Metastable zone determination of lipid systems: Ultrasound velocity versus optical back-reflectance measurements
Abstract
The metastable zone width (MZW) of a multi-component system as influenced by the process parameters cooling rate, agitation speed, and additive concentration was determined via ultrasound velocity measurements. The results were compared with those obtained by optical back-reflectance measurements (ORM) using coconut oil as a model substance. Increasing the cooling rate led to the shift of the nucleation point to lower temperatures. This tendency was better visualized by the ultrasonic curves while a significant disturbance of the ORM signal could be observed. Agitation led to an increase of the nucleation temperature and hence a narrower metastable zone. The influence of an additive on the MZW was found to strongly depend on its concentration. The MZW detected by the ultrasound technique was narrower compared to that obtained by the ORM method, indicating the faster response to the phase transition of the ultrasound technique. Another advantage of the ultrasound technique was the in situ evaluation of the experimental data, while ORM needed a linear fitting to estimate the saturation temperature. Furthermore, ultrasound velocity measurements are based on density determination of the medium whereas the ORM sensor is able to detect only particles that are located within the measuring zone and possess a well-defined size.
Practical applications: MZW is one of the most important parameters that determine the characteristics of crystalline products. However, a proper technique that can be used in MZW detection in fat systems has rarely been reported, due to the difficulties in dealing with natural fats. The findings of this study can greatly help those who are involved in the field of fat crystallization from both the academic and the practical point of view. This is due to the fact that new and promising techniques for the online and in situ determination of the MZW of fats, with high accuracy, and reproducibility, under most process conditions, were clarified in this work. The readers can easily follow the procedure developed in this paper. Also information about the influence of process parameters and additives on the MZW is included.
Abbreviations:
MZW,
metastable zone width
ORM,
optical back-reflectance measurement
Introduction
Fats and oils can be regarded as multi-component systems consisting of various triglycerides and minor components (phospholipids, waxes, vitamins, etc.). The diverse compositions lead to broad melting point ranges and complex physical properties. Fats and oils are often modified via physical or chemical processes to extend their utilization, especially in food applications. Dry fractionation is a promising technique for this purpose. It is a physical process based on two main steps: partial crystallization of high-melting triacylglycerols (TAG) from a melt without solvents and a subsequent separation step of the crystals from the mother liquid. The characteristics of the fractionated products are strongly influenced by the crystallization step, which significantly depends on the process conditions and the parameter settings 1.
In general, crystallization includes two kinetic steps: nucleation and crystal growth. Nucleation can be referred to the birth of crystals. Once the nuclei are formed, they grow and develop into crystals. According to previous studies, nucleation has the strongest predetermining influence on product properties, such as the purity of the desired products, solid fat content, crystal habit, particle size, and size distribution 2.
Similar to solutions, melts must be supersaturated (supercooled) to initiate nucleation, which is achieved by cooling the melt to a temperature below the melting point. The temperature level of supercooling when nuclei are spontaneously formed is called metastable limit. In contrast to the saturation limit, the metastable limit is not thermodynamically defined 2 and strongly depends on process parameters such as cooling rate, agitation, and impurities. Operating a crystallization process at the temperature close to the metastable limit has the risk to result in large amounts of fine crystals. Furthermore, fast growth rates lead to liquid inclusions and diminished purities. Reversely, operating the crystallizer at a temperature close to the saturation limit results in slow growth rates and, hence, high purities. However, the latter case is less favored because of economic considerations. For this reason, controlling a crystallization process is a prerequisite to have a good compromise between product quality and cost efficiency. According to Hofmann 3, crystallizers should be operated approximately in the middle of the metastable zone. As a consequence, having a convenient way to detect the metastable zone width (MZW) of fat systems is essential in the field of industrial crystallization.
In order to determine the MZW, on- and in-line techniques are needed that enable a fast detection of nucleation and exhibit direct applicability in slurry systems 4-6. However, only limited knowledge is available on feasible practical techniques in melt crystallization, and especially in fat systems, which can be attributed to the complexity of fat compositions and the difficulties in obtaining an accurate state estimation.
Turbidimetry was applied to determine the MZW of palm oil in solvent fractionation 6. However, the authors concluded that the obtained results cannot be directly transferred to dry fractionation based on melt crystallization.
