Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production
Abstract
In the first part of this review, the biochemistry of lipid accumulation in the oleaginous microorganisms is depicted. Lipid biosynthesis form sugars and related substrates is a secondary anabolic activity, conducted after essential nutrient (usually nitrogen) depletion in the medium. Due to this exhaustion, the carbon flow is directed towards the accumulation of intracellular citric acid that is used as acetyl-CoA donor in the cytoplasm. Acetyl-CoA generates cellular fatty acids and subsequently triacylglycerols. Lipid accumulation from hydrophobic substrates is a growth associated process, being independent from nitrogen exhaustion in the medium. Medium fatty acids are incorporated with various incorporation rates and are either dissimilated for growth needs or become “substrate” for intracellular biotransformations. “New” fatty acid profiles (in both extra- and intracellular lipids) that did not previously exist in the medium are likely to be produced. Oleaginous microorganisms consume their own storage lipids when their metabolic abilities cannot be saturated by the extracellular carbon source. Reserve lipid breakdown is independent from the type of the carbon source used for lipid accumulation. In most cases it is accompanied by lipid-free biomass production. Lipid mobilization is a specific process, as preferential degradation of the neutral lipid fractions is observed.
Abbreviations:
Aox, acyl-CoA oxidases; DOT, dissolved oxygen tension (in %, v/v); Fru, fructose (in g/L); Glc, glucose (in g/L); Glol, glycerol (in g/L); L, total intra-cellular lipid (in g/L); PUFAs, polyunsaturated fatty acids; S, substrate fat (in g/L); SCO, single cell oil; TAGs, triacylglycerols; X, total biomass (in g/L); Xf, lipid-free biomass (in g/L)
Introduction
The utilization of oleaginous microorganisms (these that can accumulate lipids to more than 20% w/w, in their dry cell mass) as sources of oils and fats (the so-called single cell oils, SCOs) in large-scale operations presents a great industrial interest. Although the production price of SCOs is higher compared with the traditional utilization of common oils and fats due to the obligatory maintenance of aseptic conditions 1, several alternative options for large-scale production of SCOs exist, since the price of various naturally occurring lipids and fats of the plant and animal kingdom can tremendously vary (from 0.3 to up to 100 US $ per kg—see: Ratledge and Wynn 2). Therefore identification of microorganisms capable of producing in increased quantities lipids with structure and composition similar to that of high-value fats, and subsequent large-scale production can present an enormous financial interest 2. Furthermore, various oleaginous microorganisms have the potentiality to present remarkable growth and production of SCO on wastes and by-products of the argo-industrial sector 3-8; thus, valorization of these residues together with production of potentially high-added value lipid could increase the viability of the process being simultaneously beneficial for the environment. Moreover, the continuously increasing demand and utilization of the so-called “1st generation biodiesel” (fatty acid methyl-esters—FAMEs deriving from trans-esterification of principally plant oils) that has occurred the last 15 years has increased the price of various foodstuffs, and this situation has led to the necessity of discovery of non-conventional sources of oils, that could be subsequently converted into biodiesel. The oleaginous microorganisms (yeasts, molds, and algae) are considered as potential candidates for the production of this lipid that would result in the generation of the so-called “2nd generation” biodiesel 7, 9-16. Finally, specifically the oleaginous yeasts, with their unicellular form, are considered as the more appropriate organisms that have been used as tools in the comprehension of numerous phenomena of lipid biochemistry 2, 4, 17-20.
A very large variety of substrates has been used as carbon sources for the oleaginous microorganisms such as analytical-grade or industrially-derived (therefore, of low-cost) sugars or sugar-enriched wastes or residues, polysaccharides, N-acetylglucosamine, hydrolysates of various products or by-products, vegetable oils, pure free fatty acids, FAMEs, fatty byproducts or wastes, n-alkanes, ethanol, glycerol, and organic acids 4, 7, 8, 10, 13-18, 21-30.
The microbial lipids are mainly composed of triacylglycerols (TAGs) 1, 2, 27, 31, 32. Other components presented in non-negligible quantities are free fatty acids 25, 26, 31-34, other neutral lipids (such as monoacylglycerols, diacylglycerols, and steryl-esters), sterols and polar fractions (e.g. phospholipids, sphingolipids, glycolipids) 5, 27, 31, 32, 35. As a general remark it should be stressed that when growth is carried out on various hydrophobic substances utilized as substrates (the so-called “ex novo” lipid accumulation), the microbial lipid produced contains lower quantities of TAGs compared with growth elaborated on sugar-based substrates (the so-called “de novo” lipid accumulation) 5, 25, 31, 32, 36-40. Additionally, the oil accumulated by the oleaginous fungi is more unsaturated than that of the yeasts 2, 18-20, 28-30, 41. This is the main reason for which the oleaginous molds have been principally used in order to produce lipids rich in polyunsaturated fatty acids (PUFAs) of medical and dietetical interest like γ-linolenic acid (Δ6,9,12C18:3) 5, 6, 28-32, 42-49, dihomo-γ-linolenic acid, arachidonic acid, docosahexaenoic acid, eicosapentanoic acid, etc (for reviews see: Ratledge 4; Čertik and Shimizu 50; Ratledge and Wynn 2; Sakuradani and Shimizu 51; Sakuradani et al. 52). In contrast, few oleaginous yeast strains have been revealed capable to synthesize such types of “rare” PUFAs 2, 18, 20, 30. Nevertheless, the last years, the utilization of genetic engineering has resulted in the construction of genetically modified yeast strains (principally belonging to the species Yarrowia lipolytica), that are capable of (super)-expressing various desaturases (e.g. Δ5-, Δ6-, Δ8-desaturate), elongases and acyl-transferases, therefore, being, capable to synthesize the above mentioned “rare” PUFAs and to transfer these newly synthesized molecules into TAGs 53-56.
In the present review article, we will be interested in the biochemical events related with the lipid accumulation in the oleaginous yeasts when various carbon sources (hydrophilic or hydrophobic ones) are utilized as substrates. Also the events related with storage lipid degradation (turnover) in the oleaginous microorganisms will be assessed and comprehensively discussed.
The biochemistry of lipid accumulation and degradation in the oleaginous microorganisms
Lipid production from fermentation of sugars and related substrates used as carbon source
Substrates used
De novo accumulation of cellular lipids is an anabolic biochemical process in which, by virtue of quasi-inverted β-oxidation reaction series, acetyl-CoA issued by the intermediate cellular metabolism, generates cellular fatty acids. Fatty acids are then esterified with glycerol generating structural (phospholipids, sphingolipids, etc) and reserve (mainly TAGs) lipids 2, 3, 7, 18-20, 23.
With the notable exception of cellulose and methanol a very high number of carbon sources have been considered as substrates for the de novo lipid biosynthesis from oleaginous microorganisms. Concerning the conversion of cellulose into SCO, few papers have recently appeared in the international literature 57, 58. Sugar-based media such as simple sugars (e.g. glucose and fructose), lactose, sucrose, whey, glucose-enriched wastes, molasses, etc 5, 6, 10, 16, 31, 32, 46, 47, 59-68 have been used. Xylose-based media have been recently considered as substrates of noticeable importance due to the abundance of xylose as substrate, deriving after chemical hydrolysis of various lignocellulosic materials 11, 28, 69, 70. In addition, the utilization of sugar-based substrates more complicated compared with glucose, such as polysaccharides (e.g. starch and pectin), has been studied. Although the above substrates are similarly metabolized, in some cases the results that have been achieved, in terms of both lipid and fatty acid composition of the SCO produced, presented notable differences 45, 48, 71.
The stoichiometry of glucose (and similar sugars such as lactose, fructose, etc) metabolism indicates that about 1.1 moles of acetyl-CoA are generated from 100 g of glucose (∼0.56 moles) catabolized 18, 28, 30. As far as xylose is concerned, this compound can be either metabolized through the phosphoketolase reaction, which is the most efficient pathway yielding around 1.2 moles of acetyl-CoA per 100 g of xylose (∼0.66 moles) utilized, or the pentose phosphate pathway, where around 1.0 mole of acetyl-CoA is formed per 100 g of xylose utilized 18, 28. Therefore, if all the acetyl-CoA produced is channeled towards lipid synthesis, the maximum theoretical yield of SCO produced per glucose consumed is around 0.32 g/g 18. This value is somewhat higher concerning the fermentation of xylose (around 0.34 g/g), assuming that oleaginous microorganisms utilize exclusively the phosphoketolase pathway for xylose assimilation. With reference to glycerol, the maximum theoretical yield of SCO is around 0.30 g/g 18. However, even under ideal conditions for SCO production (e.g. highly aerated chemostat cultures) lipid yield on glucose consumed can rarely be higher than 0.22 g/g 1, 2, 7, whereas in other reports this threshold is considered to be in the level of 0.20 g/g or even lower 28, 46, 47. However, in Thamnidium elegans grown on sucrose in shake flasks, the conversion yield of lipid produced per sugar consumed was ∼0.24 g/g, while utilization of other sugars (glucose or fructose) equally resulted in exceptional conversion yields, i.e. >0.20 g/g 65. Maximum conversion yields of the same magnitude compared with growth of T. elegans on sucrose (∼0.23 g/g) have been reported for Cunninghamella echinulata cultivated on xylose in shake-flask experiments 28. The conversion threshold of glycerol into SCO is around 0.10 ± 0.02 g/g, in general lower than that obtained on glucose, xylose or other sugars, regardless of the oleaginous strain used 28, 29, 49, 72-78, presumably due to the relatively poor regulation of the enzymes involved in the primary metabolic steps of glycerol assimilation (e.g. glycerol kinase, 3-P-glycerol dehydrogenase) 7, 76. In contrast, the respective value is slightly higher (e.g. ∼0.15 g/g) for the case of algae like Schizochytrium limacinum growing heterotrophically on glycerol 79-81, while only in two cases so far conversion of glycerol into SCO has been performed with a conversion yield higher than 0.20 g/g; it was either the case of Aspergillus sp. strains that stored remarkable amounts of intra-cellular lipids and also produced quantities of extra-cellular oxalic acid cultured in shake flasks 82, or the case of the fungus T. elegans that produced indeed high SCO quantities (from 5.9 to 11.6 g/L corresponding to lipid in dry weight ranging between 60 and 70% w/w), with conversion yield ranging between 0.16 and 0.22 g/g, equally in shake-flask experiments 83.
