–C10:0) was detected in the rat tissues (Supplementary Tables S1–S4), our results confirmed that these FAs from dairy origin have been rapidly taken up and oxidized by the liver.
In the present study, we next wanted to examine the effect of a small enrichment of dietary ALA (from 2.5 to 3.4% of total dietary fatty acids, i.e., from 0.6 to 0.8% of energy) when combined with dairy fat. The results showed indeed an additional accumulation of ALA in adipose tissue (Fig. 3A). However, unexpectedly, ALA in the liver and plasma of the rats fed the commercial butterfat-enriched diet (containing 0.8% energy of ALA) was similar to the level found in rats fed the fractionated butterfat-enriched diet (containing 0.6% energy of ALA) (Fig. 3B and C). Moreover, ALA in RBC was significantly decreased in these animals (Fig. 3D). Therefore, the diet with the higher amount of ALA did promote an increased accumulation of ALA only in adipose tissue. These unexpected results led us to wonder what could explain this decreased or similar incorporation of ALA in specific tissues: A higher β-oxidation rate or an increased conversion to longer n-3 PUFAs. In the liver, n-3 LC-PUFAs such as EPA (Fig. 4A), DPA (Fig. 4D) and DHA (Fig. 4G) were significantly higher in the rats fed the CBF diet, compared with the two other diets. This result showed that, at least in the liver, ALA has been more converted to longer n-3 PUFAs. Therefore, these results suggested that consumption of SFAs (42% of total FAs in the CBF diet, i.e., 9% energy) from dairy origin associated with an adequate intake level of ALA, near its optimal level corresponding to 0.8–1% energy 15, 16, is beneficial first for the tissue bioavailability of ALA itself and additionally for its conversion to longer n-3 PUFA, as well. In ALA-deficient post-weaning rats fed with a diet containing 2.3% of ALA (0.5% of energy) in a dairy blend, a further increase in brain DHA level was exhibited that reached the values obtained with pure rapeseed diet (8.3% of ALA). This effect could be ascribed to both ALA increase (from 0.3 to 0.5% energy) and LA/ALA ratio decrease (from 9.1 to 5.8) 23, 24. Therefore, the results obtained in different rat models are in agreement with the usefulness of increasing even slightly the intake of ALA when mixed in a matrix which is adapted to its protection against β-oxidation (presence of short and medium chain SFAs) and to the potentialization of its conversion to n-3 LC-PUFAs (presence of myristic acid). In the present study, due to the lack of an additional experimental group with a diet supplying 0.8% ALA and no dairy fat, the relative importance of the crucial increase in ALA and the beneficial presence of the dairy fat matrix is however difficult to define.
These results also present several additional arguments to fight against the caricatural exclusion of SFAs from the diet. Dietary SFAs are usually thought to have negative consequences for human health. Indeed, early epidemiological studies have shown that high intake (more than 15% of daily energy intake) of SFAs is positively associated with increased levels of blood cholesterol and high coronary heart disease mortality rates 39, 40. This is the reason why dietary guidelines still promote diets low in saturated fat. According to this negative assumption, SFA are still considered as a single group even though it is now well known that they do not have similar levels in common foods. Moreover, SFA do not have similar metabolic fates 28, 41, and each SFA possesses specific functions 42. In addition, as opposed to the early epidemiological studies 39, 40, 3 recent meta-analyses suggested that there is no significant evidence for concluding that saturated fat is associated with an increased risk of CVD 43-45.
To conclude, we show that, when mixed with a dairy fat blend, a slight increase in dietary ALA has a great impact on its conversion to EPA, DPA and even DHA in the liver of rats. These results support the hypothesis that fatty acids from dairy fat with adequate intake of ALA may contribute to a significant increase in and mainly the tissue storage of n-3 polyunsaturated fatty acids.
The authors are indebted to CNIEL (Centre National Interprofessionnel de l'Economie Laitière, France) for constructive scientific discussion and financial support. We are grateful to X. Blanc (UPAE, INRA, Jouy-en-Josas, France) for the preparation of the diets. We thank N. Boulier-Monthéan and F. Boissel for able technical assistance and animal cares.
The authors declare that this work was partly supported by research grants from the French Dairy Council.