The ultrasound velocity detector was introduced by Ulrich and Omar 2, 4 as a feasible online measuring device to detect the MZW of salt solutions by monitoring the ultrasound velocity as a function of temperature.
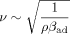 ((1))
((1))The density of oils was reported to show a reverse proportionality to temperature 8, while adiabatic compressibility is linearly proportional to temperature 9. However, according to Strege 7, the adiabatic compressibility has the stronger determining impact on the ultrasound velocity. Ultrasonic velocity increases with increasing temperature and varies with pressure and concentration.
In fat crystallization, ultrasound velocity measurements have been reported as an alternative technique for determining the solid fat content of fat systems 10, 11. However, its application to MZW determination of fat systems has not been widely investigated so far.
Laser light reflectance measurement techniques like optical back-reflectance measurements (ORM) are commonly used methods to determine the particle size distribution. They enable both online and in situ determinations, even in systems exhibiting high solid concentrations 12-15. These measurements are based on the laser light backscattering technique, which determines the chord length distribution (CLD). The laser light randomly travels through the measurement zone. As the beam hits the surface of a particle (crystal), light is backscattered into the probe. Accordingly, the number of counts depends on the concentration of solid particles present in the suspension. So far, laser reflectance measurement has been used in various applications, such as the determination of the aggregation and particle size in salt solutions and emulsion systems 12. Although this technique has rarely been reported in the context of fat crystallization and MZW determination of fat, it possesses a great potential for detecting the MZW in fat systems, due to the wide range in particle size detection from 0.14 to 2400 µm 16.
In this study, the MZW of a multi-component system was determined via ultrasound velocity measurements and the results were compared to those obtained by ORM, using coconut oil as a model substance. The effects of process parameters such as cooling rate, agitation speed, and additive concentration on the MZW were also investigated using the above-mentioned techniques.
Materials and methods
Fat melts of refined, bleached, and deodorized (RBD) coconut oil (Ostthüringer Nahrungsmittelwerk Gera, Gera, Germany) and lauric acid (Sigma–Aldrich Laborchemikalien, Seelze, Germany) as an additive were chosen as model systems. The same batch of the substances was used throughout the experiments.
In situ measurement of the MZW
The ultrasound velocity was detected as a function of temperature by using a low-energy, high-frequency Liquisonic 30 immersion sensor (2 MHz, 0.1 W) developed by SensoTech (Magdeburg, Germany). ORM were based on the detection of reflected laser light counting the hit particles per second as a function of temperature (Messtechnik Schwartz, Düsseldorf, Germany).
Of melt, 200 g was filled into a crystallizer (400 mL double-wall beaker) with the sensor immersed into the melt, which was agitated by a magnetic stirrer. The temperature of the crystallizer was controlled by a programmable thermostat. The experimental setup is shown in Fig. 1.
Experimental setups for ultrasound velocity measurements (A) and ORM (B).
Effect of cooling rate and agitation speed
Prior to the experiments, coconut oil was conditioned at 35°C for 1 h to destroy its crystal memory, and subsequently cooled down in a linear mode until the first nuclei were formed. The melt was immediately heated up to the initial temperature at the same rate. The effect of the cooling rate was studied by varying the cooling rate of the programmable thermostat from 5 to 15 K/h at a constant agitation speed of 500 rpm. The effect of the agitation rate was studied by varying the agitation rate from 0 to 700 rpm at a constant cooling rate of 10 K/h.
Effect of additive concentration
The mixture of coconut oil and lauric acid was prepared by generating homogeneous melts heated at 80°C for 30 min. The concentration of lauric acid ranged from 0 to 15 wt%. Cooling rate and agitation speed were kept constant at 10 K/h and 500 rpm, respectively, in all experimental runs. Three parallel measurements were carried out for each condition and the average value is presented in the Results and discussion section.