Ethanol has been considered as a potential substrate for the de novo lipid biosynthesis of the oleaginous microorganisms by a number of investigators 18, 59, 84-86, given that it is considered as a very proper one since no residual carbon arises from its uses in fermentation processes 18. Taking into consideration that ethanol is by far the more reduced substrate by any other considered yet for the process of de novo lipid accumulation, the final stoichiometric balance for SCO synthesis from ethanol could result in a theoretical yield of 0.54 g of lipid per 1 g of ethanol consumed 18. Nevertheless such high conversion yields have never been achieved in the literature with the conversion threshold of ethanol into SCO being around 0.31 g/g 18, 29, 84.
Coming back to glycerol, even though this substrate presents a slightly lower theoretical conversion yield compared with glucose, this carbon source is of substantial and increasing importance due to its appearance into the market volume in continuously growing quantities due to application into an industrial scale of the bio-diesel production process. In general, the utilization of glycerol as a microbial substrate, refers mainly to the production of 1,3-propanediol by bacteria (for review see: Papanikolaou 87), whilst to the best of our knowledge, only some investigations have been conducted so far with (bio-diesel derived waste) glycerol utilized as substrate (or co-substrate) by microorganisms in order to produce SCO 26, 28, 29, 49, 72-83. In the above-cited papers trials were performed with Cryptococcus curvatus, S. limacinum, Y. lipolytica, Mortierella isabellina, C. echinulata and T. elegans strains. Moreover, in a recent investigation, Chatzifragkou et al. 83 have performed a relatively extensive screening of yeast and Zygomycetes strains (species Candida boidinii, C. curvata, C. oleophila, C. pulcherrima, C. echinulata, M. isabellina, M. ramanniana, Mucor sp., Pichia membranifaciens, Rhodotorula sp., T. elegans, Y. lipolytica, Zygosaccharomyces rouxii, Zygorhynchus moelleri) cultivated on bio-diesel derived glycerol in conditions favoring the production of SCO (nitrogen-limited conditions) and they have found that although yeast strains in general produced higher quantities of biomass compared with the molds, the production of lipid was increased in the fungal strains. The more promising SCO producer amongst all strains tested was the Zygomycete T. elegans 83.
Citric acid 88, acetic acid 89-91, or other low-molecular weight organic acids 90, 92, 93 have been equally considered as substrates for SCO production. Specially, as far as acetic acid is concerned, it is considered as a remarkable pollutant, generated either in the process water of Uranium bleaching or as effluent issued from the Fischer–Tropsch reaction 90, 93; the investigations, thus, concerning its biotransformation in SCO are very interesting in both economical and ecological terms.
Biochemistry of de novo lipid accumulation
All microorganisms are capable to synthesize lipids, though only the oleaginous strains may accumulate inside their cells significant lipid quantities (i.e. >20% w/w, on dry cell basis). In a series of investigations, it has been demonstrated that the oleaginous microorganisms do not possess a hyperactive system of fatty acid biosynthesis, but they are capable of producing in significant quantities, acetyl-CoA, the basic unit of fatty acid biosynthesis 3, 20. The biochemistry, thus, of de novo lipid biosynthesis, may be divided in two distinct parties: the intermediate cellular metabolism and the biosynthesis of TAGs.
The net product of glycolysis is pyruvic acid, which passes through the mitochondrial membrane to the mitochondrion matrix. Pyruvate-dehydrogenase catalyzes the formation of acetyl-CoA from pyruvic acid, and acetyl-CoA either enters inside the Krebs cycle, or is transported again into the cytoplasm in order to enhance biosynthesis of cellular fatty acids 2, 3, 18, 20. Since the mitochondrial membrane is not permeable by acetyl-CoA, the transformation of this compound to acetyl-carnitine (catalyzed by carnitine-acyl-transferase) should be necessary for the transport of this unit into the cytosol. Though, the capital role carnitine-acyl-transferase is exactly the opposite, namely the transport of the acetyl-CoA, issued by β-oxidation, inside the mitochondrion matrix. Minimal amounts of acetylcarnitine may pass through the mitochondrion matrix in order to enter the cytosol, and this is the case of de novo lipid biosynthesis of the non-oleaginous microorganisms 2, 4, 20. In the oleaginous microorganisms, acetyl-CoA that constitutes the precursor of intracellular biosynthesis of fatty acids derives from breakdown of citric acid that under some circumstances has been previously accumulated inside the mitochondria and then is transported into the cytosol (for reviews see: Ratledge 18, 20; Ratledge and Wynn 2; Fakas et al. 30).
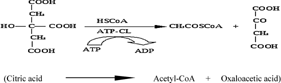
The key step for lipid accumulation in the oleaginous microorganisms is the change of intracellular concentration of various metabolites, conducted after exhaustion of some nutrients into the culture medium 2, 7, 18, 20. In most of the performed studies, the essential nutrient the depletion of which induced the accumulation of reserve lipid is that of nitrogen, whereas the biochemistry of de novo lipid accumulation of lipid has been completely elucidated only when extracellular nitrogen in the limiting factor of microbial growth 1-4, 7, 8, 30. Nitrogen exhaustion provokes a rapid decrease of the concentration of intracellular AMP (adenosine monophosphate), since, by virtue of AMP-desaminase, the microorganism cleaves AMP in IMP (inosine monophosphate) and NH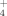 ions. The NH
ions. The NH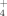 ions constitute a complementary nitrogen source, necessary for synthesis of cell material after the extracellular nitrogen limitation 94. The excessive decrease of intra-cellular AMP concentration alters the Krebs cycle function; NAD+- (and in various cases also NADP+-) isocitrate dehydrogenase, enzyme responsible for the transformation of isocitric to α-ketoglutaric acid, losses its activity, since it is allosterically activated by intracellular AMP 47, 95-97. Thus, iso-citric acid is accumulated inside the mitochondrion. This acid is found in equilibrium with citrate (reaction catalyzed by isocitrate acotinase). When the intra-mitochondrial citric acid concentration reaches a critical value, citrate enters the cytoplasm in exchange with malate 98. Finally, citric acid is cleaved by the ATP-citrate lyase (ATP-CL), the enzyme-key of lipid accumulation process in the oil-bearing microorganisms, in acetyl-CoA and oxaloacetate 94, 96, 99 (for reviews see: Ratledge 18, 20; Ratledge and Wynn 2). Acetyl-CoA, by a quasi-inverted β-oxidation process, will generate the cellular fatty acids (for reviews see: Ratledge and Wynn 2; Papanikolaou and Aggelis 7; Fakas et al. 30). NADPH, indispensable fatty acid biosynthesis, is provided by the intermediate cellular metabolism, in which the importance of malic enzyme has been considered as crucial for various oil-bearing microorganisms, specifically in the cases in which NADPH is produced exclusively by virtue of the reaction catalyzed by the above mentioned enzyme under nitrogen-limited conditions 2, 97. Schematically, the intermediate cellular metabolism of the oleaginous microorganisms in which lipid accumulation is performed after nitrogen exhaustion from the medium, is presented in Fig. 1 (adapted by Davies and Holdsworth 3).
ions constitute a complementary nitrogen source, necessary for synthesis of cell material after the extracellular nitrogen limitation 94. The excessive decrease of intra-cellular AMP concentration alters the Krebs cycle function; NAD+- (and in various cases also NADP+-) isocitrate dehydrogenase, enzyme responsible for the transformation of isocitric to α-ketoglutaric acid, losses its activity, since it is allosterically activated by intracellular AMP 47, 95-97. Thus, iso-citric acid is accumulated inside the mitochondrion. This acid is found in equilibrium with citrate (reaction catalyzed by isocitrate acotinase). When the intra-mitochondrial citric acid concentration reaches a critical value, citrate enters the cytoplasm in exchange with malate 98. Finally, citric acid is cleaved by the ATP-citrate lyase (ATP-CL), the enzyme-key of lipid accumulation process in the oil-bearing microorganisms, in acetyl-CoA and oxaloacetate 94, 96, 99 (for reviews see: Ratledge 18, 20; Ratledge and Wynn 2). Acetyl-CoA, by a quasi-inverted β-oxidation process, will generate the cellular fatty acids (for reviews see: Ratledge and Wynn 2; Papanikolaou and Aggelis 7; Fakas et al. 30). NADPH, indispensable fatty acid biosynthesis, is provided by the intermediate cellular metabolism, in which the importance of malic enzyme has been considered as crucial for various oil-bearing microorganisms, specifically in the cases in which NADPH is produced exclusively by virtue of the reaction catalyzed by the above mentioned enzyme under nitrogen-limited conditions 2, 97. Schematically, the intermediate cellular metabolism of the oleaginous microorganisms in which lipid accumulation is performed after nitrogen exhaustion from the medium, is presented in Fig. 1 (adapted by Davies and Holdsworth 3).
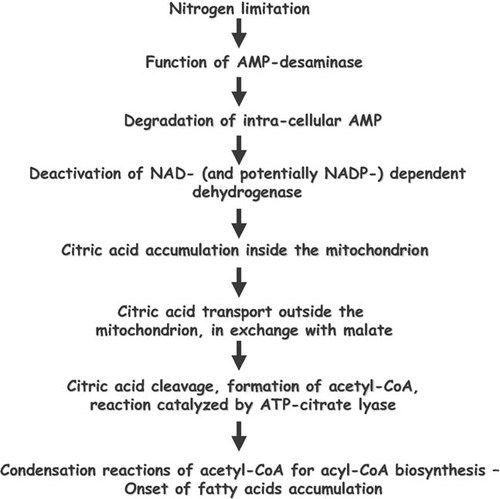
Consecutive steps leading to the de novo lipid biosynthesis in oleaginous microorganisms growing under nitrogen-limited conditions (from Davies and Holdsworth 3, adapted).