Graphic interpretation of the experimental results
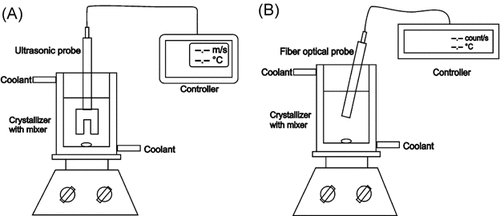
Figure 2 demonstrates the typical graphs of ultrasound velocity versus temperature (A) and time (B). The relationship between ultrasound velocity and temperature is characterized by a curve exhibiting one shoulder (Fig. 2A). The nucleation temperature is defined as the temperature value at which a sudden increase of ultrasonic velocity was detected in the cooling cycle (point 1). This point is in good agreement with the crystallization exotherm obtained from the temperature–time curve shown in Fig. 2B. The temperature at the intersection of the ultrasonic velocity signal from the cooling and reheating cycle was defined as the saturation point of the systems (point 2). The maximum allowable supercooling (MZW) is determined as the temperature difference between the saturation and nucleation temperatures.
Ultrasound velocity as a function of temperature (A) and time (B).
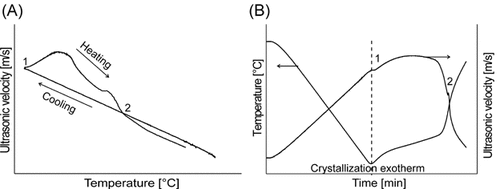
As shown in Fig. 3A, temperature–time curves obtained from the ORM measurements exhibited the same character as detected via the ultrasound velocity measurements (Fig. 2B), possessing a sharp crystallization exotherm where nucleation occurs. This point coincides with the last point of the particle–time curve prior to the great increase in particle number, indicating that the sensitivity of ORM is sufficient to detect the first solid particles crystallizing in the melt (nucleation point, point 1). The saturation temperature can be estimated by linear fitting of the temperature–time curve (point 2). The saturation temperature (intersection obtained by linear fitting) is equal to the temperature where the number of the crystals decreases to the initial value. The MZW can also be determined from the curve of particle counts versus temperature (Fig. 3B) presenting an oscillation at the end. The first point of the peak is related to the nucleation point while the point where the signal of the reheating cycle is equal to that of the cooling cycle is the saturation point. In this work, the MZW is determined based on Fig. 3A because of the great fluctuation of the crystal counts per second versus the temperature curve presented in Fig. 3B which leads to an inaccuracy of the measurements under certain experimental conditions.
Crystal counts per second as a function of time (A) and temperature (B) determined by ORM measurements.
Shear viscosity determination
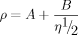 ((2))
((2))Characterization of crystal morphology
Approximately 3 mL melt of the mixture was filled into the microscope cell (diameter of 3.6 cm) equipped with a programmable thermostat. The sample was heated at 50°C for 15 min and cooled down to the crystallization temperature with a cooling rate of 1 K/min. When the crystallization temperature was reached, the sample was kept isothermal and the crystal morphology was observed after 60 min by a light microscope (magnification 3.3 × 5; Olympus BH2-UMA, Olympus Optical Co., Tokyo, Japan).
Results and discussion
Influence of the cooling rate on the MZW of coconut oil
In this study, the nucleation and saturation points were determined by using the techniques described above. Ultrasound velocity increases linearly through the melt with decreasing temperature until the nuclei are formed (Fig. 4A), which can be explained by the fact that nucleation leads to a decrease in the density of the liquid phase 17. In Fig. 4B, the sharp increase of the peak refers to the presence of crystals, while the signal intensity falls back to the baseline value when all particles are molten in the reheating cycle.
Effect of cooling rate on the MZW of coconut oil detected by ultrasound technique (A) and by ORM (B).
The ultrasonic sensor detected the nucleation point at higher temperatures than the ORM, which can be attributed to the higher sensitivity of the ultrasonic probe (Table 1). As a consequence, the MZW of the coconut oil detected by the ultrasound velocity sensor is significantly narrower compared to the data obtained by ORM. The results summarized in Table 1 indicate that both of the applied measuring techniques detected a broadening of the MZW with increasing cooling rate, which is in good agreement with the findings of Ulrich and Strege 2.
| Nominal cooling rate [K/h]a) | Tc [°C]b) | Ts [°C]b) | MZWb) | |||
|---|---|---|---|---|---|---|
| Ultrasound | ORM | Ultrasound | ORM | Ultrasound | ORM | |
| 5 | 20.51 ± 0.18 | 20.30 ± 0.00 | 27.12 ± 0.03 | 27.07 ± 0.02 | 6.61 | 6.77 |
| 10 | 20.00 ± 0.21 | 19.73 ± 0.06 | 27.75 ± 0.11 | 27.66 ± 0.05 | 7.76 | 7.93 |
| 15 | 19.53 ± 0.06 | 18.87 ± 0.35 | 28.21 ± 0.09 | 28.79 ± 0.37 | 8.68 | 9.92 |
- Data expressed as mean ± SD (n = 3).