Net product of the action of ATP-CL, therefore, is acetyl-CoA, that will be further converted into intra-cellular fatty acids. If ATP-CL enzymatic complex does not exist, nitrogen exhaustion leads in the accumulation of citric acid inside the cytoplasm. In this case, citric acid either will be excreted into the culture medium (case of citric acid production by Aspergillus niger and Candida sp. strains—see: Ratledge 20; Papanikolaou and Aggelis 7) or will provoke the inhibition of the 6-phosphoro-fructokinase, having as result intracellular accumulation of polysaccharides based on 6-phosporo-glucose (case of Aureobasidium pullulans—see: Galiotou-Panayotou et al. 100). Due to the significant biochemical similarity between the intracellular de novo lipid accumulation and the extracellular secretion and production of citric acid, the last years, the yeasts have been divided and classified by various authors as either lipid-accumulating or citric acid-producing ones 49, 101-103. The pattern of intermediate metabolism and de novo lipid biosynthesis is presented in Fig. 2.
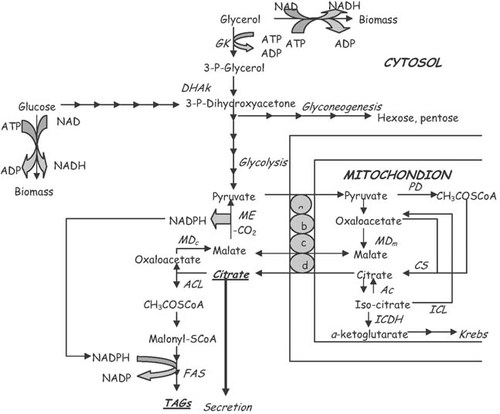
Intermediate metabolism in the oleaginous microorganisms. (a–c) Systems of pyruvate transport from cytoplasm to mitochondrion and inversely for the malate. (d) System of citrate and malate transport between cytoplasm and mitochondrion. Enzymes: Ac, acotinase; ACC, acetyl-CoA carboxylase; ACL, ATP-citrate lyase; FAS, fatty acid synthetase; ICDH, iso-citrate dehydrogenase; MDc, malate dehydrogenase (cytoplasmic); MDm, malate dehydrogenase (mitochondrial); PD, pyruvate dehydrogenase; PFK, phospho-fructo-kinase; PK, pyruvate kinase (from Ratledge 18; Papanikolaou and Aggelis 7, adapted).
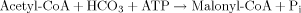
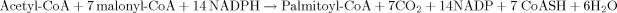
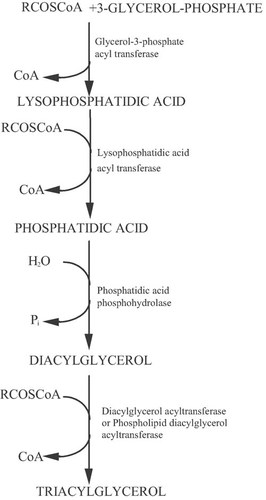
Generally, the multi-enzymatic complexes of FAS and ATP-CL are inhibited by the presence of exogenous long aliphatic chains (e.g. fatty acids, n-alkanes, etc) found into the culture medium 34, 105. Whatever the origin of the intra-cellular aliphatic chains, after the biosynthesis of fatty-CoA esters, an esterification with glycerol takes place in order for the reserve lipids to be stocked in the form of TAGs 18, 20. This synthesis is conducted mainly by the so-called pathway of α-glycerol phosphate acylation (for reviews see: Ratledge 18; Davies and Holdsworth 3; Athenstaedt and Daum 106; Müllner and Daum 107; Fakas et al. 30). In this metabolic pathway, free-fatty acids are activated to coenzyme A and are subsequently used for the acylation of the glycerol backbone to synthesize TAGs. In the first step of TAGs assembly, glycerol-3-phosphate (G-3-P) is acylated by G-3-P acyltranferase (GAT) at the sn-1 position to yield 1-acyl-G-3-P (lysophospatidic acid-LPA), which is then further acylated by lysophosphatidic acid acyltransferase (also named 1-acyl-G-3-P acyltransferase-AGAT) in the sn-2 position to yield phosphatidic acid (PA). This is followed by dephosphorylation of PA by phosphatidic acid phosphohydrolase (PAP) to release diacylglycerol (DAG). In the final step DAG is acylated either by diacylglycerol acyltransferase or phospholipid diacylglycerol acyltransferase to produce TAGs (for reviews see: Ratledge 18; Davies and Holdsworth 3; Athenstaedt and Daum 106; Müllner and Daum 107; Fakas et al. 30). The steps of TAG assembly through the α-glycerol phosphate acylation pathway, a pathway that is very commonly used in the oleaginous microorganisms, are depicted in Fig. 3. Alternatively, PA can be synthesized through the dihydroxyacetone-phosphate (DHAP) pathway as follows (studies performed principally on the non-oleaginous yeast Saccharomyces cerevisiae—for reviews see: Athenstaedt and Daum 106; Müllner and Daum 107): DHAP is acylated at the sn-1 position by the enzyme DHAP acyltransferase (DHAPAT). The product, 1-acyl-DHAP, is reduced by 1-acyl-DHAP reductase (ADR) to yield 1-acyl-G-3-P, which is further acylated to yield PA, reaction catalyzed by AGAT. Finally, as far as the structure of the TAGs produced is concerned, although their final composition could theoretically be a random substitution of acyl-CoA groups into glycerol, in the case of the oleaginous microorganisms that have been examined, the glycerol sn-2 position in most of the cases is occupied by unsaturated fatty acids (therefore, vegetable-type TAGs are produced—see: Thorpe and Ratledge 36; Ratledge 18, 20; Guo and Ota 108).
In batch experiments, lipid accumulation involving de novo biosynthesis pathway in the oleaginous microorganisms is essentially a secondary anabolic activity, since, according to the biochemical steps previously illustrated, it is enhanced after the nitrogen depletion of the culture medium due to “metabolic overflow” at carbon excess conditions (for reviews see: Ratledge 4; Ratledge and Wynn 2; Dyal and Narine 41; Fakas et al. 30; Papanikolaou and Aggelis 8). However, it should be stressed in this point that the limitation of nitrogen is not the sole factor of crucial importance for accumulation of storage lipid during growth of oleaginous microorganisms on sugars or similarly metabolized compounds. For instance, in recent investigations it has been demonstrated that the oleaginous yeast Rhodosporidium toruloides can present efficient production of biomass and accumulation of storage lipid in nitrogen-rich media, provided that phosphorus or sulphate is the limiting factor of cell growth 14, 109. Interestingly enough, the cultivation on sulphate- or phosphate-limited nitrogen-rich media induced remarkable changes in the total fatty acid composition of storage lipids produced, with (noticeable) increment of the cellular saturated fatty acid content of R. toruloides when sulphate-limited conditions were imposed 109. In the later case, thus, with a simple fermentation technique and based on this property of R. toruloides, a cocoa-butter substitute was produced (for more details see part II 155 of the current review-article). In any case, the property of microorganisms to store significant quantities of SCO in nitrogen-rich and phosphate- or sulphate-limited media, can be exploited in industrial level for the valorization of sugar-rich and nitrogen-rich agro-industrial residues 14, 109. In general, growth of oleaginous microorganisms on sugar (or similarly metabolized compounds) based nitrogen- (or sulphate- or phosphate-) limited media can be divided into two distinct phases: (a) phase in which all nutrients are available for the microbial growth (the balanced growth phase). During this phase, cell growth (including total biomass—X and lipid-free biomass—Xf production) is carried out, and substrate (sugar) is consumed with relatively high assimilation rates. In this phase, cellular lipids (L) that include quantities of TAGs are produced but, in general, they contain polar fractions (e.g. sphingolipids, phospholipids, etc.) corresponding to membrane lipids 5, 31. In this phase, total lipid in dry matter corresponds to a value of 5–10% w/w; (b) lipid accumulation phase. In this phase, neutral lipids (mostly TAGs) are accumulated inside the microbial cells or fungal mycelia, while substrate (sugar) uptake could potentially present somehow lower uptake rate compared with the balanced growth phase. It should also be stressed that in several cases (e.g. experiments performed with the oleaginous molds M. isabellina and T. elegans or also with other oleaginous microorganisms), during nitrogen-limiting phase besides lipid, also lipid-free biomass is synthesized. This lipid-free biomass increment despite nitrogen-limited conditions into the medium indicates partial cell growth (and cell proliferation) and principally accumulation of non-lipid storage materials like intra-cellular polysaccharides 28, 29, 46. A pattern of lipid accumulation kinetics in an oleaginous microorganism during growth on sugar-based nitrogen-limited media is presented in Fig. 4 (data from Papanikolaou et al. 65). It is easily understood that the onset of lipid accumulation is given after exhaustion of assimilable nitrogen into the culture medium. Furthermore, it can be seen that after complete exhaustion of sugar from the fermentation medium, some quantities of previously stored lipid are degraded (lipid turnover) in favor of lipid-free material formation.
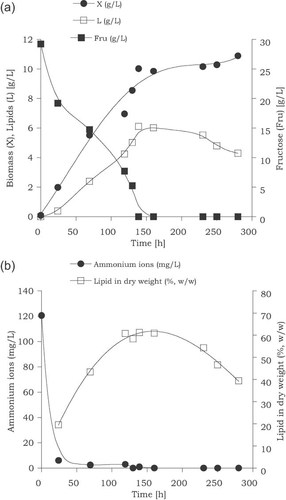
Kinetics of the oleaginous fungus Thamnidium elegans growing on fructose in shake-flask nitrogen-limited experiments (initial fructose at 30 g/L). Representation of biomass production (X, g/L), lipid production (L, g/L), fructose consumption (Fru, g/L) (a), ammonium ions assimilation and lipid in dry weight evolution (b) (data from Papanikolaou et al. 65).
In general, de novo synthesized yeast lipid is composed of C16 and C18 fatty acids. Palmitic acid (C16:0) constitutes the 15–25% w/w, of total lipids, whilst palmitoleic (Δ9C16:1) is, in general, presented in percentages inferior than 5% w/w 4, 7, 20, 24. Likewise, stearic acid (C18:0) is generally a minor component of the yeast lipid (5–8% w/w). Oleic acid (Δ9C18:1) is the principal fatty acid accumulated inside the yeast cells (amounts sometimes higher than 70% w/w), while linoleic (Δ9,12C18:2) is found in the second position (15–25% w/w) 4, 20. More unsaturated fatty acids (e.g. α-linolenic acid—Δ9,12,15C18:3) are not frequently synthesized into the yeast lipid reserves 22. It may be assumed, therefore, the yeast lipid produced is, in general, composed of unsaturated fatty acids. Only in rare cases (e.g. case of Lipomyces starkeyi DSM 70295 growing on glucose-enriched sewage sludge—see: Angerbauer et al. 10) the quantity of saturated fatty acids accumulated by the oleaginous yeasts (principally C16:0 and to lesser extent C18:0) is higher than 50% w/w, of total lipids. In fact, the above event (the potentiality of producing through de novo fatty acid accumulation process globally saturated microbial lipids) is considered to be the limiting step for the synthesis of microbial analogous of expensive exotic fats (e.g. cocoa-butter) 3, 8, 20, 110. Various strategies have been performed in order to alleviate the above disadvantage. The fatty acid profiles of several oleaginous yeasts when de novo lipid accumulation is performed are depicted in Table 1.
| Strain | Lipid (% w/w) | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | Reference |
|---|---|---|---|---|---|---|---|---|
| Candida sp. 107a) | 37.1 | 37 | 1 | 14 | 36 | 7 | T. |
Gill et al. 104 |
| Candida sp. 107 | n.r. | 28 | n.r. | 8 | 41 | 17 | 17 |
Davies 22 |
| Candida sp. | 40.3 | 23 | 13 | 3 | 54 | 5 | 2 |
Aggelis et al. 24 |
| Rhodotorula gracilis | 41.0 | 21 | T. | 13 | 51 | 11 | 3 |
Choi et al. 111 |
| Candida curvatab) | 29.1 | 36 | T. | 14 | 40 | 7 | T. |
Evans and Ratledge 59 |
| Candida curvatab) | 28.0 | 37 | T. | 10 | 44 | 6 | T. |
Evans and Ratledge 59 |
| Apiotrichum curvarumb) | 31.0 | 34 | T. | 10 | 43 | 7 | 2 |
Hassan et al. 113 |
| Cryptococcus curvatusb) | 38.0 | 24 | T. | 10 | 46 | 9 | 6 |
Hassan et al. 114 |
| Cryptococcus curvatusb) | 25.0 | 18 | T. | 16 | 50 | 16 | T. |
Meesters et al. 72 |
| Cryptococcus curvatusb) | 50.0 | 31 | - | 22 | 42 | 1 | n.r. |
Wu et al. 15 |
| Cruptococcus albidus | 46.3 | 14 | T. | 9 | 53 | 18 | 2 |
Hansson and Dostálek 112 |
| Cruptococcus albidus | n.r | 20 | n.r | 11 | 59 | 6 | 6 |
Davies 22 |
| Yarrowia lipolytica | 43.2 | 15 | 2 | 11 | 47 | 21 | 3 |
Papanikolaou and Aggelis 74 |
| Yarrowia lipolytica | 30.7 | 12 | 11 | 9 | 57 | 11 | T. |
André et al. 75 |
| Yarrowia lipolytica a) | 22.3 | 13 | 17 | 6 | 55 | 7 | n.r. |
Makri et al. 76 |
| Rhodosporidium toruloides | 67.5 | 20 | 1 | 15 | 47 | 13 | 3 |
Li et al. 9 |
| Rhodosporidium toruloides | 65.2 | 34 | T. | 13 | 48 | 1 | T. |
Hu et al. 115 |
| Rhodosporidium toruloides | 62.1 | 26 | 2 | 5 | 62 | 3 | T. |
Wu et al. 14 |
| Rhodosporidium toruloides | 55.6 | 43 | T. | 16 | 35 | 2 | T. |
Wu et al. 109 |
| Lipomyces starkeyi | 68.0 | 56 | 2 | 14 | 26 | T. | T. |
Angerbauer et al. 10 |
| Lipomyces starkeyi | 61.5 | 37 | 4 | 6 | 49 | 1 | T. |
Zhao et al. 11 |
| Rhodotorula mucilaginosa | 48.6 | 22 | 2 | 9 | 55 | 11 | T. |
Zhao et al. 116 |
| Trichosporon capitatum | 37.6 | 12 | 1 | 2 | 74 | 9 | n.r. |
Wu et al. 16 |
| Rhodotorula sp. | 22.0 | 22 | 1 | 7 | 56 | 12 | n.r. |
Chatzifragkou et al. 83 |
| Candida oleophila | 15.3 | 13 | 3 | 7 | 66 | 11 | n.r. |
Chatzifragkou et al. 83 |
- T. <0.5% w/w; n.r.: not reported.
- a) Representation of the neutral fraction of microbial lipids produced.
- b) Cryptococcus curvatus was formely Candida curvata and then Apiotrichum curvatum; thus these microorganisms in fact are the same species.
Lipid production from fermentation of hydrophobic substrates used as carbon source
Substrates used
A somehow restricted number of yeast strains have been recorded to be capable of growing on fats and at the same time accumulate significant lipid quantities. These yeasts belong to the genera Torulopsis (T. versatilis, Torulopsis sp.), Candida [C. tropicalis, C. guilliermondii, Yarrowia (C.) lipolytica], Trichosporon, Geortichum and to the species Pichia methanolica, Apiotrichum curvatun and Rhodosporidium toruloides 21, 25, 33, 37, 38, 40, 117-128. It is evident that the number of microorganisms that are capable to consume soaps and free-fatty acids is higher, since culture on these materials is done regardless of the lipolytic capacity of the microorganism used. In contrast, the microorganisms that are able to proceed with TAG or fatty-esters break-down, should obligatory possess an active lipase system into their enzymatic arsenal 8, 17, 122, 129, 130. As far as the yeast Y. lipolytica is concerned, in various reports in the past years it was considered as a non-oleaginous microorganism, since it had been assumed as ineffectual of accumulating significant lipid quantities from sugars or similarly metabolized compounds during submerged growth in nitrogen-limited media 20. However, the capacity of at least some Y. lipolytica strains to accumulate high lipid quantities (up to 60% w/w, in dry weight) when various fats or oils were used as sole carbon and energy source is out of question indicating the oleaginicity of this microorganism 25, 33, 37, 40, 122, 124, 125, 128, 131 (for reviews see: Beopoulos et al. 27; Papanikolaou and Aggelis 8; Sabirova et al. 132). It should also be noticed that in at least one case a strain of Y. lipolytica produced significant intra-cellular fat quantities (23–43% w/w, lipid in dry weight) when cultivated on glucose or glycerol (de novo fatty acid accumulation) in highly aerated bioreactor experiments 64, 74, 76, whereas on the other hand, small production of lipids occurred in shake-flask experiments, in which the nitrogen limitation imposed led to remarkable production of extra-cellular citric acid 49, 133.
The fatty materials utilized as substrate from the oleaginous strains may be vegetable oils 33, 37, 119, 122, 129, 130, 134, fatty esters (methyl-, ethyl-, butyl-, or vinyl-esters of fatty acids) 21, soap-stocks 120, pure free-fatty acids 117, 118, 126, 127, industrial fats composed of free-fatty acids of animal or vegetable origin 25, 40, 124, 125, 133 and crude fish oils 38, 39, 108. In the case of the growth of various microorganisms on media in which hydrophobic materials are found as substrate (or co-substrate), the quantity of TAGs accumulated into the total accumulated lipids, can be substantially lower compared with the lipids produced through the de novo lipid accumulation pathway; for instance, in the case of strains of the molds Amylomyces rouxii and Cunninghamella blakesleeana and the yeast C. lipolytica cultured on soybean oil utilized as the sole substrate, intra-cellular lipid produced contained significant free-fatty acids quantities ranging between 30 and 83% w/w, of total intra-lipid produced, while TAGs represented in various cases a marginal compound of SCO produced 37. Likewise, in the case of the oleaginous yeast Candida sp. 107, fractionation of the lipids produced from this microorganism grown on various n-alkanes showed that, although the TAGs still constituted the major SCO fraction, their relative proportion was less than when glucose was utilized as the sole growth substrate, while loss in TAGs was compensated for by corresponding increase in the quantity of phospholipids 36. Yeast lipid containing TAGs but also considerable quantities of free-fatty acids has been reported during growth of C. lipolytica 1094 on corn oil utilized as the sole substrate 33. Likewise, Aoki et al. 123 have studied the effect of the addition of various hydrophobic materials (e.g. TAGs, fatty acid ethyl-esters or free-fatty acids) used as glucose co-substrates upon the biochemical behavior of the yeast P. methanolica, and it was revealed that the intra-cellular lipid compounds were almost of the same chemical structure with the fatty material that was added into the medium. It was also interesting to state that in the case of the addition of free-fatty acids, fat accumulation ten-fold increased compared with the addition of methyl-esters or TAGs (lipid accumulation around 20% w/w, in dry matter against 2.5–2.8% w/w, respectively), while in the case of added free-fatty acids as co-substrate, SCO produced contained around 93% w/w, free-fatty acids into the total microbial lipids stored 123. Growth of Geotrichum sp. and C. guilliermondii on crude fish oils resulted in the accumulation of TAGs in quantities 30–60% w/w, into the total SCO produced (substantially lower TAG quantities compared with de novo lipid accumulation from sugars—see i.e.: Ratledge 18, 20), while polar lipids or non-identified fatty compounds were also found in noticeable concentrations 38, 108. Finally, growth of Y. lipolytica ACA-DC 50109 on industrial fats composed of free-fatty acids (a fully saturated derivative of tallow called “stearin”, or on mixtures of stearin with fully hydrolyzed oleic rapeseed oil), resulted in SCO production in which TAGs were the principal lipid fraction of the accumulated fat (45–55% w/w, of total lipids), while free-fatty acids were produced in significant amounts as well (about 30–40% w/w, of total lipids) 25, 40. Likewise, fundamental biochemical differences exist between de novo lipid accumulation from nitrogen-limited sugar-based substrates and ex novo lipid production from fats or other hydrophobic compounds utilized as sole carbon and energy source. These differences will be depicted in the following chapter.