- a) Nominal cooling rates were set on the programmable thermostat.
- b) Tc = nucleation temperature; Ts = saturation temperature; MZW, =ΔT = Ts − Tc.
Figure 4 clearly demonstrates the shift of the nucleation point to lower temperatures when increasing the cooling rate for both measuring techniques. This tendency, however, is better visualized by the ultrasonic curves (Fig. 4A), while Fig. 4B reveals a high disturbance of the ORM signal. Ulrich 18 reported that the nucleation point of a binary system can be shifted by varying the process parameters such as the cooling rate, whereas the process conditions have no impact on the saturation point in case of aqueous solutions. Our results revealed that a cooling rate of 15 K/h increased the saturation point of the model system. This observation is in good agreement with the results of Smith 6 who reported a significant change in the saturation temperature of a mixed system containing diverse triglycerides with the variation of the cooling rate. However, it must be noted for fat systems that different components tend to co-crystallize and form mixed crystals, depending on the crystallization parameters such as the cooling rate. Consequently, different cooling rates might result in solid fractions crystallizing in a different way and exhibiting various chemical compositions. Hence, the saturation point of coconut oil can be affected. However, the dependency of the saturation temperature on the cooling rate might result from the measuring technique due to non-isothermal conditions. In order to give a plausible explanation for this observation, special efforts are necessary to identify the exact chemical composition of the solid fraction.
Effect of agitation
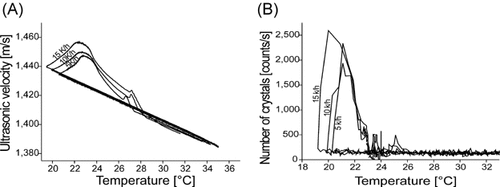
The results included in Table 2 indicate that both techniques detect nucleation at higher temperatures in an agitated melt. This can be explained by the fact that agitation provides a rapid and hence more efficient heat and mass transfer. Therefore, nucleation also occurs faster in an agitated system.
| Agitation speed [rpm] | Tc [°C] | Ts [°C] | MZW | |||
|---|---|---|---|---|---|---|
| Ultrasound | ORM | Ultrasound | ORM | Ultrasound | ORM | |
| 0 | 18.31 ± 0.01 | 18.97 ± 0.06 | 28.89 ± 0.01 | 27.1 ± 0.94 | 10.57 | 8.14 |
| 200 | 20.53 ± 0.18 | 19.70 ± 0.17 | 28.83 ± 0.06 | 28.2 ± 0.15 | 8.31 | 8.46 |
| 500 | 20.00 ± 0.21 | 19.73 ± 0.06 | 27.75 ± 0.11 | 27.7 ± 0.05 | 7.76 | 7.93 |
| 700 | 20.07 ± 0.12 | 19.57 ± 0.06 | 27.70 ± 0.04 | 27.9 ± 0.16 | 7.63 | 8.30 |
- Data expressed as mean ± SD (n = 3).
On the other hand, it is well known that the first nuclei crystallize near to the wall and the bottom of the reactor. As a consequence, a static supercooled melt is not homogeneous. The nuclei can easily sediment without agitating the melt. Considering that the ORM sensor has a measuring zone of 1 mm, it is reasonable to assume that there are some particles located out of this zone that cannot be detected by the sensor in a static system.
The evaluation of the experimental results (Table 2) reveals that the ultrasound velocity measurements detected a significant increase of the nucleation temperature and a narrower metastable zone when applying an agitation rate of 200 rpm, while increasing the agitation rate above this value has no considerable effect on the nucleation point.
Figure 5 also demonstrates that the nucleation temperature increases when applying agitation during the crystallization process. The graphic interpretation of the data also confirms that the variation of the agitation rate (500 or 700 rpm) has no significant effect on the nucleation point. Fig. 5B, however, shows a sequence of large peaks at an agitation rate of 700 rpm. In addition, a shoulder appears in the ultrasound curve under these conditions. These observations allow the conclusion that the agitation rate might have a rather disturbing effect on the measuring signal above a certain limit.