The biochemistry of ex novo lipid accumulation
The free-fatty acids (existed as initial substrate or produced after lipase-catalyzed hydrolysis of the TAGs/fatty esters) are incorporated, with the aid of active transport, inside the microbial cell. The incorporated fatty acids are either dissimilated for growth needs or become a substrate for endo-cellular bio-transformations (synthesis of “new” fatty acid profiles which did not exist previously in the substrate) 25, 38-40, 122, 125, 130. The dissimilated free-fatty acids will be degraded, by virtue of the process of β-oxidation, into smaller chain acyl-CoAs and acetyl-CoA, reactions catalyzed by the various acyl-CoA oxidases (Aox) (for reviews see: Fickers et al. 135; Beopoulos et al. 27), providing, thus, firstly the necessary energy for cell growth and maintenance (channel of acetyl-CoA inside the Krebs cycle), and secondly the formation of organic substances (intermediate metabolites) which constitute the precursors for the synthesis of cellular materials 4.
The degradation of hydrophobic materials has been studied in enzymatic and molecular level in significant details by strains of the non-conventional yeast Y. lipolytica 35, 118, 126, 127, 136-140. This yeast when cultivated on TAG-type substrates has been reported to secrete an extra-cellular lipase called Lip2p, encoded by the LIP2 gene 137. This gene encoded for the biosynthesis of a precursor pre-mature protein with Lys-Arg cleavage site. The secreted lipase was reported to be a 301-amino-acid glycosylated polypeptide, which belongs to the TAG hydrolase family (EC 3.1.1.3). The Lip2p precursor protein was processed by the KEX2-like endoprotease encoded by the gene XPR6, whereas deletion of the above gene resulted in the secretion of an active but fewer stable pro-enzyme 27, 135, 137. Simultaneously other intra-cellular lipases (Lip7p and Lip8p) may also be secreted into the culture medium, that present different fatty acid specificities, with maximum activity being displayed against Δ9C18:1, C6:0 (capronic acid) and C10:0 (capric acid) fatty acids (for reviews see: Fickers et al. 135; Beopoulos et al. 27). Then, the released free-fatty acids, produced after lipase-catalyzed hydrolysis of the TAGs would be incorporated inside the yeast cells. It is interesting to state that for the case of Y. lipolytica yeast, the various individual substrate fatty acids are incorporated inside the microbial cell with different rates 25, 40, 125, before being subjected to degradation performed by the various intra-cellular Aox. In fact, it has been revealed that the aforementioned biochemical process is a multi-step reaction requiring different enzymatic activities of five acyl-CoA oxidase isozymes (Aox1p through Aox5p), encoded by the POX1 through POX5 genes 118, 126, 127, 135, 136, 139, 140. Aox3p is specific for short chain acyl-CoAs, Aox2p preferentially oxidizes long-chain acyl-CoAs while Aox1p, Aox4p and Aox5p do not appear of being sensitive in the chain length of the aliphatic acyl-CoA chain 27, 135, 138, 139. Moreover, the physiological function of the above-mentioned oxidases has been investigated by gene disruption 136; mutations in Aox4 and Aox5 resulted in an increase in total Aox activity. The growth of mutant Y. lipolytica strains was analyzed and in the presence of POX1 gene only, strains did not grow on fatty acids, whereas POX4 alone elicited partial growth, while the growth of the double POX2-POX3-deleted mutant was normal on media containing pure Δ9C18:1 as the sole carbon source 136, 139. β-Oxidation contributes one mole of NADH and one mole of FADH2 for every 1 mole of acetyl-CoA generated, before the entering of acetyl-CoA inside the Krebs cycle 4, 20, and is depicted in Fig. 5.
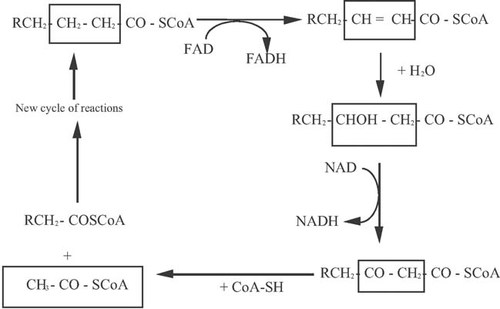
β-Oxidation process of a fatty acid with even number of carbon atoms (from Ratledge 4, adapted).
Principal biochemical differences exist between de novo and ex novo lipid biosynthesis; in the later case, lipid accumulation occurs simultaneously with cell growth, being entirely independent from nitrogen exhaustion from the culture medium 25, 40, 122, 129-131, 134. When fats or other hydrophobic materials are utilized as the sole carbon and energy source, accumulation of reserve lipid is a growth-coupled process 25, 40, 129, 131 in which lipid is accumulated simultaneously with lipid-free material formation in the presence of assimilable nitrogen into the culture medium. A pattern of lipid accumulation kinetics in an oleaginous microorganism during growth on fat-based media (is presented in Fig. 6 (data from Papanikolaou et al. 131). Moreover, in order to further demonstrate the independence of the process of ex novo lipid accumulation from the extra-cellular nitrogen availability of the culture medium, Papanikolaou et al. 131 have performed submerged cultures of Y. lipolytica strain ACA-DC 50109 on stearin at two initial concentrations (S0 = 10 or 20 g/L) and different nitrogen concentrations [(NH4)2SO4 = 0.0–10.0 g/L] and indeed, it has been demonstrated that in all instances significant biomass and SCO quantities were produced (Fig. 7—data from Papanikolaou et al. 131). However, although total cellular lipid was produced in significant quantities regardless of the nitrogen availability into the medium, the higher quantities of lipid were produced when substantially low initial nitrogen concentration media were employed (Fig. 7). Moreover, it should be stressed that with the aid of genetic engineering, there has been a construction of “obese” yeast strains (strains belonging to the species Y. lipolytica), in which the various genes encoding to the Aox have been subjected to disruption 126, 127. Therefore mutant Y. lipolytica strains in which various Aox were deleted, have been constructed 126, 127. These strains were the JMY 798 (MTLY 36-2P) and JMY 794 (MTLY 40-2P) deriving from the wild strain W29 (ATCC 20460), and despite the disruption of the genes encoding for the synthesis of the various Aox, the genetically modified strains presented significant and comparable growth with the W29 wild strain during growth on pure fatty oleic acid, but in the former case noticeable quantities of microbial lipids were accumulated as high-size intra-cellular lipid droplets, the so-called lipid bodies 126, 127. The lipid bodies are considered as depots of neutral lipids (TAGs and to lesser extent steryl-esters) enclosed by polar fractions, while in a relatively recent development, extensive proteomic analysis of the lipid-particle proteins has been elaborated 35.
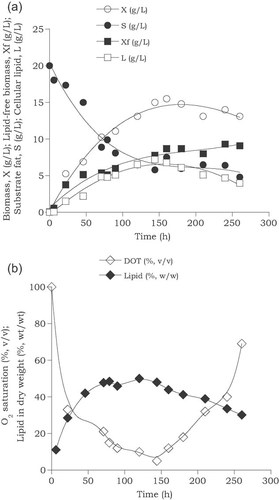
Kinetics of the oleaginous yeast Yarrowia lipolytica on an industrial derivative of tallow composed of free-fatty acids (stearin) used as the sole carbon source. Representation of biomass production (X, g/L), lipid production (L, g/L), lipid-free material production (Xf, g/L), substrate fat consumption (S, g/L) (a), dissolved oxygen tension (DOT, % v/v) and lipid in dry weight evolution (b) (data from Papanikolaou et al. 131).
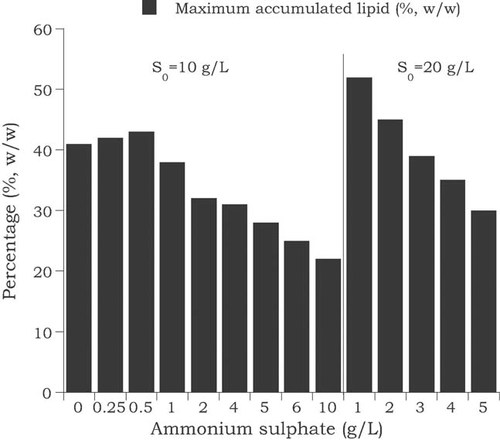
Kinetics of the oleaginous yeast Yarrowia lipolytica on an industrial derivative of tallow composed of free-fatty acids (stearin) used as the sole carbon source. Representation of maximum SCO produced (% w/w, in dry mass) during growth at initial stearin concentration 10 or 20 g/L and variations in the initial concentration of ammonium sulphate (data from Papanikolaou et al. 131).
When fats are used as sole carbon and energy source, there is not any de novo lipid biosynthesis from the acetyl-CoA, since fatty acid synthetase (FAS) and ATP-citrate lyase (ACL) are strongly inhibited by the presence of exogenous aliphatic chains. Therefore, the presence of hydrophobic materials in the culture medium should be generally incompatible with de novo biogenesis of cellular lipids even if glucose or other hydrophilic materials presented into the medium and were co-metabolized with the exogenous fatty acids 34, 105. For instance, Meyer and Schweizer 105 proceeded with growth of S. cerevisiae and C. lipolytica strains on mixtures of glucose and pentadecanoic acid. It has been reported that, although the extra-cellular concentration of pentadecanoïc acid was indeed low (about 0.3% w/v), the fatty acid synthetase activity of the strains was completely repressed, having as a result, only the presence of odd fatty acids into the cellular lipids. However, in more recent investigations it has been demonstrated that some de novo biosynthesis of fatty acids from glucose or glycerol could potentially occur, in spite of the presence of significant exogenous quantities of fatty materials; for instance, the biochemical behavior of P. methanolica HA-32 was studied in media composed of glucose (at 50 g/L) and fish oil (or its derivatives ethyl-esters or free-fatty acids) (at 30 g/L). The fish oil (or its derivatives) contained significant quantities of docosahexanoic acid (DHA—Δ6,9,12,15,17,19C22:6), a fatty acid that through the de novo lipid accumulation from glucose can never be synthesized in P. methanolica 123. Although the extra-cellular presence of TAGs or free-fatty acids into the culture medium resulted in an intra-cellular fatty acid profile similar to that of the substrate fat, and, therefore, significant DHA quantities were detected into the SCO produced presumably through its direct incorporation from the fatty substrate, the presence of fatty acid ethyl-esters resulted in the synthesis of a cellular lipid containing very restricted quantities of DHA, and presenting similarities with the one that had been synthesized when glucose was used as the sole carbon source 123. Therefore, the yeast P. methanolica could perform de novo fatty acid biosynthesis from glucose, in spite of the presence of exogenous aliphatic onto the culture medium 123. Likewise, in investigations performed with the yeast Y. lipolytica ACA-DC 50109 in submerged cultures, in which growth was supported by the simultaneous use of stearin (mixtures of free-fatty acids and mainly of C16:0 and C18:0) and industrial glycerol or glucose, extensive studies of the intra-cellular lipid profile of microbial lipid produced, equally suggested some de novo synthesis of intra-cellular fatty acids, in spite of the presence of long-chain fatty acids found into the culture medium 26, 102; when Y. lipolytica ACA-DC 50109 had been cultured on stearin utilized as the sole substrate, no dehydrogenation or elongation reactions were conducted in cellular level 40, while the microbial lipid produced was almost completely saturated and was composed mainly of C18:0 fatty acid 40, 131. Given that growth of Y. lipolytica on glucose or glycerol used as the sole substrate is accompanied by synthesis of a lipid that is globally unsaturated 49, 74, 102, 133, enrichment of the reserve lipid with unsaturated fatty acids during growth on glucose/stearin or glycerol/stearin mixtures (principally Δ9C18:1 and Δ9,12C18:2) that occurred, indicates de novo fatty acid biosynthesis despite the presence of long aliphatic chains into the medium (see relevant results in Table 2).
| Fatty acid composition of stearin (% w/w) | |||||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | ||||
| 25 | 52 | 2 | T. | ||||
| Fatty acid composition of microbial lipid produced (% w/w) | |||||||
|---|---|---|---|---|---|---|---|
| S0 (g/L) | Glol0 (g/L) | Glc0 (g/L) | C16:0 | C18:0 | C18:1 | C18:2 | Reference |
| 10 | – | – | 13 | 83 | 3 | T. |
Papanikolaou et al. 25 a) |
| – | 30 | – | 15 | 10 | 45 | 20 |
Papanikolaou et al. 133 a) |
| – | – | 30.0 | 11 | 6 | 53 | 10 |
Papanikolaou et al. 102 a) |
| – | 50 | – | 15 | 11 | 47 | 21 |
Papanikolaou and Aggelis 74 b) |
| 10 | 11 | – | 16 | 72 | 7 | 2 |
Papanikolaou et al. 40 c) |
| 10 | 10 | – | 15 | 76 | 5 | 2 |
Papanikolaou et al. 26 a) |
| 10 | 23 | – | 16 | 68 | 7 | 2 |
Papanikolaou et al. 26 a) |
| 10 | 34 | – | 14 | 67 | 10 | 3 |
Papanikolaou et al. 26 a) |
| 11 | – | 21 | 15 | 68 | 7 | 3 |
Papanikolaou et al. 102 a) |
| 11 | – | 35 | 15 | 68 | 8 | 3 |
Papanikolaou et al. 102 a) |
| 9 | – | 29 | 17 | 56 | 18 | 8 |
Papanikolaou et al. 102 a) |
- T. <0.5% w/w. Representation of cellular fatty acid composition in the fermentation time in which the maximum concentration of microbial lipid had been achieved. S0, initial concentration of fatty material (stearin, that is a fully saturated tallow derivative composed of free-fatty acids); Glol0, initial concentration of glycerol (utilization of bio-diesel derived waste glycerol as substrate); Glc0, initial concentration of glucose (utilization of commercial-glucose as substrate). Fatty acids and glucose (or glycerol) were simultaneously consumed by the microorganism.
- a) Batch flask culture.
- b) Single-stage continuous culture.
- c) Batch bioreactor culture.
The oleaginous yeasts growing on various hydrophobic materials (e.g. oils, fats, free-fatty acids, FAMEs), accumulate and at the same time modify the fatty acid composition of the employed fatty substance utilized as substrate 21, 25, 39, 40, 108, 117, 119, 122, 125. The phenomena that are controlling the fatty acid composition of the cellular lipids are the specific rate of incorporation of substrate aliphatic chains inside the microbial cells and the intra-cellular changes of fatty acids defined by the enzymatic capabilities of the microorganism 25, 122. In addition, the (typo- and stereo-) specificity of the microbial lipases versus the substrate fat (in the case in which TAGs are used as carbon source), can equally potentially lead to a biomodification of the substrate fatty acid composition as a function of the bioconversion time 33, 122, 125. For all of the above cited reasons, the fatty acid profile of the accumulated reserve lipid can potentially be significantly distinguishable compared with that of the initial fat substrate employed. Various oleaginous yeasts, possessing active systems of intra-cellular desaturations (desaturases Δ9 and Δ12), can potentially be implicated in the accumulation of a more unsaturated fat compared with the substrate 21, 117, 120, 141. In other cases (growth on various fish oils) yeast strains may consume more rapidly from the substrate the saturated (C14:0 and C16:0) and oligounsaturated (Δ9C18:1) fatty acids, storing into their cells the polyunsaturated fatty acids (e.g. DHA) fatty acids, resulting equally in the synthesis of a SCO more unsaturated compared with the substrate fat 39. On the other hand, various yeasts present the tendency to dissimilate for growth and maintenance the unsaturated and lower aliphatic chain fatty acids, and accumulate the saturated ones, resulting, thus, in the synthesis of cellular lipids more saturated compared with the substrate 25, 40, 122, 125. Likewise, in other cases, the accumulated lipid composition is almost the same with that of the substrate 33, 37, 119, whereas, finally, it is interesting to indicate that in some cases the fatty acid composition of the intra-cellular free-fatty acids can present remarkable differences in comparison with the stored TAGs 128. The fatty acid profiles of the initial substrate fat used and the accumulated lipid for fermentations performed by oleaginous microorganisms is presented in Table 3. Likewise, in various cases growth of oleaginous microorganisms on fatty mixtures may result in selective uptake of the various individual fatty acids from the culture medium, and during fermentation the extra-cellular fat also may present significant compositional differences compared with the initial substrate fat 37, 125, 142. The fatty acid profiles of the initial lipid substrate and the remaining lipid fraction after fat fermentations performed by eukaryotic microorganisms are presented in Table 4.