Effect of agitation on the MZW of coconut oil detected by ultrasound velocity technique (A) and by ORM (B).
The descent of the saturation point with the increment of the agitation speed could be detected by ultrasound technique, while ORM provided an unclear trend due to scattered signals. The effect of process parameters on the saturation point of multi-component systems has already been assumed in the previous section.
Effect of additive
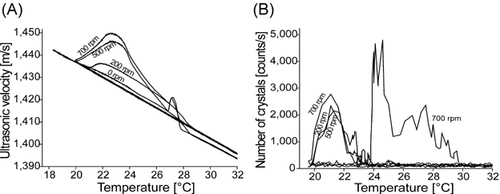
Figure 6A demonstrates higher values of ultrasound velocity in a pure melt compared to those containing an additive (lauric acid). The effect is more pronounced when increasing the additive concentration. In addition, the shoulder becomes smoother when increasing the lauric acid content. The data obtained by the ORM technique display a similar tendency (Fig. 6B). This observation can be explained by the results of the viscosity measurements, which indicate an exponential decrease in viscosity with an increasing concentration of lauric acid (Fig. 6C). The addition of lauric acid increases the density of the melt, resulting in lower velocities of the ultrasound. Previous studies reported that viscosity reduction (e.g., by using additives) accelerates nucleation. However, systems are known where the viscosity of the bulk solution may be modified by additives without a significant influence on nucleation 19.
Influence of lauric acid on the MZW of coconut oil determined by ultrasound technique (A) and ORM (B) and influence of lauric acid concentration on the viscosity of coconut oil (C).
According to Table 3, both methods detected similar values for the nucleation and saturation temperatures of the mixtures. However, the ultrasound technique still detected the temperature change earlier than the ORM method. Increasing the concentration of lauric acid to 10% results in a depression of the nucleation point although the viscosity of the melt decreased.
| Lauric acid [%] | Tc [°C] | Ts [°C] | MZW | |||
|---|---|---|---|---|---|---|
| Ultrasound | ORM | Ultrasound | ORM | Ultrasound | ORM | |
| 0 | 20.00 ± 0.21 | 19.73 ± 0.06 | 27.75 ± 0.11 | 27.66 ± 0.05 | 7.76 | 7.93 |
| 5 | 20.21 ± 0.10 | 19.43 ± 0.06 | 27.36 ± 0.06 | 27.26 ± 0.02 | 7.15 | 7.83 |
| 10 | 19.37 ± 0.31 | 17.77 ± 0.06 | 26.35 ± 0.06 | 26.21 ± 0.17 | 6.98 | 8.45 |
| 15 | 22.51 ± 0.05 | 20.43 ± 0.25 | 26.80 ± 0.10 | 26.79 ± 0.08 | 4.29 | 6.36 |
- Data expressed as mean ± SD (n = 3).
The particle curve generated at 15% lauric acid concentration shows a great fluctuation in the particle number, and the intersection of the signal obtained from the heating and cooling cycles cannot be identified (Fig. 6B). The ultrasound velocity measurements performed at the highest additive concentration (15%) also provided a curve displaying a different character than that at lower concentrations (Fig. 6A).
It was observed that there were colloid-like particles suspended in the mixture during the cooling cycle that caused a sudden decrease in the ultrasound velocity signal at the temperature of 22.51°C. However, further cooling of the mixture resulted in a second stage of nucleation at a temperature of about 19°C without the detectable change of ultrasonic velocity signal. The nucleation at this temperature is similar to the one of the coconut oil mixture in the presence of low lauric acid concentrations. Therefore, the nucleation point matching this mixture in Table 3 results from the temperature where the change of the ultrasound velocity signal can be detected.
The effect of lauric acid on nucleation is assumed to be related to the strength of the molecular interactions between the triglycerides of coconut oil and lauric acid, which strongly depends on concentration. It is well known that lauric acid is the main fatty acid component of coconut oil triglycerides. Therefore, when present in relatively small concentrations, lauric acid can form strong physical bonds with the coconut oil triglycerides. Lauric acid incorporates into the growing embryo prior to the nucleation, which results in an inhibiting effect on the nucleation process. At higher concentrations, however, lauric acid molecules that cannot be incorporated into the embryo associate together in the mixture 20. During cooling, they form their own crystals at a higher temperature level. Those crystals can cause the nucleation at the higher temperature before the temperature reaches the true nucleation point. As a result it can be concluded that the effect of lauric acid on the MZW of coconut oil is strongly influenced by concentration.
An addition of lauric acid affects the saturation temperature of the melt in the same manner as described for the nucleation temperature. The saturation temperature decreases as the amount of lauric acid is increased up to 10% in concentration. When more than 15% lauric acid is present, the saturation temperature increases, but less dramatically than the nucleation temperature.
The characterization of crystal morphology performed by light microscopy supported the hypothesis described above. The crystal morphology obtained after 60 min of isothermal crystallization at 21°C revealed that coconut oil crystallizes as spherulites consisting of needle-like single crystals (Fig. 7A). No significant change of crystal habit was found when lauric acid was present at concentrations of up to 10%. However, plate needles were observed on the surface of the melt without the formation of spherulites when increasing the lauric acid concentration to 15% (Fig. 7B).

Crystal morphology of coconut oil (A) and plate needle-like crystals of the coconut oil + 15% lauric acid (B) crystallized after 60 min at 21°C.
Two stages of nucleation of coconut oil in the addition of lauric acid at 15% concentration were observed at a crystallization temperature of 18°C (Fig. 8). Plate needle crystals were present on the surface of the melt during the first 20 min of the crystallization process. Afterward, the typical spherical crystal agglomerates of coconut oil were formed in the melt and the plate needle-like crystals were observed to nucleate at the edge and the surface of the spherulites, allowing the simultaneous crystallization of multiple components of coconut oil and lauric acid. According to Smith 6, the higher-melting additive may act as a seeding material and initiates early nucleation. However, our results allow the hypothesis that lauric acid does not act as a seeding material, even though it is applied in high concentrations, since it operates in the nucleus and retards the true nucleation of coconut oil. The excess amount of lauric acid seems to result in separate crystallization of the two components without inducing the nucleation of the coconut oil.
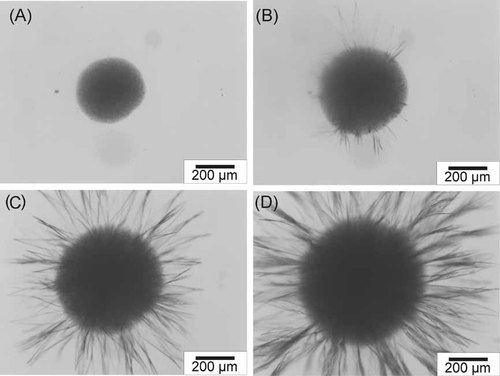
Stages of crystallization of coconut oil +15% lauric acid at 18°C: (A) spherulite in the melt at 30 min, (B) at 48 min, (C) at 50 min, and (D) at 60 min.
This allows the conclusion that, up to a concentration of 10%, lauric acid is incorporated into the coconut oil crystal, whereas by increasing the concentration to 15% lauric acid might be crystallized separately. In order to confirm this hypothesis, the exact chemical composition of the solid phase should be investigated.
Conclusions
It can be concluded that the MZW detected by the ultrasound velocity measurements was narrower compared to those obtained by the ORM method. This can be attributed to a higher accuracy of the ultrasonic technique, which is due to the fact that a direct evaluation of the ultrasound curves is possible. Furthermore, the ultrasonic measurements exhibited a better graphical reproducibility. The ORM curves, on the other hand, enable a simple determination of the nucleation temperature while the saturation point can be estimated via a linear fitting giving the possible inaccuracy. It must be noted that the ultrasonic measurements are based on density determination while ORM detects particles possessing a well-defined size and located within the measuring zone of the optical sensor, which can also be considered as a limiting factor concerning accuracy. Therefore, the applicability of the ORM technique is limited for the determination of the MZW of fat systems, whereas the ultrasound velocity measurements proved to be an accurate method for the MZW detection, proven by the model system based on a number of parameter changes examined. Furthermore, the above-mentioned measuring methods are not suitable to provide qualitative information on the nucleation process. For instance, the mechanism of the additive influence cannot be clearly determined, neither by the ultrasound apparatus nor with the ORM technique.
The authors have declared no conflict of interest.