| Substrate | Fatty acid composition (% w/w) | Strain | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:5 | C22:6 | ||||
| Methyl-stearate | S | – | 100 | – | – | – | – | – | Torupopsis sp. |
Matsuo et al. 21 |
| C | 4 | 40 | 48 | 5 | 1 | – | – | |||
| Vinyl-esters | S | 35 | 65 | – | – | – | – | – | Torupopsis sp. |
Matsuo et al. 21 |
| C | 26 | 37 | 22 | 8 | T. | – | – | |||
| Methyl-esters | S | 30 | 70 | – | – | – | – | – | Candida tropicalis |
Matsuo et al. 21 |
| C | 35 | 17 | 32 | 6 | 2 | – | – | |||
| Methyl-esters | S | 30 | 70 | – | – | – | – | – | Trichosporon sp. |
Matsuo et al. 21 |
| C | 39 | 18 | 32 | 5 | 1 | – | – | |||
| Pure stearic acid | S | – | 100 | – | – | – | – | – | Rhodospodidium toruloides |
Gierhart 117 |
| C | 13 | 49 | 26 | 4 | T. | – | – | |||
| Corn oil | S | 12 | 2 | 22 | 62 | 1 | – | – | Candida lipolytica |
Glatz et al. 119 |
| C | 11 | 2 | 36 | 41 | T. | – | – | |||
| Palm oil | S | 30 | 2 | 45 | 11 | 2 | – | – | Candida lipolytica |
Glatz et al. 119 |
| C | 27 | 8 | 47 | 10 | T. | – | – | |||
| Olive oil | S | 13 | 3 | 69 | 11 | T. | – | – | Candida lipolytica |
Glatz et al. 119 |
| C | 12 | 3 | 70 | 13 | T. | – | – | |||
| Palmitate soaps | S | 52 | 5 | 31 | 7 | T. | – | – | Candida lipolytica |
Montet et al. 120 |
| C | 34 | 3 | 38 | 17 | 2 | – | – | |||
| Refined soybean oil | S | 10 | 4 | 22 | 56 | 8 | – | – | Aspergillus flavus |
Koritala et al. 37 |
| Ca) | 9 | 2 | 9 | 66 | 12 | – | – | |||
| Soybean oil | S | 10 | 4 | 22 | 56 | 8 | – | – | Candida lipolytica |
Koritala et al. 37 |
| Ca) | 7 | 7 | 21 | 60 | 9 | – | – | |||
| EPOb) | S | 7 | 2 | 11 | 69 | 7 | – | – | Langermania gigantea |
Aggelis et al. 122 |
| C | 7 | 1 | 9 | 74 | 7 | – | – | |||
| EPO | S | 7 | 2 | 11 | 69 | 7 | – | – | Rhodotorula sp. |
Aggelis et al. 122 |
| C | 30 | 5 | 36 | 25 | T. | – | – | |||
| EPO | S | 7 | 2 | 11 | 69 | 7 | – | – | Candida lipolytica |
Aggelis et al. 122 |
| C | 8 | 1 | 13 | 76 | T. | – | – | |||
| Crude fish oil | S | 16 | 6 | 22 | 3 | 1 | 9 | 13 | Geotrichum sp. |
Guo et al. 38 |
| C | 20 | 7 | 21 | 5 | 2 | 7 | 16 | |||
| Crude fish oil | S | 16 | 6 | 22 | 3 | 1 | 9 | 13 | Candida guillermondii |
Guo and Ota 108 |
| C | 20 | 7 | 29 | 6 | 2 | 4 | 10 | |||
| Refined sardine oil | S | 17 | 5 | 15 | 2 | – | 22 | 13 | Geotrichum sp. |
Kinoshita and Ota 39 |
| C | 7 | 3 | 13 | 4 | – | 26 | 21 | |||
| Tuna head oil | S | 18 | 4 | 26 | 1 | – | 7 | 27 | Geotrichum sp. |
Kinoshita and Ota 39 |
| C | 11 | 2 | 31 | 5 | 8 | 37 | ||||
| Tuna head oil | S | 18 | 4 | 26 | 1 | – | 7 | 27 | Candida guilliermondii |
Kinoshita and Ota 39 |
| C | 22 | 2 | 38 | 4 | 2 | 14 | ||||
| Stearin/HOROc) | S | 15 | 26 | 38 | 10 | 3 | – | – | Yarrowia lipolytica |
Papanikolaou et al. 25 |
| 50/50 | C | 14 | 50 | 24 | 4 | – | – | – | ||
| Stearin | S | 25 | 52 | 2 | T. | – | – | – | Yarrowia lipolytica |
Papanikolaou et al. 40 |
| C | 16 | 80 | 1 | – | – | – | – | |||
| Stearin/HORO | S | 20 | 38 | 22 | 5 | 1 | – | – | Yarrowia lipolytica |
Papanikolaou and Aggelis 125 |
| 70/30 | C | 21 | 68 | 8 | 1 | – | – | – | ||
| Stearin/HORO | S | 13 | 22 | 44 | 9 | 5 | – | – | Yarrowia lipolytica |
Papanikolaou and Aggelis 125 |
| 40/60 | C | 16 | 57 | 23 | 4 | T. | – | – | ||
| Olive oil | S | 11 | 5 | 79 | 4 | T. | – | – | Yarrowia lipolytica |
Najjar et al. 128 |
| Ca) | 3 | 1 | 83 | 8 | T. | – | – | |||
| Olive oil | S | 11 | 5 | 79 | 4 | T. | – | – | Yarrowia lipolytica |
Najjar et al. 128 |
| Cd) | 30 | 24 | 35 | 5 | T. | – | – | |||
| Waste cooking olive oil | S | 16 | 2 | 72 | 7 | T. | – | – | Aspergillus sp. |
Papanikolaou et al. 143 |
| C | 9 | 19 | 65 | 6 | T. | – | – | |||
| Waste cooking olive oil | S | 16 | 2 | 72 | 7 | T. | – | – | Aspergillus niger |
Papanikolaou et al. 143 |
| C | 14 | 3 | 78 | 5 | T. | – | – | |||
- T. <0.6% w/w.
- a) Representation of the TAG fraction of total cellular lipids produced.
- b) EPO is evening primrose oil.
- c) Stearin: Industrial derivative of tallow composed of fully saturated free fatty acids; HORO: Fully hydrolyzed oleic rapeseed oil.
- d) Representation of the free-fatty acids fraction of total cellular lipids.
| Substrate | Fatty acid composition (% w/w) | Strain | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:5 | C22:6 | ||||
| Refined soybean oil | S | 10 | 4 | 22 | 56 | 8 | – | – | Candida lipolytica |
Koritala et al. 37 |
| SRa) | 12 | 5 | 23 | 51 | 7 | – | – | |||
| SRb) | 8 | 4 | 24 | 58 | 6 | – | – | |||
| Refined soybean oil | S | 10 | 4 | 22 | 56 | 8 | – | – | Amylomyces rouxii |
Koritala et al. 37 |
| SRa) | 12 | 4 | 22 | 55 | 8 | – | – | |||
| SRb) | 4 | 1 | 24 | 59 | 8 | – | – | |||
| Refined soybean oil | S | 10 | 4 | 22 | 56 | 8 | – | – | Cunninghamella blakesleeana |
Koritala et al. 37 |
| SRa) | 18 | 7 | 24 | 46 | 6 | – | – | |||
| SRb) | 5 | 2 | 24 | 61 | 8 | – | – | |||
| EPOc) | S | 7 | 2 | 11 | 69 | 7 | – | – | Langermania gigantea |
Aggelis et al. 122 |
| SR | 9 | 2 | 10 | 72 | 7 | – | – | |||
| EPO | S | 7 | 2 | 11 | 69 | 7 | – | – | Rhodotorula sp. |
Aggelis et al. 122 |
| SR | 7 | 2 | 10 | 72 | 8 | – | – | |||
| EPO | S | 7 | 2 | 11 | 69 | 7 | – | – | Candida lipolytica |
Aggelis et al. 122 |
| SR | 7 | 2 | 11 | 71 | 9 | – | – | |||
| Refined sardine oil | S | 17 | 5 | 15 | 2 | – | 22 | 13 | Geotrichum sp. |
Kinoshita and Ota 39 |
| SR | 5 | 4 | 10 | 2 | – | 37 | 19 | |||
| Stearin/HOROd) | S | 20 | 38 | 22 | 5 | 1 | – | – | Yarrowia lipolytica |
Papanikolaou and Aggelis 125 |
| 70/30 | SR | 22 | 74 | 4 | T. | – | – | – | ||
| Stearin/HORO | S | 16 | 33 | 29 | 7 | 3 | – | – | Yarrowia lipolytica |
Papanikolaou and Aggelis 125 |
| 60/40 | SR | 15 | 76 | 8 | T. | – | – | – | ||
| Stearin/HORO | S | 13 | 22 | 44 | 9 | 5 | – | – | Yarrowia lipolytica |
Papanikolaou and Aggelis 125 |
| 40/60 | SR | 17 | 71 | 11 | T. | T. | – | – | ||
| Sunflower oil | S | 5 | 5 | 34 | 53 | n.r. | – | – | Yarrowia lipolytica |
Kamzolova et al. 142 |
| SR | 5 | 24 | 27 | 35 | n.r. | – | – | |||
| Waste cooking olive oil | S | 16 | 2 | 72 | 7 | T. | – | – | Aspergillus niger |
Papanikolaou et al. 143 |
| SR | 5 | 4 | 88 | 3 | T. | – | – | |||
| Waste cooking olive oil | S | 16 | 2 | 72 | 7 | T. | – | – | Penicillium expansum |
Papanikolaou et al. 143 |
| SR | 11 | 2 | 83 | 4 | T. | – | – | |||
- T. <0.5% w/w; n.r.: not reported.
- a) Free-fatty acid fraction of the residual oil.
- b) TAG fraction of the residual oil.
- c) EPO is evening primrose oil.
- d) Stearin: Industrial derivative of tallow composed of fully saturated free-fatty acids; HORO: Fully hydrolyzed oleic rapeseed oil.
Degradation of reserve lipid in the oleaginous microorganisms
After exhaustion or decrease in the uptake rate of the carbon source in the culture medium the oleaginous microorganisms, as a general rule, consume their own lipid reserves. Several types of studies have been performed for this purpose using oleaginous yeasts, molds and bacteria and under various environmental conditions, breakdown (turnover) of the previously produced lipophilic materials was conducted, with this process being performed simultaneously with biosynthesis of new lipid-free biomass 5, 6, 25, 37, 40, 47, 48, 124, 125, 129-135, 143, 144. Likewise, a significant part of work has been performed in relation the mobilization of neutral lipids in non-oleaginous yeasts (in most cases research performed with S. cerevisiae—see i.e.: Athenstaedt et al. 145; for review see: Müllner and Daum 107). Utilization of TAGs and steryl-esters (STEs) (the main lipid compounds found in both oleaginous and non-oleaginous microorganisms—see: Ratledge 20; Müllner and Daum 107) from the lipid particles requires the action of TAG lipase(s) (EC 3.1.1.3) and STE hydrolase(s) (EC 3.1.1.13). An intra-cellular lipase's and hydrolase's system is expressed either constitutively or inducibly 146, 147, being responsible for the cleavage of the esters and the generation of fatty acids that will be subsequently catabolized 34, 146, 147. Moreover, in the case of plant or mammalian cells, in which storage lipid mobilization is also routinely observed, some proteins associated with the phospholipid monolayer of lipid particles, such as perilipins (in the case of mammalian cells) and oleosins (in the case of the plants), were assumed to be involved in the mobilization of the neutral lipid core of the particle by serving as docking and/or activating proteins for TAG lipases and STE hydrolases 107. Analysis of the most abundant yeast lipid particle proteins by mass spectrometry led to the identification of several polypeptides with unknown function 145, but in any case, no one of these polypeptides, was homologous to perilipins and oleosins 107.
The presence of lipases and STE hydrolases is a prerequisite in order for onset of storage lipid turnover to be given 107, 146, 147, while carbon starvation conditions are also required 25, 32, 124, 131, 134, 143, 144, 146. In a series of investigations it has been demonstrated that the phenomenon of cellular lipid degradation in the oleaginous microorganisms was independent of the culture “pre-history” (meaning, in fact, independent of the type of the carbon source assimilated by the microorganism in order to proceed with reserve lipid accumulation), since such turnover has been observed even though SCO accumulation previously occurred through de novo 30, 32, 67, 144, 148 or ex novo mechanism 25, 40, 124, 129, 131, 134, 143. In any case, the released cellular fatty acids will be catabolized via the process of β-oxidation and the produced acetyl-CoA will be further converted via the Krebs cycle and the anaplerotic by-pass of glyoxylic acid 40, 67, 97, 148; in the case in which de novo lipid accumulation had been previously performed, during lipid turnover period, sugar (that could potentially exist into the culture medium), is no longer assimilated, while the function of Krebs cycle through the utilization of NAD+- (and potentially NADP+)-isocitrate dehydrogenase had already been suppressed due to extra-cellular nitrogen limitation 47, 99. In general, the activity of glyoxylic acid by-pass enzymes (carnitine acetyl-transferase and iso-citrate lyase) increases considerably in cells growing on C2 compounds (e.g. ethanol) or on substrates leading to C2 unit's formation (i.e. previously accumulated TAGs, extra-cellular TAGs, hydrocarbons or free fatty acids), while this activity is indeed reduced during growth on glucose or other sugars 67, 142, 148, 149. Therefore, even though microbial growth had been performed on sugars or similarly metabolized compounds, SCO turnover should be principally performed through the glyoxylic acid by-pass pathway, while cellular nitrogen (obtainable via AMP-desaminase) should secure the biosynthesis of new lipid-free material 47. On the other hand, degradation of accumulated lipid when ex novo lipid accumulation had been previously performed is conducted indisputable principally through the glyoxylic acid anaplerotic by-pass pathway; in fact, reserve lipid turnover generally occurs when the extra-cellular flow rate of aliphatic chains is considerably decreased. This fact can be either due to the apparent absence of substrate fat from the culture medium 134, 143 or due to the presence of fat that is substantially rich in the fatty acid C18:0 and cannot be easily catabolized by the yeast strains due to discrimination against this fatty acid 25, 40, 121, 124, 125. In the case of ex novo lipid accumulation, culture conditions could be still favorable for microbial growth since extra-cellular nitrogen may be presented into the medium even during reserve lipid degradation 40. Additionally, biosynthesis of carnitine acetyl-transferase and iso-citrate lyase had been previously induced due to the cultivation on fatty materials 142, 143, 150. Moreover, in recent investigations it has been demonstrated that lack of some nutritional components such as iron and magnesium from the fermentation medium, can partially or even completely suppress the degradation of microbial lipids 47. On the other hand, utilization of multiple-limited media did not negatively influence the process of storage lipid accumulation, and therefore utilization of such types of media presents obvious interest for the process of SCO fermentation in industrial scale 47.
Microbial lipid turnover has been extensively studied in C. echinulata and Y. lipolytica. It has been demonstrated that C. echinulata tended to selectively degrade its stored TAGs instead of the other neutral lipid fractions (e.g. diacylglycerols, free-fatty acids, STE etc) 32. Moreover, the intra-cellular saturated and monounsaturated fatty acids were rapidly and selectively consumed, while the polyunsaturated ones (e.g. Δ9,12C18:2, Δ6,9,12C18:3) were not. Therefore, after accomplishment of SCO turnover, the remaining cellular lipid was drastically more unsaturated compared with the originally accumulated lipid 32. On the contrary, Y. lipolytica tended to dissimilate for growth and energy requirements the unsaturated fatty acids regardless of their concentrations into the stored lipid reserves, and thus, after storage lipid turnover, the remaining cellular lipid was almost completely saturated 125. Mobilization of microbial lipophilic compounds has also been studied in oleaginous bacteria; in this case the tested strains had previously accumulated both TAGs and poly-hydroxy-alkanoates (PHAs), and as in the case of oleaginous yeasts and molds turnover of storage materials occurred due to shifting from carbon excess to carbon limited conditions 146. In this case, it has been revealed that simultaneous consumption of TAGs and PHAs at the stationary growth phase was performed 146.
Reserve lipid turnover was in general accompanied by generation of lipid-free material (Xf) providing evidence that accumulated lipids act as precursor in the biosynthesis of cell materials. Holdsworth and Ratledge 144 have proceeded with a two-stage fermentation of the oleaginous yeast C. curvata growing on glucose. In the first stage, lipid accumulation was enhanced (growth on glucose, initial molar ratio C/N = 60), while, in the second stage, no extra-cellular carbon source was employed. The microorganism used its accumulated lipids; in this case, the obtained yield YXf/L (g of lipid-free biomass formed per 1 g of cellular lipid consumed) was about 1.9 g/g 144 that can be considered as somehow elevated, since the maximum theoretical yield of conversion of TAGs into lipid-free biomass is around 1.7 g/g 30, 32, 144. The same yield presented drastically lower values (0.6–0.8 g/g) during growth of Y. lipolytica ACA-DC 50109 on industrial fats composed of mixtures of saturated and unsaturated fatty acids 25, 40, 124, whilst growth of Mucor circinelloides on vegetable oils was accompanied by a yield YXf/L 0.63–0.66 g/g 134. Finally, growth of the mold C. echinulata on glucose enriched tomato waste hydrolysate was accompanied by cellular lipid degradation with yield YXf/L value 0.95 g/g 32, with the respective value for M. isabellina cultured on glucose being around 1.0 ± 0.1 g/g 47 and on starch being slightly lower (0.7–0.8 g/g) 48.
Concluding remarks—future perspectives
The production of SCOs presents continuous expansion the last years. Amongst microbial lipids, yeast lipids present a high importance in both academic and industrial point of view. Due to their unicellular nature and their potentiality to grow on a plethora of hydrophilic or hydrophobic substrates, the oleaginous yeasts are considered as perfect “tools” for studying phenomena of advanced lipid biochemistry and biotechnology 4, 8, 132, 135. Interestingly, the last years there have been works indicating interesting analogies between lipid metabolism in the yeast cells and the gastro-intestinal tract and vascular system 128, 151.
Fundamental differences in biochemical level exist between de novo lipid accumulation from hydrophilic substrates and ex novo lipid accumulation from hydrophobic substrates. In the former case, lipid production is a secondary anabolic activity occurring after nitrogen had been depleted from the growth medium. On the other hand, ex novo lipid production is a growth associated process occurring simultaneously with cell growth, being entirely independent from nitrogen exhaustion from the culture medium. The major industrial applications related with the de novo accumulation of storage lipid in yeasts refer to the production of “non-specified” lipids that will be further converted into FAMEs (2nd generation bio-diesel), since the conventional utilization of edible oils as starting materials for the synthesis of bio-diesel has resulted in significant increase in their price the last years 1. Moreover, through the de novo lipid accumulation, it is possible to produce yeast lipids that have a fatty acid composition similar to that of cocoa-butter 3, 4, 19, 20. The prospective (significant) rise in the price of cocoa-butter indicates again the potentiality of the utilization of oleaginous yeasts to produce cocoa butter-like cellular lipids. Needless to indicate that for both the above mentioned potentialities, the utilization of low- or even negative-cost hydrophilic materials as substrates should be envisaged (hemicelluloses hydrolysates, bio-diesel derived waste glycerol, whey, sugar-based wastes, etc.), in order for an economically viable bioprocess to be performed.
The process of lipid production from fatty materials is critically influenced by the fatty acid composition of the fat used as substrate. “New” fatty acid profiles (in both extra- and intra-cellular level) that did not previously exist in the substrate fat may be produced during fat fermentation led by the oleaginous yeasts. An important application of the ex novo lipid production refers to the valorization of fatty wastes and the production of lipid-rich microbial biomass (containing in several cases valuable PUFAs) that will further be used as food or feed supplement 33, 38, 40, 108, 119, 120, 124, 131, 143. On the other hand, the most important application of the ex novo lipid accumulation process in the oleaginous microorganisms refers to the “improvement” and the “up-grade” of the fatty materials utilized as substrates in order to produce “tailor-made” lipids of high-added value. Such cases refer to the valorization of low-cost saturated fatty acids so as to produce intra-cellular substitutes of cocoa-butter or other high-value exotic fats like shea butter, sal fat etc (for review see: Papanikolaou and Aggelis 8) or the valorization of low- or negative-cost unsaturated fatty substances in order to produce lipids containing the medically important γ-linolenic acid 129, 130, 152.
The number of research investigations on the topic of SCO fermentation presents a significant expansion the last years. Research teams work on the identification and selection of “new” naturally occurring oleaginous strains capable of storing huge lipid quantities inside their cells or mycelia. Optimization of culture conditions related with the maximization of SCO production is being currently performed. These studies present interest for future experiments. Likewise, studies that concern advanced lipid biochemistry, identification of limiting steps related with the accumulation of storage lipid inside the cells or mycelia of the oleaginous microorganisms and extensive analysis of microbial lipid produced are also performed, while, equally, these studies present interest for future experimental work. The same is stressed in relation with investigations dealing with cloning of specific genes coding for lipid-accumulating key enzymes 153, kinetic modeling 29, 102, 124, 129, 130 and metabolic flux analysis and finally proteomic—genomic analysis 154 for several oleaginous microorganisms.
Acknowledgements
Financial support concerning the results achieved by our research team has been kindly provided by: Agricultural University of Athens; University of Patras; Dracoil SA; Project BIOSIS (INTERREG III GREECE, ITALY); State Scholarship Foundation (Athens, Greece); General Secretary of Research and Technology (Ministry of Development, Greek Government).
The authors have declared no conflict of interest.




